Anti-corrosion and electrically conductive inorganic conversion coatings based on aligned graphene derivatives by electrodeposition
Wenxing Fei ,Jinn Cui ,Yhui Sun ,Junhe Yng ,Shnglin Go ,Jing Li,*
a School of Materials Science and Engineering,University of Shanghai for Science and Technology,No.516 Jungong Road,Shanghai 200093,China
b Shanghai Jian Qiao University,No.1111 Huchenghuan Road,Shanghai 201306,China
c Fengye Holding Group,Zhuji 311814,China
Keywords:Corrosion protection Electrical conductivity Electrodeposition Graphene
ABSTRACT Ultrathin conversion coatings,made from aligned graphene derivatives and ammonium zirconium carbonate(AZC),were fabricated on stainless steel by electrodeposition.Sulfonated graphene oxide(SGO)provided electron pathways and physical barriers to corrosive molecules.Electrodeposition ensured the alignment of SGO and the facile fabrication of the coatings.AZC is an environmental-friendly crosslinking agent,water-repellent and corrosion inhibitor.Upon dehydration reactions,AZC improved the cohesion between SGO layers and anchored the conversion coatings on metal substrates.When the mass ratio of SGO to AZC was 2:1,the corrosion current density of the composite coatings reached 0.098 μA cm-2,while that of the bared stainless steel was 1.04 μA cm-2,given a coating thickness of only 500 nm.The electrical conductivity of SGO/AZC composite coatings can be tailored from 3.84 × 10-5 to 2.28 × 10-3 S·cm-1 by heat treatment and HI reduction,which satisfied the electrical conductivity requirement of wide applications in electronic industry,office appliances and petroleum storage.
1.Introduction
Coatings with corrosion protection and electrostatic dissipation properties were highly demanded in electronic industry,office appliances and petroleum storage[1].The electrical conductivity of antistatic coatings should be higher than 10-8S cm-1[2].The addition of conductive fillers,like carbon materials[3]and metallic particles[4],in polymer matrix coatings were one of the most common methods for improving the electrical conductivity.Formation of electron conductive pathways in insulating polymer matrix required relative high filler content,which usually brought about poor filler dispersion and high viscosity of the coatings [5].The electrical conductivity of the composite coatings was still limited by the contact resistance between the neighbouring conductive particles,even if the high filler content was applied.
Graphene coatings had been applied on copper[6]and stainless steel[7]surfaces for corrosion protection,due to its excellent physical barrier properties and electrical conductivity [8].The fabrication methods of graphene coatings included chemical vapor deposition [9],Langmuir Blodgett method [10],electrodeposition [11] and transfer printing method [12].Debate has been raised over the years on the corrosion protection properties of graphene coatings [13–15].The argument against the application of graphene anticorrosion coatings included:1)graphene was cathodic to most metals.The galvanic coupling between graphene and metal may promote corrosion;2) defects of the graphene coatings leaded to the formation of large cathode and small anode,which may accelerate dangerous localized corrosion.To solve the problems,it was proposed that either defect-free continuous graphene coatings should be fabricated,or insulating materials should be applied to break the galvanic contact between graphene and metal [16].However,fabrication of defect-free graphene coatings was difficult and expensive,while application of insulating materials,like polymers,sacrificed the intrinsic electrical properties of graphene materials.
Alternatively,inorganic inhibitors can break the galvanic contact between graphene and metal substrate by forming conversion layers at the metal/coating interfaces [17].Inorganic conversion coatings had been widely applied for corrosion protection of aluminum[18],stainless steel [19],zinc [20] and other metals [21].To replace toxic chromate passivating agents,environmental-friendly inorganic inhibitors were studied,including ammonium zirconium carbonate (AZC) [22],phosphates[23],titanium salts[24],molybdenum salts[25],rare earth metal salts [26].The corrosion protection efficiency of the chrome-free conversion layers was usually inferior to the chromate treatment.For example,it was reported that the corrosion current density(Icorr)of zinc phosphate conversion layers prepared on Q235 carbon steel was about 4.70 μA cm-2with a thickness of 1 μm[27],while the Icorrof conversion layers prepared on mild carbon steel after chromate treatment reached 0.38 μA cm-2with a thickness of 1.5 μm [28].
