Ovarian hyperstimulation syndrome following the use of GnRH agonist trigger of final oocyte maturation and freeze-all strategy: A case report and review of the literature
Dalia Khalife, Suleiman Ghunaim✉, Lina El Taha, Omar Odeh, Natasha Habr, Johnny Awwad
1Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, Haifa Idriss-ART Unit, American University of Beirut,Beirut, Lebanon
2School of Medicine, The University of Jordan, Amman, Jordan
3Division of Plastic and Reconstructive Surgery, American University of Beirut Medical Center, Beirut, Lebanon
ABSTRACT
Rationale: The current literature has a surprising controversy regarding the use of low-dose human chorionic gonadotropin (hCG)for luteal support as an explanation for the development of ovarian hyperstimulation syndrome, and this is because of the gap in the listing of the predisposing factors that put women at an increased risk of ovarian hyperstimulation syndrome.
Patient concerns: A case of 25-year-old woman presented with abdominal pain, distention, dyspnea, and nausea with a 6.5 kg increase in weight from baseline. Ultrasonographic examination showed bilaterally enlarged multicystic ovaries after gonadotropinreleasing hormone (GnRH) agonist triggering and cycle segmentation with no hCG rescue administration.
Diagnosis: Moderate/severe ovarian hyperstimulation syndrome.
Interventions: The woman was admitted to the hospital for medical management of moderate/severe ovarian hyperstimulation syndrome, and pain management was advanced to patient-controlled anesthesia with the start of low molecular weight heparin. On day 2, albumin therapy followed by a furosemide chase was started due to an increase in abdominal girth. On day 1, Cabergoline was maintained, and on day 2 the GnRH antagonist Cetrorelix was started.
Outcomes: The woman’s clinical condition improved, and a clinical pregnancy was eventually achieved during the first cryo-warmed blastocyst cycle.
Lessons: Ovarian hyperstimulation syndrome can still happen even after the use of GnRH agonist and avoidance of hCG support.Segmentation of in vitro fertilization with complete avoidance of hCG for luteal support remains the best approach.
KEYWORDS: Ovarian hyperstimulation syndrome;Gonadotropin releasing hormone agonist; In vitro fertilization;Human chorionic gonadotropin support; Ovulation induction;Reproductive endocrinology
1. Introduction
Ovarian hyperstimulation syndrome (OHSS) is a serious complication of controlled ovarian stimulation. It is characterized by an exaggerated ovarian response and a clinical spectrum resulting from third space sequestration of protein-rich fluid.Signs and symptoms include abdominal discomfort, ascites,hemoconcentration, electrolyte imbalance, and oliguria. In extreme cases, thromboembolism, renal failure and death may occur[1].
Human chorionic gonadotropin (hCG) has been traditionally used to trigger ovulation. With a similar biological activity as luteinizing hormone (LH), hCG can activate LH receptors. However, its prolonged half-life (24-36 hours) and sustained luteotropic effect have led to an increased incidence of OHSS[2]. Increased vascular permeability is mediated mainly by vascular endothelial growth factor (VEGF) and other factors including prostaglandins, prolactin,cytokines and the renin-angiotensin-aldosterone system[3].
The overall incidence of severe OHSS has been reported to be 2% to 3% in hCG-triggered ovarian stimulation cycles[4,5].Several preventive strategies to improve cycle safety have been proposed which include withholding of hCG administration and cycle cancelation. The recent adoption of gonadotropin-releasing hormone (GnRH) agonist triggering for final oocyte maturation has significantly reduced the overall incidence of OHSS[6]. The use of the GnRH agonist trigger, although a safer alternative to hCG, has been associated with a significant reduction in pregnancy outcome when a fresh embryo transfer is considered. Lower implantations and higher miscarriages were attributed to the luteolytic effect associated with a shorter exposure to the endogenous LH surge elicited by GnRH agonist. The addition of a single 1 500 IU hCG luteal rescue dose at the time of oocyte retrieval has significantly improved clinical outcomes, albeit a reported severe OHSS incidence of 0.72% in selected high-risk patients. In the absence of well-defined clinical guidelines for the use of the hCG rescue protocol, the incidence of severe OHSS has been reported to attain 26% in poorly selected high-risk patients[7]. For this reason, a segmented approach consisting of GnRH agonist triggering and a freeze-all policy has been recommended for the complete prevention of OHSS in women with extreme ovarian response[7-9].
