In situ polymerization preparation and mechanical properties of nanocomposites based on PA10T/10I-block-PEG copolymer and graphene oxide
Xio-o Fu ,Xin Tong ,Ji-co Yng ,Gng Zhng ,Mei-lin Zhng ,Xio-jun Wng,c,** ,Jie Yng,d
a College of Polymer Science and Engineering,Sichuan University,Chengdu,610065,China
b Analytical and Testing Center,Sichuan University,Chengdu,610064,China
c Jiangsu JITRI Advanced Polymer Materials Research Institute Co.,Ltd,China
d State Key Laboratory of Polymer Materials Engineering,Sichuan University,Chengdu,610065,China
Keywords:Graphene oxide Thermoplastic elastomer In-situ polymerization Composite
ABSTRACT Poly (decamethylene terephthalamide/decamethylene isophthalamide)-block-polyvinyl alcoho) (PA10T/10IPEG) copolymer/graphene oxide (GO) composites were prepared via in-situ melt polymerization and two different nano-filler addition approaches were compared.The relationship between the micro-structure and performance of the elastomer composites prepared by one-step and two-step methods was explored.The results show that the two-step method significantly promoted the dispersion of the GO in a polymer matrix,and facilitated the grafting of more hard molecular chains.Thus,the elastic modulus and tensile strength of the nanocomposite have been significantly improved by the presence of GO.This was because of the strong interaction between the functional groups on the surface of the GO and the hard molecular chains.This would be also be favorable to load transfer across the interface.Additionally,the elongation at the break of composites increased by 10% with the addition of a small amount of GO(0.2% wt).This is because hard domains tend to be enriched on the surface of GO in composites and act as a lubricating layer at the interface between the GO and matrix,leading to increased deformation ability.This work provides an effective strategy to prepare elastomer composites with high strength and toughness.
1.Introduction
As a derivative of graphene,graphene oxide(GO)has a single atomic layer structure similar to graphene,and exhibits the ultra-high mechanical properties of graphene[1–5].The oxygen-containing functional groups of GO greatly increase the polarity of graphene.This ameliorates the dispersion problem of graphene and enhances the interaction between fillers and matirx,and has been widely applied into polymers for high performance nanocomposites [6–12].For example,Xin et al.prepared GO/nitrile–butadiene rubber (NBR) composites by a solution-blending method [13].The addition of 0.44 vol% of GO nanosheets enhanced the tensile strength and modulus at 200%elongation of HXNBR by more than 50%and 100%.Xu et al.prepared nylon-6-(PA6-)GO composites by in situ polymerization by a melt spinning process,and found that the tensile strength increased 2.1 fold and Young's modulus increased 2.4 fold with a graphene loading of 0.1 wt%only,revealing an excellent reinforcement to composites by GO[14].Wang et al.prepared poly (hexamethylene terephthalamide)-co-polycaprolactam(PA6T/6)/GO composites via in-situ polycondensation and improved the tensile strength to 73.6 MPa (by 45.7%) because of strong interaction,excellent dispersion and physical entanglement with the PA6T/6 matrix even with a small amount of GO(0.2 wt%),and a balance of strength and toughness was obtained[15].

Fig.1.Schematic diagram of the process of preparing composites for GO-H samples.
As an important branch of the polymer,thermoplastic elastomers(TPEs) are also research hotspots of GO reinforced polymer nanocmposites.The TPEs come out as an alternative to rubbers,having gained great attention because of their outstanding performace and recyclability.This can reduce the use of fossil fuels and cut down the carbon footprint compared to rubber [16,17].In general,the TPEs are copolymers composed of a hard chain segment which forma a hard region acting as the crosslinking point of rubber and a soft chain segment which behaves like a rubber chain segment in the crossliking point[18–20].It has been widely used in many fields such as electronic,electrical,automotive,construction,household and daily life [21–23].In recent years,using GO as a reinforcing filler to improve the mechanical strength and thermal stability of elastomers has becoming an important topic for TPE research.Paszkiewicz et al.[24] prepared a polytrimethylene terephthalate-block-propylene oxide (PTT-PTMO) copolymer composite filled with the GO by in-situ polymerization and found that the presence of graphene oxide can effectively increase the Young's modulus and yield stress of the thermoplastic elastomer.Qiu et al.[25] prepared a thermoplastic polyester elastomer(TPEE)by melting mixing and results show that the presence of GNS improves the mechanical performance of TPEE,and the surface functionalization of GNS further improves the reinforcing effect because of enhanced interfacial interactions.
In our previous study,the poly (decamethylene terephthalamide/decamethylene isophthalamide) -block-polyvinyl alcoho) (PA10T/10IPEG)(for simplification,copolymer PA10T/10I-PEG below is replaced by TPAE directly) has been synthesised and proved to be a highperformance TPE with excellent heat resistance [26,27].In this work,GO was applied to further enhance the comprehensive performance of TPAEs via in situ synthesis.In addition,two different addition methods of GO,the one and two step methods,were compared.Specifically,the GO is fully mixed with the hard segment monomer,and then polycondensed with PEG to prepare an elastomer by the two-step method,named GO-H.The GO and PEG are premixed,and then hard segment monomer is added to synthesize the elastomer composite by a one-step method,named GO-S.We explore the relationship between structure and performance by two methods of preparing composite,and further hope to find a suitable preparation strategy for high-performance composite materials.
2.Materials and method
2.1.Materials
Graphene oxide(GO)powder was purchased from Deyang Carbonene Technology Co.,Ltd.Terephthalic acid (TPA) was supplied by Yangzi Petrochemical Co.Ltd (Nanjing,China).Isophthalic acid (IPA) was supplied by Lotte Chemical Co.Ltd.1,10-Decanediamine (DMD) was purchased from Jiangsu Gaoming Co.Ltd (Wuxi,China).PEG(Mw =1000 g mol-1) and tetraisopropyl orthotitanate (Ti (iOC4H9)4)were obtained from Aladdin Reagent Co.Ltd(Shanghai,China).Sodium Hypophosphite was obtained from Kelong Chemical Reagent Co.Ltd(Chengdu,China).All were used as received.