We report on a case of severe early OHSS requiring in-hospital management developing after GnRH agonist triggering and cycle segmentation with no hCG rescue administration.
2. Case report
A 25-year-old (body mass index of 20.9 kg/m2) nulligravid woman,previously healthy, presented with primary infertility of one year duration. Her menarche occurred at age of 12, followed by regular and predictable menstrual cycles every 28 days. She never reported any history of acne, excessive body hair growth, or scalp hair loss.Her ultrasound findings were remarkable for polycystic ovarylike ovaries. Her husband’s semen analysis was notable for severe oligoasthenospermia with 100 000 total sperm count/mL and a motility 20%.
The GnRH antagonist Cetrorelix (Cetrotide®; Merck Serono;Netherlands) was utilized for pituitary suppression. Step-down ovarian stimulation was initiated on the third day of the menstrual cycle with a starting dose of 225 IU of recombinant folliclestimulating hormone (recFSH) (Gonal F®; Merck Serono;Netherlands). Transvaginal ultrasound monitoring was used to measure follicle growth. Because of follicular growth stagnation on stimulation day 8, the recFSH was substituted for an equivalent dose of human menopausal gonadotropin (Menopur®; Ferring;Denmark). On day 10 of stimulation, the size of each ovary was more than 10 cm in diameter. Final oocyte maturation was triggered using a single bolus of 0.3 mg of triptorelin (Gonapeptyl®; Ferring;Denmark) when at least 3 follicles reached 18 mm in diameter.
Ovum pick-up was performed 36 hours later. Thirty-eight oocytes were retrieved, yielding 34 metaphaseⅡ oocytes and 26 zygotes.Because of excessive ovarian stimulation, about 10 follicles in the upper pole of each ovary remained inaccessible to transvaginal needle aspiration. Because of incomplete follicle aspiration, the dopamine agonist Cabergoline (Dostinex®) was started at daily dose of 0.5 mg for the next 10 days. Cycle segmentation was discussed with the couple at this stage, and a total of 11 blastocysts were cryopreserved.
3. Clinical findings
Twenty-four hours following oocyte pick-up, the patient presented to the emergency department with abdominal pain, distention,dyspnea and nausea. She had 6.5 kg increase in body weight from baseline 24 hours ago. Physical exam was remarkable for bilaterally decreased basilar breath sounds and a tender tense abdomen.Ultrasonographic examination showed bilaterally enlarged and kissing multi-cystic ovaries (left: 16.1 cm × 8.4 cm × 9.7 cm and right: 13.5 cm × 8.0 cm × 8.9 cm), occupying most of the pelvis and abdomen reaching almost 3 cm above the umbilicus. A moderate amount of non-echogenic ascitic fluid was also visualized. Doppler studies confirmed adequate blood flow through the ovarian vessels.Computed tomography scan of abdomen and pelvis (Figure 1) ruled out the presence of acute intraabdominal inflammatory processes including appendicitis. It also confirmed ultrasound ovarian findings.

Figure 1. Abdomino-pelvic computed tomography with intravenous contrast showing enlarged ovaries (Coronal cut).
4. Therapeutic intervention
The patient was admitted to the hospital for medical management of moderate/severe OHSS. Her laboratory results indicated hemoconcentration: white blood cell count 15 400/mm3; hemoglobin 15.7 g/dL; and hematocrit 46%. Pain management was advanced to patient-controlled anesthesia with a fentanyl baseline rate of 10 mcg/h, and a bolus of 10 mcg/h as needed every 6 min. Low molecular weight heparin (Lovenox®) was started at a daily dose of 4 000 IU, while maintaining Cabergoline treatment.
On day 2 of admission, abdominal girth increased significantly(2 kg weight gain over 24 h). Urine output was determined at 25-30 mL/h. At this stage, albumin therapy (20%) was administered in doses of 50 g in 4-hour infusions repeated every 12 h and followed by a furosemide chase (Lasix 20 mg) as per protocol. The GnRH antagonist Cetrorelix (Cetrotide®, Merck Serono, Netherlands) was also started at a daily dose of 0.25 mg.
5. Follow up and outcome
The patient’s clinical condition and symptoms improved gradually over the next 5 days. She was discharged from the hospital on day 5 of hospitalization.
A clinical pregnancy was achieved during the first cryo-warmed blastocyst cycle which carried to term and resulted in a live male delivery at 39 weeks of gestation.
6. Discussion
Our patient underwent an antagonist protocol stimulation as it has been reported to decrease the incidence of OHSS by 20% in high risk patients[10,11], and final oocyte maturation was achieved using GnRH agonist with embryo freezing in lieu with the principal of segmentation of in-vitro fertilization (IVF) treatment to achieve an OHSS-free clinic[9]. Despite being associated with a high safety profile, the GnRH agonist trigger and freeze-all policy have been associated with sporadic cases of severe OHSS (Table 1).
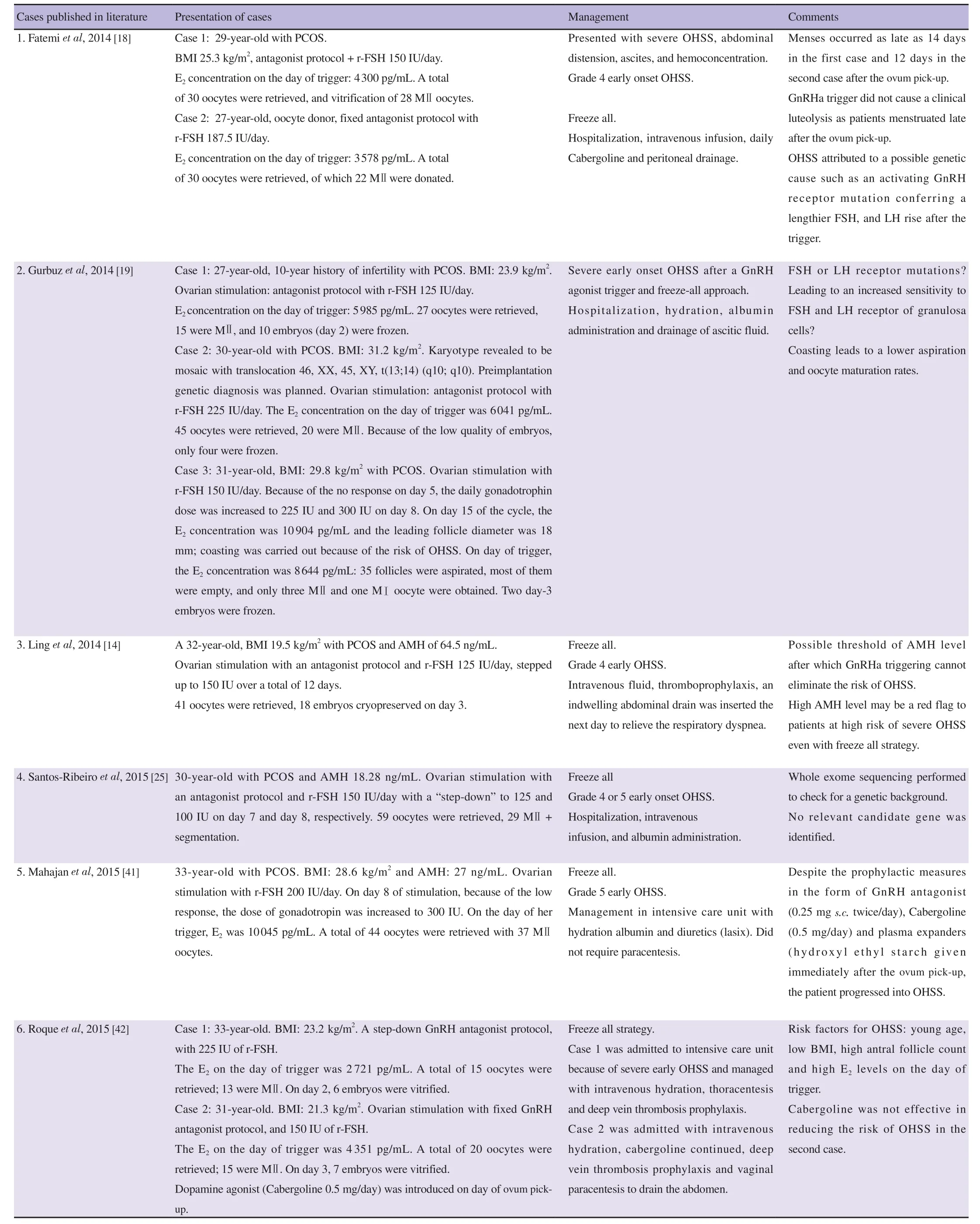
Table 1. Ten ovarian hyperstimulation syndrom cases published after GnRH agonist triggering and no hCG rescue during the luteal phase.