2.2.Preparation of TAPE nanocomposites
GO and hard segment pre-mixed elastomer composite(GO-H)by the two-step method:
The synthetic route for GO-H samples by the two-step method is shown in Fig.1.The reaction monomers of the polyamide segment were mixed with the GO and pre-polymerized in situ first and then the prepolyamide/GO reacted with the PEG for the polymerization of the composite elastomer.A typical polymerization procedure of the two-step method is described as follows:the GO was dispersed in deionized water by stirring and ultrasound and then added into the reactor together with certain amount of terephthalic acid,isophthalic acid,1,10-decanediamine and a catalyst.The prepolymerization was carried out at 260°C in airtight condition for 3 h.Then the valve was opened to remove the water vapor and by-products in the kettle for 1 h.Subsequently,products would be carefully taken out until the temperature in the kettle drops to room temperature and dried under vacuum at 100°C for more than 24 h.In the second stage,the prepolymer from the first stage and the PEG were added to the reaction kettle and melt polymerized at 275°C and 90 Pa for 3 h to prepare the TPAE/GO composite.The amount of graphene oxide added is 0.05 wt%,0.1 wt%,0.2 wt%,0.3 wt%,0.4 wt% and 0.5 wt%,and the corresponding composite elastomers are named GO-H 0.05,GOH 0.1,GO-H 0.2,GO-H 0.3,GO-H 0.4 and GO-H 0.5,respectively.By repeatedly washing using phenol solution at 60°C and filtering through a PTFE membrane while hot to remove any ungrafted chain,the GO extracted from GO-H samples was obtained.
GO and soft segment premixed elastomer composite material(GO-S)by the one-step method.
The synthetic route for GO-S samples by the one-step method is shown in Fig.2.The GO,reaction monomers and the PEG were added into the reaction kettle at the same time and the high-pressure prepolymerization and high-vacuum polymerization were carried out successively.In a typical polymerization procedure of the one step method,the GO and the soft segment are premixed by ultrasound and stirred,then a certain amount of terephthalic acid,isophthalic acid,1,10-Decanediamine and catalyst were added into a 2 L reactor.and reacted at 260°C in an airtight condition for 3 h,and then the valve was opened to remove the water vapor and by-products in the kettle.Melt polycondensation was carried out at 275°C and 90 Pa for 3 h to the TPAE/GO composite.The amount of graphene oxide added is 0.05 wt%,0.1 wt%,0.2 wt%,0.3 wt%,0.4 wt% and 0.5 wt%,and the corresponding composite elastomers were named GO-S 0.05,GO-S 0.1,GO-S 0.2,GO-S 0.3,GO-S 0.4 and GO-S 0.5,respectively.Those composites would also be repeatedly washed using phenol solution at 60°C and filtered through a PTFE membrane to remove any ungrafted chain.GO extracted from GO-S samples was obtained.
2.3.Characterization techniques
2.3.1.Intrinsic viscosity
The intrinsic viscosity [ŋ] of the samples was measured using a Ubbelohde viscometer at 30±0.1°C in 98 wt%sulfuric acid solution of TPAE/GO and neat TPAE with a concentration of 0.5 g/dL.

Fig.2.Schematic diagram of the process of preparing composites for GO-S samples.
2.3.2.Atomic force microscopy(AFM)
AFM was performed to investigate the morphology of the thermoplastic elastomer nanocomposite using a MultiMode 8 Bruker's microscope in tapping mode.Solvent-cast samples of ca 100 μm were prepared from a 5 wt% solution in trifluoroacetic acid.The samples were repeatedly washed using concentrated sulfuric acid and filtered to remove any ungrafted molecular chain.
2.3.3.Scanning electron microscopy (SEM)
SEM analysis was used to observe the fractured surface using a JSM-7500F field emission scanning electron microscope.Reichert Ultracut R ultramicrotome was used to obtain a sample with a thickness of about 100–150 nm under low temperature conditions.
2.3.4.Transmission electron microscopy (TEM)
TEM images were taken on a JEOL JEM 100CX II TEM microscope(Japan).The sample was immersed in liquid nitrogen for 2 h,and then quenched to obtain a cross section.The surface of the sample was sprayed with gold.
2.3.5.Fourier transform infrared spectrum (FTIR)
An infrared spectrum was used to qualitatively analyze the changes in functional groups of neat TPAE and the samples were extracted from composites using a Nicolet 670 spectrophotometer in the scanned wavenumber range of 4000-400 cm-1.The spectral resolution was 4.0 cm-1.
2.3.6.X-ray photoelectron spectroscopy (XPS)
The microstructure of nanocomposites was studied by an XSAM800 photoelectron spectrometer(Thermo Scientific Co.,USA)equipped with an Al anode XR50 source operated at 200 W.The overview spectra were recorded with a pass energy of 20 eV at 0.05 eV steps at a pressure<3.0*10-7mbar.The binding energies were referred to C1s,N1s and O1s signals and the overlapping peaks were deconvoluted using Thermo Avantage Software.
2.3.7.X-ray diffraction (XRD)
XRD analysis was carried out using a Philips X′pert Pro MPD X-ray diffractometer with voltage and current of 40 KV,35 mA,respectively(λ =0.154 nm,2θ =5–50°).The sample was compression-molded to obtain a 2 mm thick film.In order to improve the integrity of the crystal,the sample was annealed at 130°C for 8 h.
2.3.8.Mechanical property characterizations
Tensile measurements were performed with an electromechanical universal testing machine (MTS Systems China Co.Ltd) and dumbbellshaped specimens (25 mm × 4 mm × 2 mm) were prepared by compression molding.Samples were stretched at room temperature at a constant tensile rate of 50 mm min-1.
3.Results and discussion
3.1.Intrinsic viscosity
To explore the influence of GO on the molecular weight of the matrix resin,the values of intrinsic viscosity for nanocomposites and neat TPAE are shown in Fig.3.The result shows the intrinsic viscosity has a slight drop with the addition of GO,indicating the molecular weight growth of the polymer is hindered to a certain extent.This can be interpreted as the high specific surface area of GO having a certain adsorption effect on the molecular chain.This reduces the mobility of the segment and reduces the probability of reaction between functional groups [15].At a higher loading of GO(0.4 wt%),the intrinsic viscosity of GO-H series composites further decreases.It is well known that one of the important factors for the reduction of the molecular weight of the block copolymer is the imbalance of the distribution ratio of the two components.When a certain amount of the GO with a functional group enters the reaction system,the ratio of acid and amine functional groups will be significantly changed,and this affects the intrinsic viscosity of the block polymer.
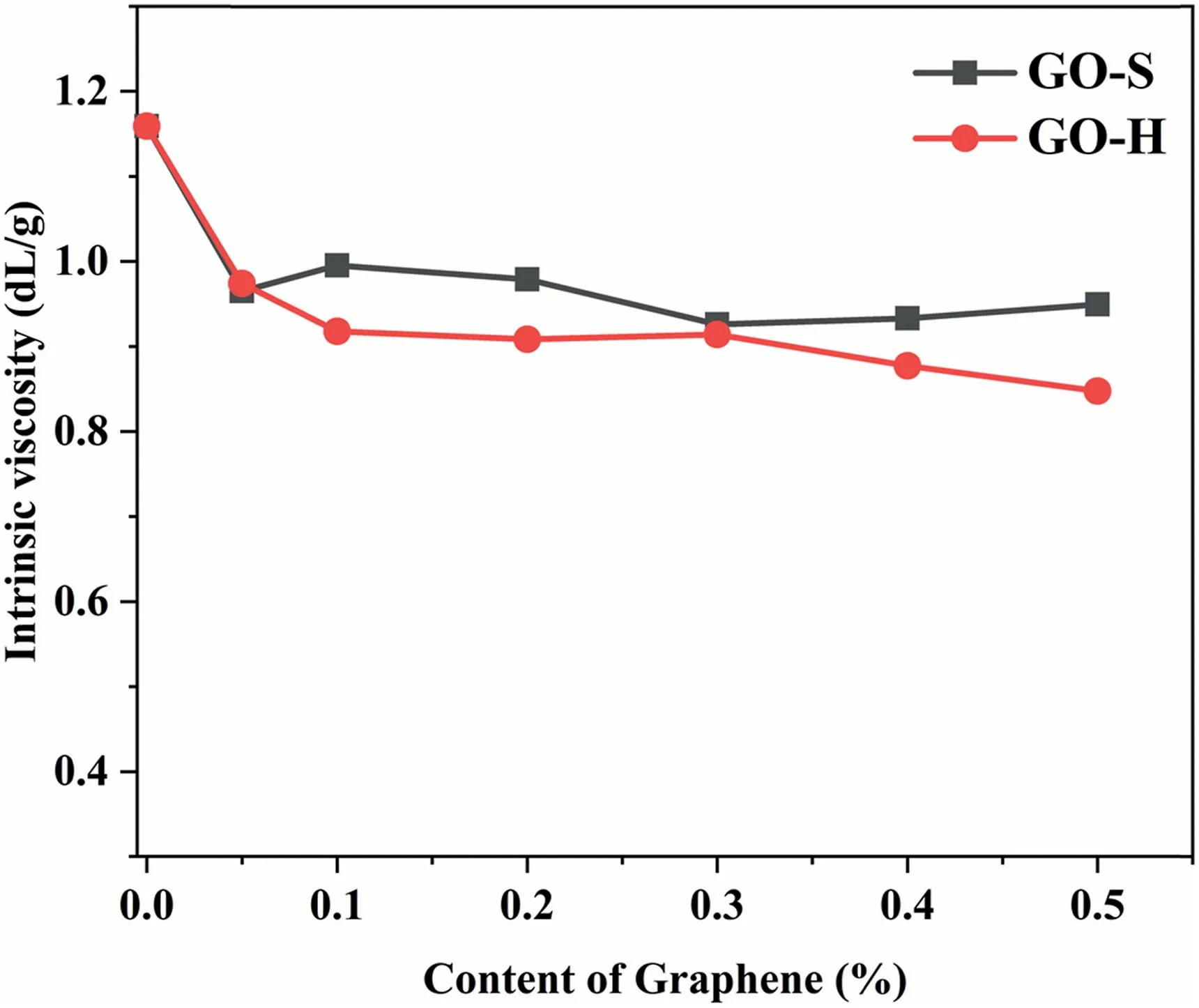
Fig.3.The intrinsic viscosity of nanocomposite and neat TPAE.
3.2.Morphology and structure
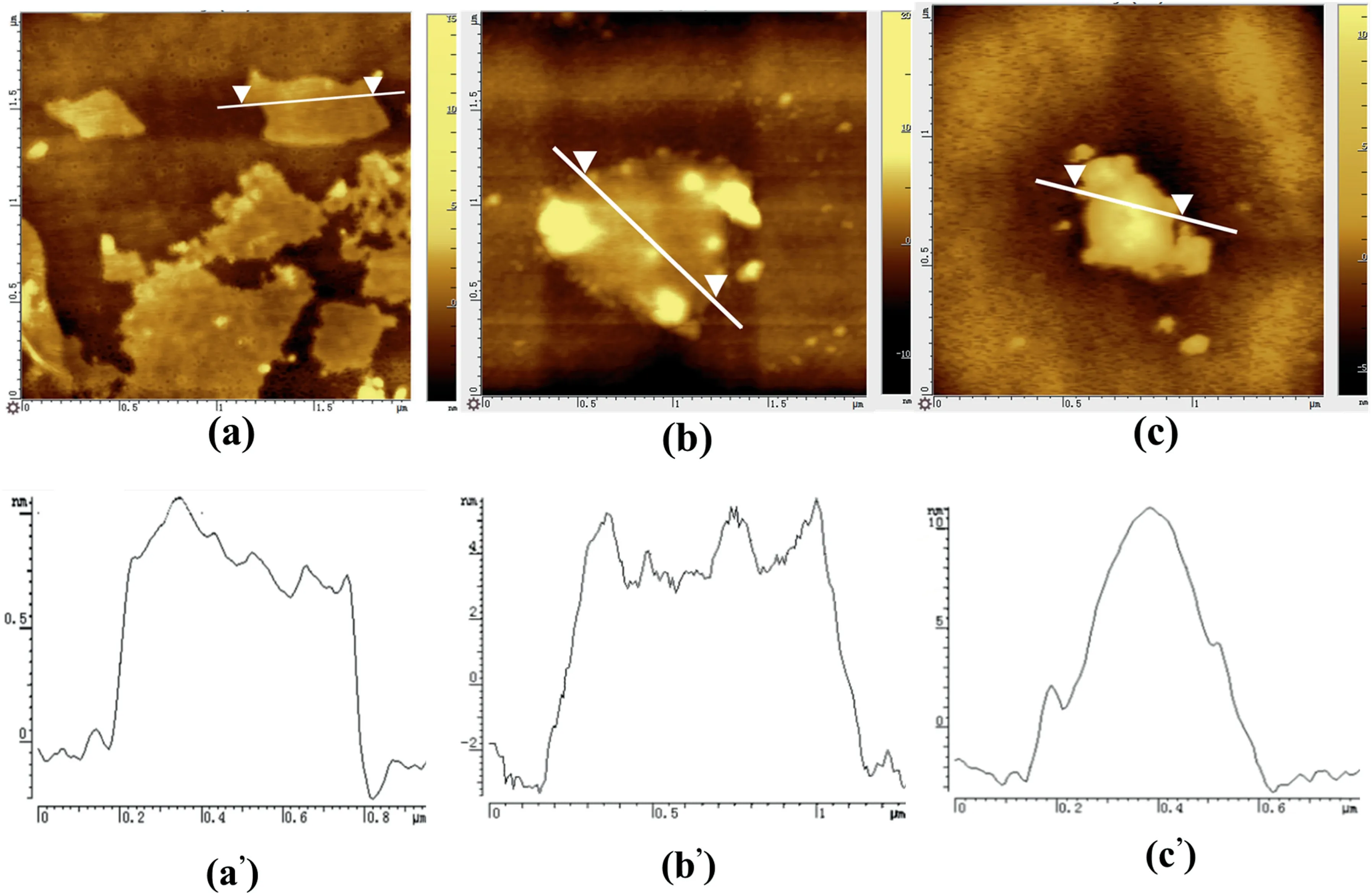
Fig.4.AFM image: (a-a’) raw GO;(b-b’) GO extracted from GO-H 0.1;(c-c’) GO extracted from GO-S 0.1.