GnRH agonist triggering of follicle maturation prevents the occurrence of OHSS by decreasing the duration of LH stimulation of the luteinized granulosa/theca cells to hours instead of days, and by inducing luteolysis leading to a rapid decrease in estrogen and progesterone levels[12].
Predisposing factors that put the patient at an increased risk of OHSS are not clearly listed in the literature. Some have labelled patients as at high risk of OHSS when the total number of follicles≥11 mm is between 15 and 25[13]. Others have found a correlation between high serum estradiol >4 000 to 6 000 pg/mL levels and a higher risk of OHSS. Other investigators have speculated the important value of the anti-Müllerian hormone (AMH) levels before proceeding to an IVF cycle. Ling et al considered that a likely threshold of AMH level exists after which agonist may not be successful in eradicating OHSS[14]. The cut-off value for AMH with 90% sensitivity and 71% specificity is 3.3 ng/mL to predict women at high risk for OHSS[15]. OHSS has also been correlated with the presence of a high number of multiple immature to intermediate follicles. During the oocyte pickup, a lot of follicles were not reachable because of the enlarged ovaries, and the progression of our case into ovarian hyperstimulation.
There is a surprising controversy regarding the use of low-dose hCG for luteal support as a possible explanation for development of OHSS[4,7,16,17] as many cases exist in the absence of utilization of hCG in the luteal support[14,18,19]. A pilot study of 12 high responder patients with ≥25 follicles ≥11 mm on the day of trigger showed that triggering with agonist for final oocyte maturation followed by hCG rescue dose avoids early OHSS, yet only one late case of moderate OHSS developed[16]. Nevertheless, a multicenter retrospective study,including 275 women at high risk of OHSS undergoing antagonist protocol with agonist trigger combined with 1 500 IU hCG at the time of oocyte retrieval reported 2 cases of severe OHSS (with an incidence of 0.72%), displaying that the risk cannot be eliminated[4].In addition, a retrospective study indicated a 26% incidence of early OHSS after agonist triggering, suggesting that hCG injection is to be avoided when patients are believed to be at high risk[7]. However,Humaidan et al[13] conducted two randomized trials including 390 women at high risk. They described a complete avoidance of OHSS despite the use of hCG rescue dose in patients with 15–25 follicles≥11 mm. The complete prevention of OHSS might have been related to the fact that patients with more than 25 follicles were excluded to be able to randomize patients between GnRH agonist and hCG trigger[13].
As for the etiology, some have considered gene mutations in GnRH receptor, FSH receptor, or LH receptor predisposing women to OHSS[20]. Interestingly, O’Brien et al reported that patients with luteinizing hormone/chorionic gonadotropin receptor (LHCGR)mutations are at higher risk of OHSS even in normal responder patients[21]. In addition, families with FSH receptor (FSHR)mutations display high sensitivity to hCG with reported occurrence of spontaneous OHSS[22]. Other possible genes include: BMP15,GDF9, AMH, VEGF, VEGR 1 and 2, ESR 1 and 2, CYP11A1,CYP19A1, SHBG, MTHFR and p53[23,24]. On the contrary, Santos-Ribeiro et al have analyzed a whole genome in a case of severe early OHSS after agonist trigger, and found no genetic variants[25]. Thus,genetic susceptibility is to be established. Assuming the restricted information on genetic factors, FSH receptor activating mutation may be a potential explanation of the occurrence of severe OHSS cases in freeze-all strategies, by the constant stimulation of hCG to the receptor leading to the release of VEGF and other mediators responsible of the development of OHSS[18]. Therefore, FSHR genotype might be a possible explanation to our case influencing the risk of iatrogenic OHSS.
Regarding management, individualization of gonadotropin dosage is suggested. In order to optimize live birth rates and to avoid the iatrogenic risk of OHSS, adjustments of FSH dosages and individualization of controlled ovarian stimulation have been established. Our concerns are the high responder patients. Algorithms to tailor dosages are lacking in the literature and some are based on the AMH levels and antral follicle count as well as the ovarian response in a previous cycle[26]. Van Tilborg et al[27] in a multicenter randomized controlled trial (RCT) study aimed to evaluate whether individualization of FSH dosage (225-450 IU/day) based on the antral follicle count is cost-effective compared to a standard dose regimen (150 IU/day). Surprisingly, their results showed that individualization based on the antral follicle count does not increase the live birth rates, but it reduces the occurrence of mild to moderate risk of OHSS[27]. Despite the failure to prove any beneficial effect in terms of pregnancy outcomes, several investigators claimed a better safety control in patients at risk of high response receiving an individualized dose according to their reserve when compared to the conventional ovarian stimulation[28,29]. Body mass index (BMI)was also one of the variables studied in the different algorithms.Inconsistencies in the literature exist regarding tailoring the dosage according to the weight of the patient, as FSH levels vary between patients even with fixed doses[30]. In addition, the combination of body weight and antral follicle count to determine the FSH dose does not seem to be beneficial[28]. Thus, using preventive therapeutic options such as using an antagonist protocol with GnRH agonist triggering for final oocyte maturation along with a freeze all strategy remains the best option for patients at risk of hyperstimulation.
Furthermore, the incidence of OHSS was shown to be lower in individualized cycles tailored to the AMH levels with 2.3% compared to 6.9% in cycles not tailored to the AMH levels (P=0.002)[31]. In a retrospective study, another model was proposed based on the age,BMI, FSH and antral follicle count and showed that the ovarian response is best predicted by only two markers (antral follicle count and FSH)[32]. In a prospective study, the usability of the CONSORT calculator was shown to be low as the calculated starting dose of FSH was not always concordant with the patient profile[33].
In high responder patients, the starting dose of gonadotropin is of utmost importance as low dosages may lead to mono-follicular recruitment and to excess response in the case of high dosages.Thus, a guidance through certain algorithms may help physicians in maintaining a safe stimulation protocol.
In predicted hyper responders, Oudshoorn et al[34] randomized 521 patients with an antral follicle count of >15 to an FSH dose of 100 IU or 150 IU/day and demonstrated that the occurrence of any grade of OHSS was lower after a lower FSH dose [5.2% versus 11.8%, relative ratio (RR) 0.44, 95% confidence interval (CI) 0.28-0.71, P=0.001], but the occurrence of severe OHSS did not differ(1.3% versus 1.1%, RR 1.25, 95% CI 0.38-4.07, P=0.728), yet results were probably limited by the lower average BMI of the recruited population along with the relatively small sample size.
In another RCT study, it was shown that once serum estradiol level was noticed to be rising above 3 500 pg/mL combined with ≥18 follicles >11 mm in diameter on a conventional gonadotropin dose in controlled ovarian stimulation through an antagonist protocol,tapering down the gonadotropin dose to 100 IU/24 h significantly decreased moderate/severe OHSS in hCG triggered cycles[35].
The administration of a dopamine analogue in our case was made on the basis that patients with polycystic ovary syndrome have lower levels of dopamine and reduced dopamine D2 receptor levels in the ovary. The use of dopamine D2 agonists (Cabergoline, Dostinex)as displayed in animal and human studies have shown to decrease ovarian VEGF production and reduce the risk of OHSS[36,37].