AFM measurement was applied to observe the microstructure of the TAPE/GO nanocomposite.Image (a),(b),(c) in Fig.4 shows the morphology of raw GO,GO extracted from CO–H 0.1 and GO-S 0.1.It can be seen from Fig.4 (a’) that the thickness of GO is about 0.8 nm,indicating completely exfoliation of GO to a single layer[28].Compared to the raw GO layer,the GO-H 0.1 raised its thickness to 5.2 nm,indicating the high efficiency of the grafting reaction occurring on the GO nanosheet surface.For the GO-S 0.1 composite,it can be seen clearly that the GO agglomerates into a spherical shape,and the height map presents a peak shape with typical agglomeration characteristics [15].
To directly compare the dispersion state and distribution of GO in a TPAE matrix prepared by two methods,SEM and TEM measurements were applied to characterize the GO-H 0.2 and GO-S 0.05.From the SEM image(Fig.5 a-b)of the fracture surface of the GO-H 0.2 sample,it can be seen that the GO layer is clearly well dispersed in the TPAE matrix with no obvious agglomeration.For the GO-S 0.05 sample,although it has a lower filler content,GO shows an obvious aggregation or layered stacking state in the matrix.To further characterize the dispersion of the TPAE/GO nanocomposite,TEM analysis was carried out on the specimens using an ultra-thin microtome and a diamond knife.As shown in Fig.5 (a’-b’),the layered GO sheet structure can be clearly observed in the GO-H 0.2 sample,indicating that GO has good dispersibility in the matrix.Correspondingly,for the GO-S 0.05 sample,the GO is in a severely agglomerated state.This is because of the differences of interaction between GO and polymer or monomer.The GO will be reduced in the process of heating.This will lead to a decrease in polarity [29].In addition,the strong π-π conjugation effect between the GO layers cause the GO layers to stack with each other[14].In the GO-S system,the GO is pre-mixed with the soft segment.It is difficult for the soft molecular segment containing a certain number of molecular weight chains(Mn =1000)to react with the GO in a state of accumulation.In the GO-H system,the GO is first dispersed in the hard segment monomers,which can react with the surface groups.Subsequently,strong molecular chain movement combined with shearing with a large amount of heat released during the in-situ polycondensation process caused the GO to be well dispersed in the matrix.
FTIR and XPS were applied to further verify the existence of a molecular chain on the GO sheet.Fig.6 shows the infrared absorption features of raw GO,GO extracted from GO-H 0.1 and GO-S 0.1 and neat TPAE.It can be easily seen that GO exhibits typical absorption peaks near 1049 cm-1,1618 cm-1and 1715 cm-1,corresponding to C–OH stretching vibration,vibration in carboxylic acid and carbonyl moieties,respectively.This all indicates the existence of -OH and-COOgroups on the surface of the GO sheet.As for the GO-H and GO-S samples,the absorption peak at 1640 cm-1corresponds to the stretching vibration of thegroup in the amide bond,and the other new peak at 1536 cm-1corresponds to the bending vibration of the N–H bond and the stretching vibration of the C–N bond of the amide group.The peaks at 2860 and 2930 cm-1are attributed to the C–H stretching vibration of the grafted polymer molecular chain.The results show that GO can be grafted onto the hard molecular chain.

Fig.5.SEM (left) and TEM (right) pictures of GO-H 0.2 (a-a’) and GO-S 0.05 (b-b’) composites.
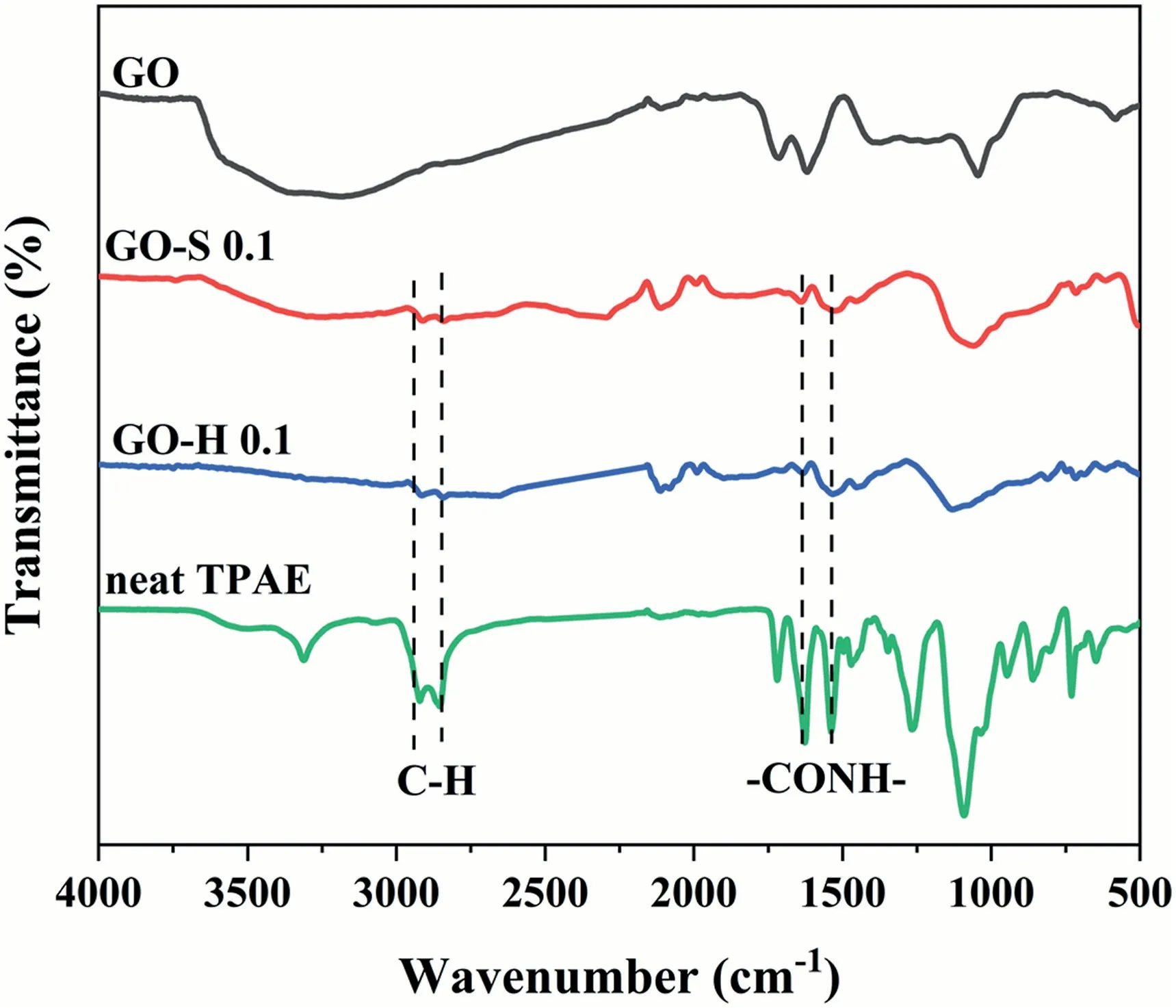
Fig.6.FTIR spectra of raw GO,GO extracted from composities GO-H-0.1,GO extracted from composities GO-S-0.1 and neat TPAE.
The XPS result(Fig.7a)shows that the C1s peaks at 288.0 eV(C 1s)and 534.0 eV (O 1s) are observed without any peak for nitrogen.However,for the GO extracted from GO-H and GO-S composities,the strong peak of N 1s was found at 401.0 eV,indicating the GO sheet surface is rich in nitrogen in these two samples.Fig.7b,c and d show the peak fitting curve of the C1s peak in GO,GO-S and GO-H,respectively.As shown in Fig.7b,for the original GO samplethe C–C (sp2hybridized C atom in the six-membered ring structure) peak appears at 284.5 eV,the C–O peak appears at 286.2 eV,andpeak appears at 287.4 eV,and the OC–O peak appears at 289.0 eV[30,31].The above polar functional groups endow a stronger physical interaction between the GO and molecular chain ensuring its favorable linkage with polymers.Compared to the C1s peak of GO,the GO-S sample(Fig.7c)exhibits a new CO–NH peak (287.5 eV) and the C–O peak disappears indicating the formation of amide bonds on the GO surface and thermal reduction of GO at that high temperature(the breakage of C–OH linkage).For the C1s peak of GO-H sample(Fig.7d),the peak representing the carboxyl group(289.5 eV) disappeared,and the intensity of the CO–NH peak further increased compared to GO-S sample,indicating that more polymer molecular chains are grafted onto the surface of GO in the GO-H sample.Table 1 summarizes the contents and ratios of different elements.The results show that the N/C and N/O ratios for GO-H and GO-S samples are significantly higher than the raw GO,confirming that a hard molecular chain was effectively grafted onto the GO nanosheet.In addition,the GO-H sample has a further increase in the ratio of N/C and N/O compared to the GO-S sample,indicating that the grafting rate of the GO-H sample is higher than that of the GO-S.

Table 1 Contents of different elements of raw GO,GO extracted from GO-H 0.1 and GO-S 0.1.
XRD analysis was performed to assess possible changes in crystalline structure and degree of crystallinity of the matrix.As shown in Fig.8,the peak positions of all composite samples,located at 2θ =19.5,21.4 and 22.7°,are similar to the pure TAPE peak position[32].It shows that the crystal structure of the composite is not changed by the presence of GO.We should also note that in the GO-H series of samples (Fig.8a),the intensity of the diffraction peak slightly increases with the increase of content of GO,especially with high loading of GO.That might be explained by the heterogeneous nucleation and promotion of matrix crystallization in the presence of GO.However,for the GO-S series of samples(Fig.8b),the change in peak intensity is not obvious.From the results of SEM and TEM,the GO presents a serious aggregation phenomenon in the matrix for the GO-S sample.This may weaken the heterogeneous nucleation effect of GO and lead to almost no promotion of crystallization.Unfortunately,with the lack of diffraction intensity of 100% amorphous PA10T or 100% crystalline PA10T,an accurate crystallinity of those composites is rendered incalculable at present,but work on this is in progress.
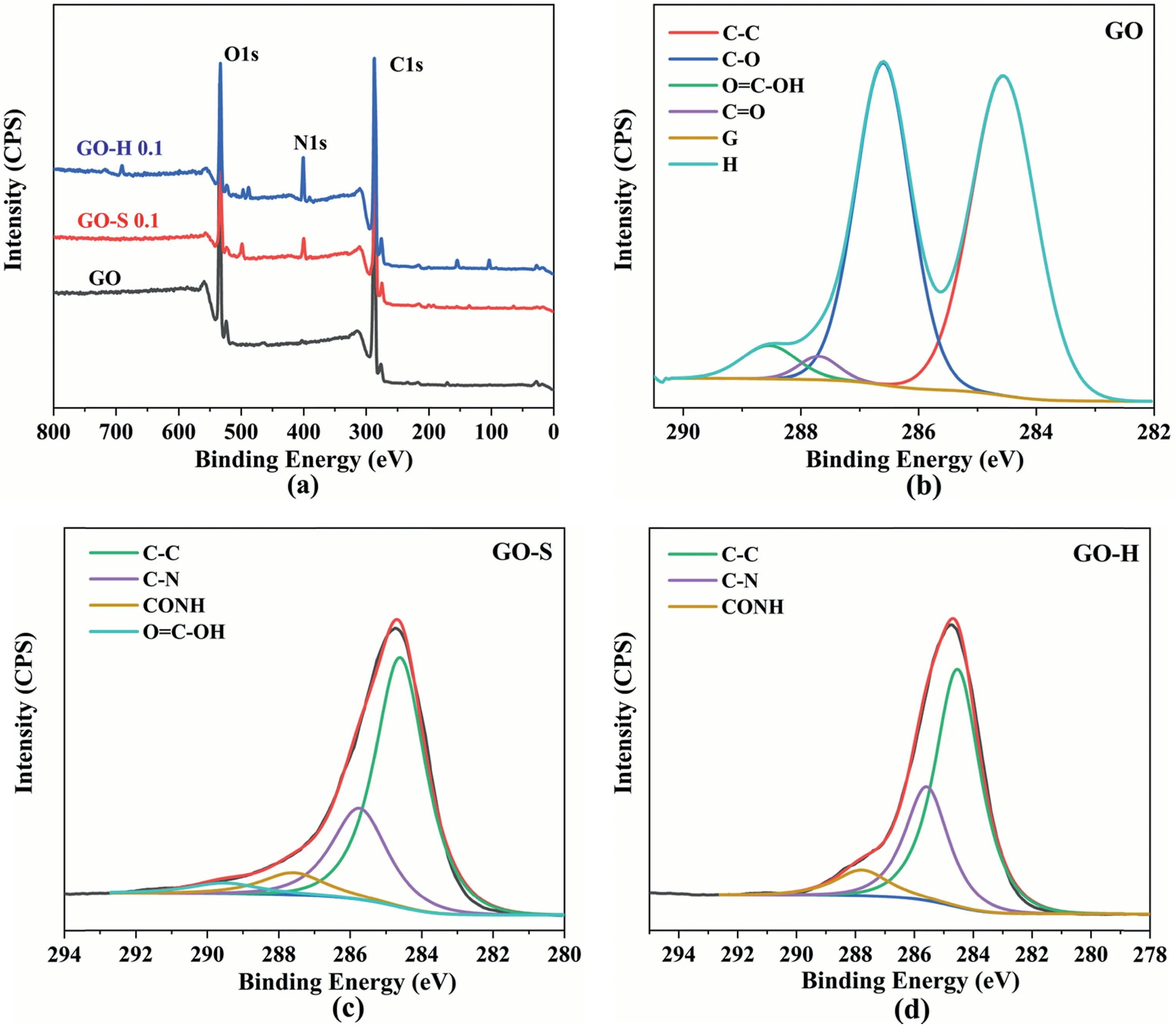
Fig.7.XPS spectra of(a)raw GO,GO extracted from GO-H 0.1,GO extracted from GO-S 0.1;and C1s peak-fitting curves of(b)raw GO,(c)GO extracted from GO-S 0.1 and (d) GO extracted from GO-H 0.1.