The effectiveness of dopamine agonists in the prevention of OHSS was evaluated in a Cochrane review. It included 16 RCTs with 2 091 high risk patients and showed with a moderate quality evidence that dopamine agonists are effective in preventing moderate to severe OHSS [odds ratio (OR) 0.27, 95% CI 0.19–0.39]. When comparing dopamine agonists to human albumin, Cabergoline was correlated to a lower risk of moderate to severe OHSS (OR 0.21,95% CI 0.12–0.38). More importantly, the use of dopamine agonists does not negatively affect the pregnancy outcomes[38]. Despite the administration of a daily dose of Cabergoline of 0.5 mg/day to our patient, her symptoms progressed over the first couple of days.
With regards to GnRH antagonist administration, the first reported attempt for the use of antagonist in the treatment of early OHSS following oocyte retrieval was for 3 patients by Lainas et al[39].This was done by administering GnRH antagonist at a dose of 0.25 mg/day starting day 3 post oocyte retrieval and continued for one week. Results have displayed a marked decrease of hematocrit,white blood cells count, ovarian volume and ascitic fluid which were noted on follow up.
Furthermore, Prapas et al[35] demonstrated in a RCT of 194 patients defined as high risk for developing moderate/severe OHSS based on a rising estradiol level ≥3 500 pg/mL combined with ≥18 follicles>11 mm in diameter that the administration of a rescue double GnRH antagonist dose the day before hCG trigger may represent a safe alternative preventive strategy for early OHSS (0% vs. 12.37%,P<0.001).
To assess the effectiveness of Cetrorelix in preventing moderate to severe OHSS, a prospective cohort study was conducted on 105 patients in which Cetrorelix was administered from day 3 to day 5 after the ovum pick-up. The occurrence of moderate to severe OHSS was significantly lower in patients receiving the antagonist compared to no intervention (18.03% vs. 37.14%, P=0.037) concluding a faster resolution of symptoms[40].
Other ovarian hyperstimulation syndrom cases[41,42] after GnRH agonist triggering and no hCG rescue during the luteal phase are shown in Table 1 as well.
In conclusion, we review a case of a clinically significant OHSS after GnRH agonist triggering and a freeze all strategy. OHSS can still happen even after the use of GnRH agonist and avoidance of hCG support. Multiple causes are identified in the literature and the best approach remains to proceed with a segmentation of the IVF treatment along with complete avoidance of hCG for luteal support.Despite self-resolving, OHSS can still have an atypical presentation that might need hospitalization and conservative treatment.
Ethics statement
This study was approved by the Institutional Review Board of the American University of Beirut.
Informed consent
Signed consent was obtained from the patient.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Funding
This study received no extramural funding.
Authors’ contributions
Doctors Dalia Khalife, Suleiman Ghunaim, Lina El Taha, and Johnny Awwad contributed into the collection of the patient’s data and clinical information. All authors contributed to the writing of the manuscript. Doctor Omar Odeh and Natasha Habr proofread the manuscript and incorporated the editor and reviewers’ comments to the manuscript. The manuscript was read and approved by all the authors. All authors have met the criteria for authorship, and they believe that the manuscript represents honest work. Given the rarity of such a case, the authors strongly believe that this is an important article to guide management of future cases; thereby, this will be a case report with review of current literature.
Publisher’s Note
The Publisher of the Journal remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
 Asian Pacific Journal of Reproduction2022年6期
Asian Pacific Journal of Reproduction2022年6期
- Asian Pacific Journal of Reproduction的其它文章
- Exogenous gonadotropin releasing hormone (GnRH) modulates scrotal and testicular biometrics, libido, endocrinological and heamatological profiles in Ganjam goat under humid tropical coastal ecosystem of Odisha
- L-arginine alleviates postmenopausal complications in female rats by stimulating ovarian dopamine beta hydroxylase
- Predictors of readiness for discharge in mothers of preterm infants: The role of stress,self-efficacy and perceived social support
- Awareness about transmission and preventive measures of COVID-19 from mother to child: A cross-sectional study among pregnant women
- Prevalence and risks of reproductive tract infections among women of urban slums in North India: A cross-sectional study
- Clinical pregnancy rate of women with unexplained infertility with or without cervical mucus aspiration before intrauterine insemination: A randomized controlled trial