3.3.Tensile properties
Table 2 summarizes the mechanical properties of neat TPAE and its GO composites.The representative stress–strain curves from tensile tests for nanocomposites are presented in Fig.9.For the GO-H samples(Fig.9a),the modulus of elasticity increases significantly with the increase of GO content from 0%to 0.5%.The tensile strength also increases from 20.8 MPa to 25.0 MPa with the increase of GO from 0% to 0.3%.The elongation at break reaches a maximum when the GO content reaches 0.2%.For the GO-S samples (Fig.9b),the modulus of elasticity is significantly improved with an increase of GO from 0 to 0.5 wt%,but the strength and elongation at break were significantly reduced.
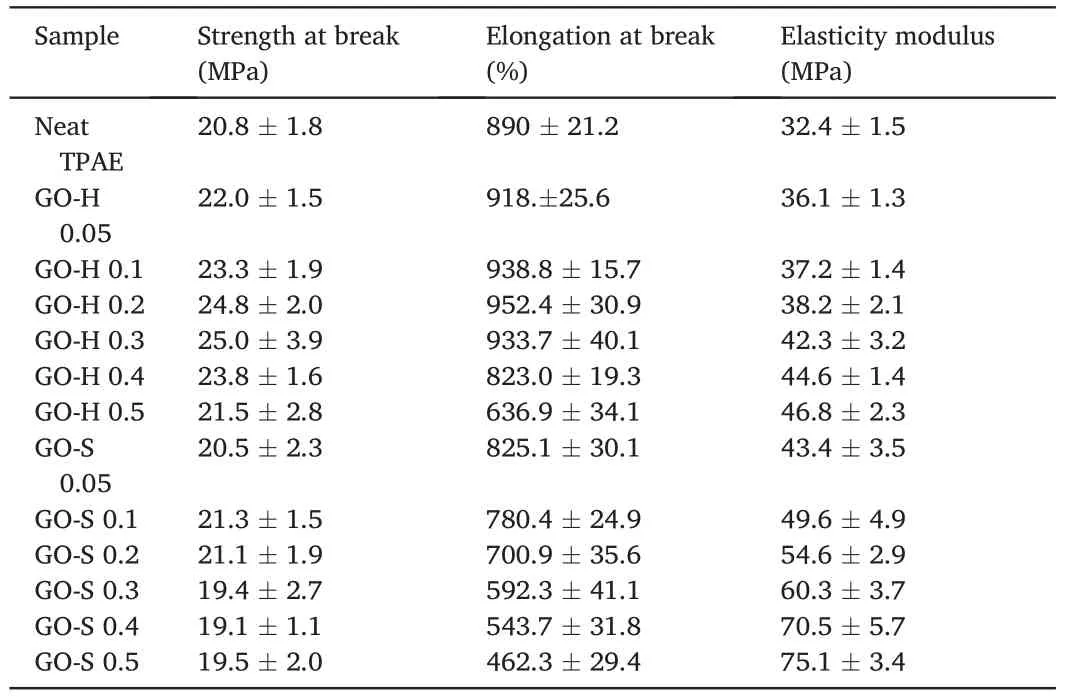
Table 2 Mechanical properties of composites.
As is well known,the reinforcing effect of fillers on polymer materials is mainly composed of two parts,namely,direct and indirect reinforcement[33].Direct reinforcement is based on the transfer of loads from the matrix to the stiffer filler particles.Indirect reinforcement is the change in the microstructure of the matrix due to the presence of filler particles.In semi-crystalline nanocomposites,the effect of nanofillers on the fracture stress depends on the interface interaction between the matrix and the nanofillers,and strong interfacial interaction leads to greater fracture stress.From literature [34],the filler particles may promote the nucleation of the polymer,increase its crystallinity,reduce the thickness of the crystallites,and affect the orientation of the platelets in the crystallites,all of which affect the mechanical properties of the nanocomposite.From the XRD results of the TAPE nanocomposite in Fig.8,it can be seen that the crystal peak intensity for the GO-H samples slightly increases with the increase of GO content and negligibly increases in degree of crystallinity.In addition,DSC analysis of composites indicates that there is no significant change of melt enthalpy with increasing concentration of GO(Fig.S1 of the Supporting Information).In fact,the crystallization behavior of GO in elastomers is not very obvious.This is mainly because of the fact that the hard segment molecular chain is a rigid chain containing aromatic rings,and its crystallinity is very low.Therefore the observed improvement in the tensile properties cannot be due to a change in crystallinity and is more likely caused by the presence of GO sheets.As discussed above,a strong interface interaction exists between the hard molecular chain and the GO in the GO-H series samples,and the stress can be transferred to the more rigid GO nanosheets.However,GO-S series samples suffer a significant decrease in mechanical properties with the incorporation of GO.This is mainly due to the serious agglomeration of graphene oxide inside the composite material and the lack of effective interaction with the matrix resin.The GO gathered inside not only fails to enhance,but forms “impurities” to cause stress concentration,which leads to a decline in the overall mechanical properties of the system.
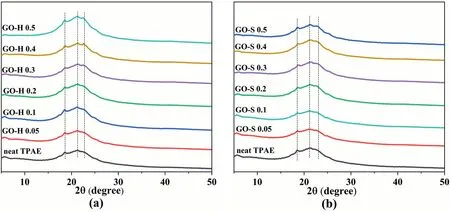
Fig.8.XRD patterns (a) GO-H samples;(b) GO-S samples.
It is well known that the presence of filler particles will increase the rigidity of the composite,resulting in a decrease in the plasticity or ductility of the composite compared to the neat polymer matrix[35].It is worth noting that the elongation at break of GO-H series composites increased with a certain amount of the GO content.The effect of nanofiller on the stress at break values depends on the interfacial interactions between polymer and graphene sheets.Strong interfacial interaction causes the enhancement in stress at break.From the results of the FTIR(Fig.6)and XPS(Fig.7),the surface of the GO grafts with a certain length of the hard segment molecular weight,and this leads to a better affinity for the free hard segment phase in the matrix.Therefore,the hard phase in the TPAE composite tends to be concentrated on the surface of the GO,as shown in the schematic(Fig.10).The approximate evolution process is that the hard molecular chains grafted on graphene attract free molecular chains around them and gradually form a spherical domain.We assume that the “spherical” structure of those enriched hard domains on the surface of GO plays a role in reducing the friction at the interface between GO sheets and the continuous hard phase.When the elastomer material is deformed by external tension,the“spherical”structure on the surface of GO sheets promotes the slippage of polymer molecular chains in the low GO content.This will increase the deformability ability of the nanocomposite.With a high content of GO,the performance of nanocomposites gradually decreases because of the agglomeration of GO.
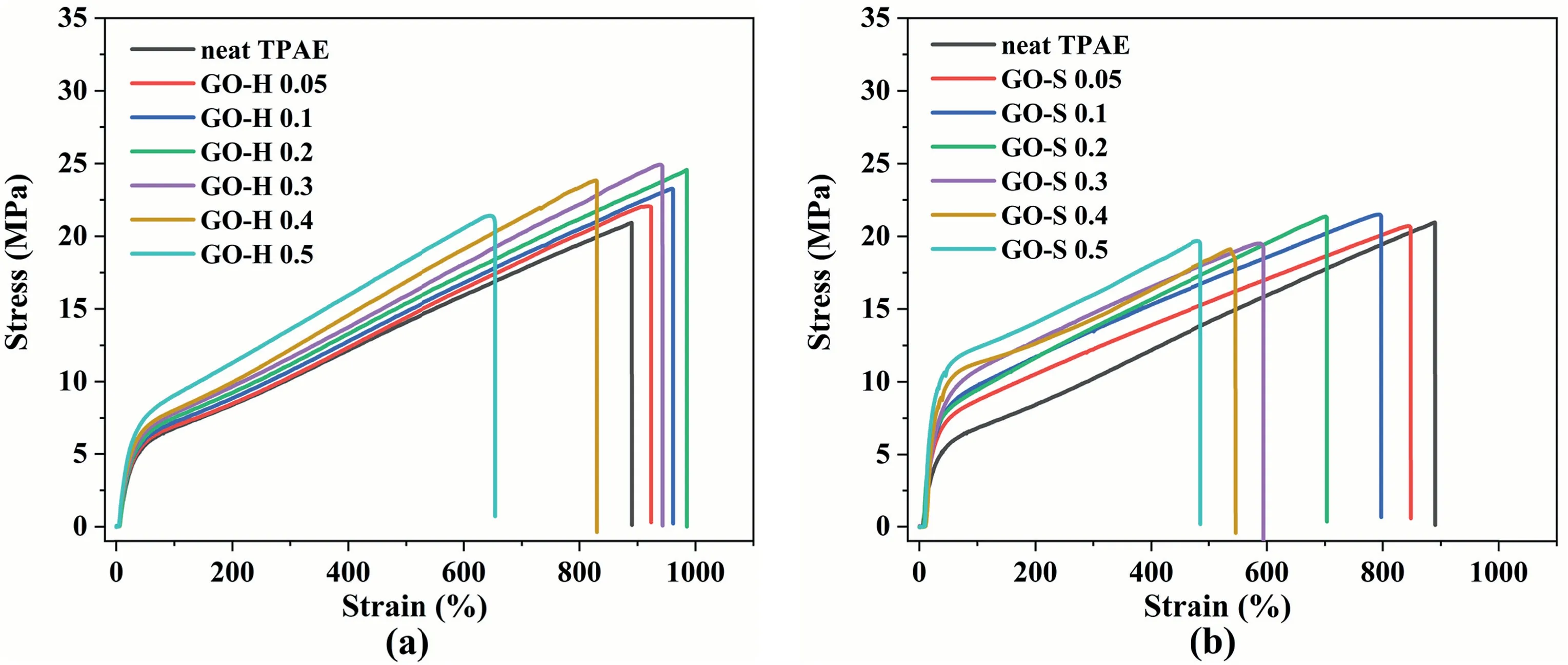
Fig.9.Stress-strain curves: (a) (a) GO-H samples;(b) GO-S samples.
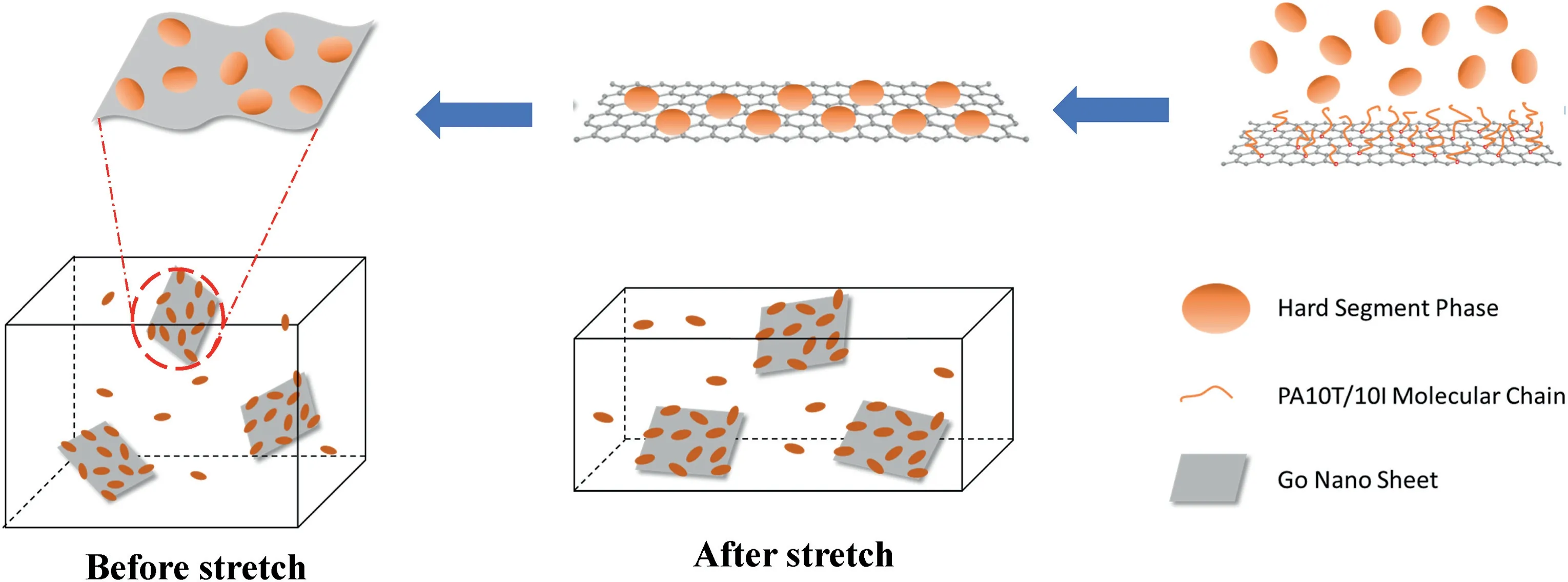
Fig.10.Schematic diagrams of enrichment of hard segment phase on the surface of GO.
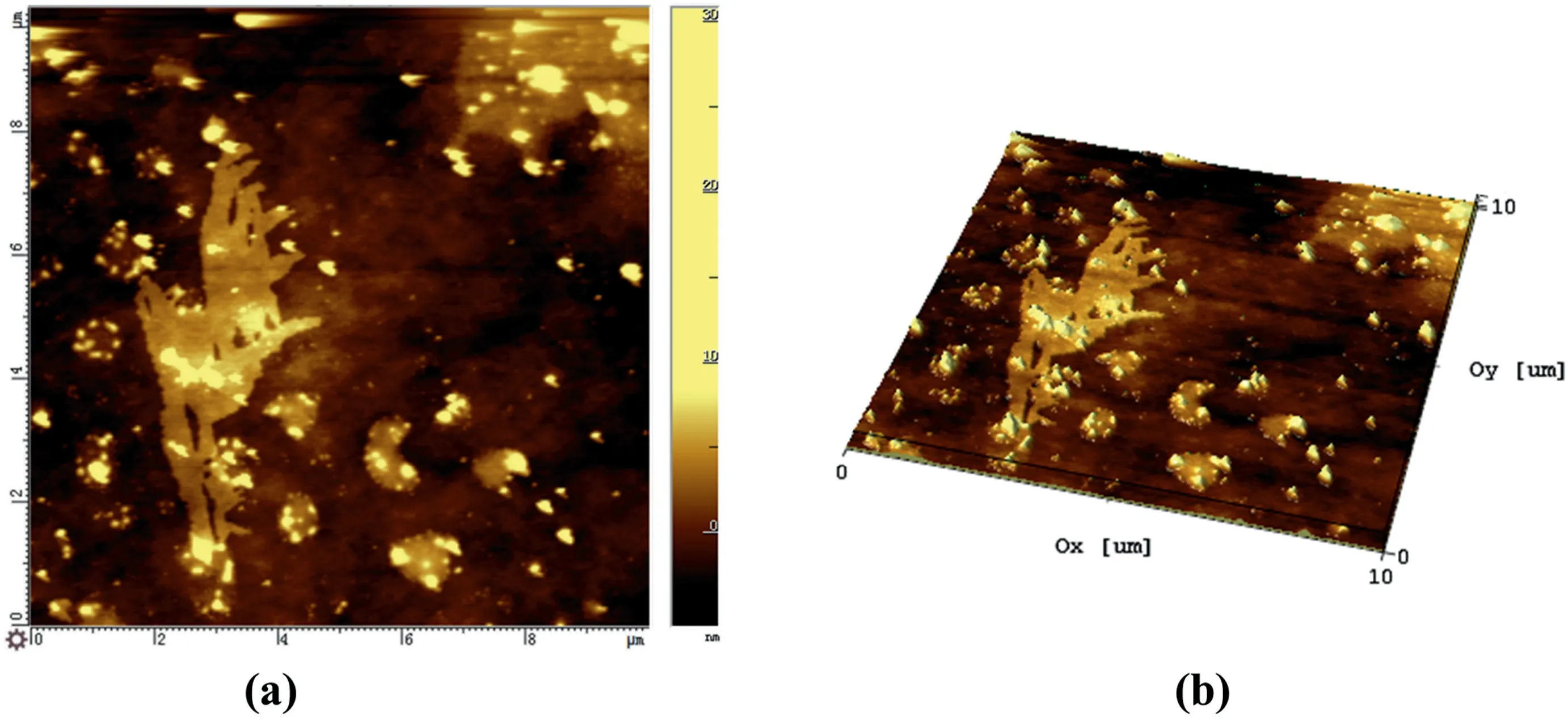
Fig.11.(a) 2D and (b) 3D AFM height images of extracted from GO-H 0.2 sample.
AFM observations confirmed the hypothesis of this “spherical”structure(Fig.11).The GO sheets embedded in the TPAE matrix can be seen.The bright spots are the discrete hard segments region dispersed on the surface of the GO.This confirms the hypothesis of those “spherical”structures on the surface of GO.Similar results were obtained by Qiu and Huang [25,36].The hard segment domains adsorbed on the surface of GO act as a lubricating layer at the interface,so that the interfacial friction between the graphene oxide particles and the continuous polymer phase is reduced.This is conducive to the deformation of the elastomer during stretching.Therefore,GO-H composites show a higher level of elongation at break than neat TPAE.
4.Conclusions
In this work,GO reinforced (PA10T/10I-PEG) elastomer nanocomposites were prepared via one-step and two-step in situ polymerization methods.The results show that the GO is well dispersed in the matrix and a stronger interface interaction exists between the hard molecular chain and GO in the composites prepared by the two-step method.The two-step method significantly improves the modulus and tensile strength of the composites.More importantly,with the addition of a low amount of GO(0.2%wt),the elongation at break increased by 10%.This is because of the free hard segment phase adsorbed on the surface of GOgrafted hard molecular chain forming a spherical domain,which can act as a lubricating layer at the interface between the GO and hard resin,leading to increased deformation ability.This work possibly provides an effective strategy for preparing elastomer composites with high strength and toughness.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors gratefully acknowledge the financial support from the Jiangsu Provincial Key Research and Development Program (Grant No.BE2019008) and the Natural Science Foundation of China (Grant No.51573103,21274094 and 21304060).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nanoms.2021.09.004.
- Namo Materials Science的其它文章
- Fabrication of segregated poly(arylene sulfide sulfone)/graphene nanoplate composites reinforced by polymer fibers for electromagnetic interference shielding
- CNT toughened aluminium and CFRP interface for strong adhesive bonding
- Abnormal enhancement to the quality factors of carbon nanotube via defects engineering
- Fracture behavior of hybrid epoxy nanocomposites based on multi-walled carbon nanotube and core-shell rubber
- Anti-corrosion and electrically conductive inorganic conversion coatings based on aligned graphene derivatives by electrodeposition
- Atomic insights into synergistic effect of pillared graphene by carbon nanotube on the mechanical properties of polymer nanocomposites