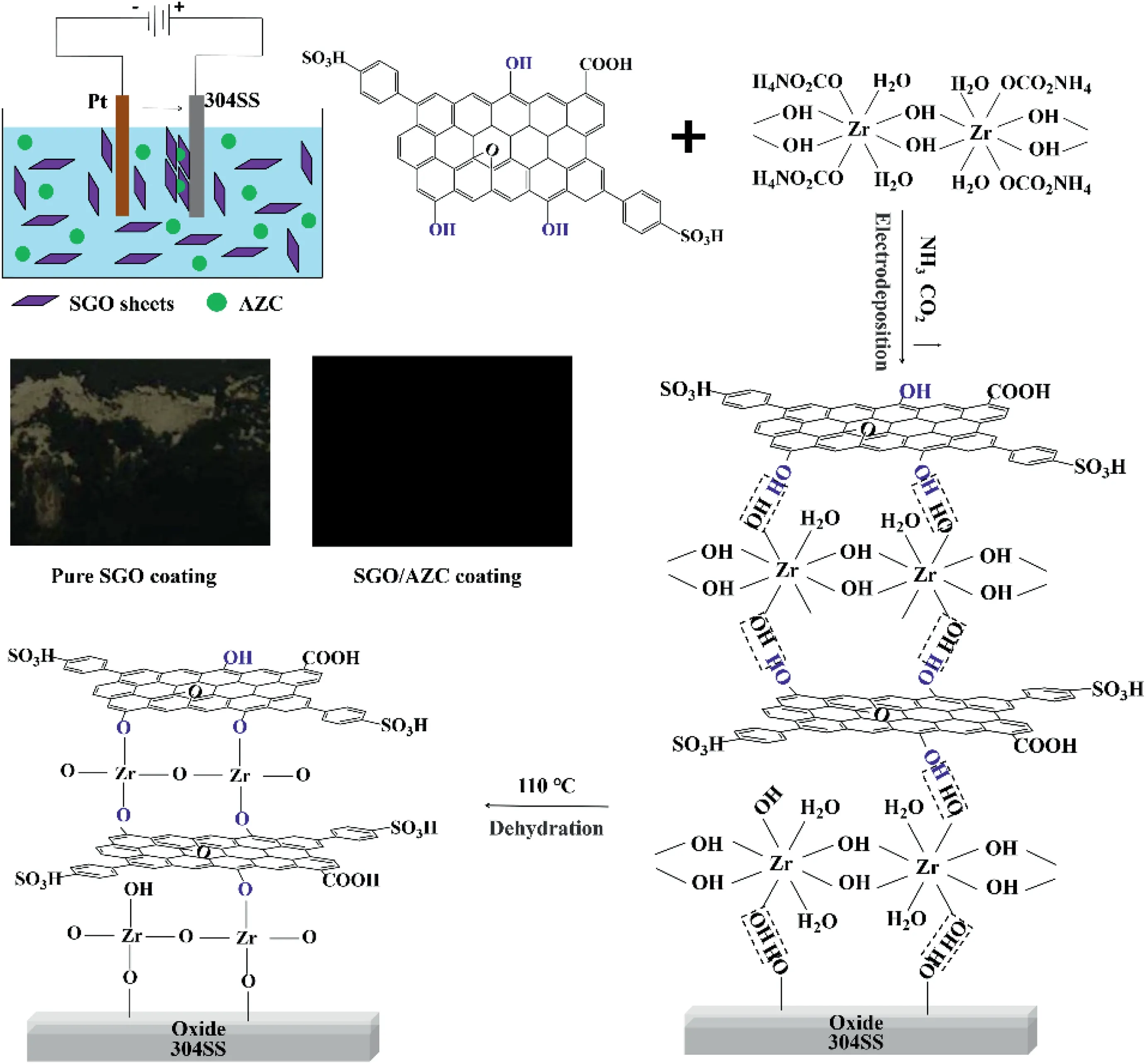
Fig.1.Processing schematics of SGO/AZC composite coatings by electrodeposition.
AZC was one of the inorganic inhibitors that can form a dense conversion coatings with polymer materials,like polyurethane [29] and cyclic-amine-containing polymer (CAP).Typically,AZC was hydrolyzed to produce zirconium hydroxyl groups,which reacted with hydroxyl or carboxyl groups to form network structure upon dehydration [30].A layer of amorphous zirconia was eventually generated on metal substrates,functioned as the corrosion protection conversion coatings.It was reported that the addition of 33.75%CAP was necessary to prepare the compact AZC conversion coatings[31].
Here,we proposed an alternative idea for fabricating the ultrathin conductive conversion coatings on metal surfaces.The inorganic conversion coatings were constructed by aligned graphene derivatives and AZC.The graphene derivatives offered electron conductive pathways and physical barriers to corrosive molecules,which compensated the barrier properties of the AZC conversion coatings.AZC was employed as the gap filler,corrosion inhibitor and crosslinking agent,which improved the cohesion of the coatings,and facilitated the anodic inhibition effects.The alignment and close-packing of the graphene derivatives was obtained by the electrodeposition method.
2.Experimental
2.1.Electrodeposition of aligned graphene derivatives/AZC coatings
Electrodeposition was conducted with 304 stainless steel (304SS,15 mm × 15 mm × 0.08 mm) as the anode and platinum sheet(15 mm × 15 mm × 0.1 mm) as the cathode,as shown in Fig.1.The distance between the electrodes was about 2 cm.Constant DC voltage of 30 V was supplied for 10 min by a DC electrical source.The electrolyte was formulated by sulfonated graphene oxide (SGO,2 mg/ml) and different concentrations of AZC aqueous dispersion.
The fabrication method of the SGO was described in our previous publication [32].In brief,500 mg graphene oxide (GO,2 mg/ml) was hydroxylated by using Na2CO3(5%) and NaBH4(4 g) at 80°C for 1 h.Aryl diazonium salts were prepared by mixing concentrated hydrochloric acid (3.5 ml,1 mol/L),p-aminobenzonic acid (307 mg) and sodium nitrite (120 mg) in an ice bath.The as-prepared aryl diazonium salt solution was added to hydroxylated graphene oxide dispersion (2 mg/ml)and stirred for 4 h.The resulted SGO was washed repeatedly and dispersed in water at a concentration of 2 mg/ml.
The SGO/AZC mass ratio was set as 8:1,6:1,4:1,2:1 in the electrolyte.The coated specimens were dried at 110°C for 10 min and referred as SGO/AZC-8,SGO/AZC-6,SGO/AZC-4,SGO/AZC-2,accordingly.To optimize the heating temperature,it was varied from 80 to 160°C to obtain the SGO/AZC-80,SGO/AZC-110,SGO/AZC-120 and SGO/AZC-160 specimens.Optionally,the SGO/AZC composite coatings were reduced by hydrogen iodic acid vapor(HI,10 mg/ml)for 2 h,4 h and 6 h at room temperature,referred as SGO/AZC-2h,SGO/AZC-4h and SGO/AZC-6h.
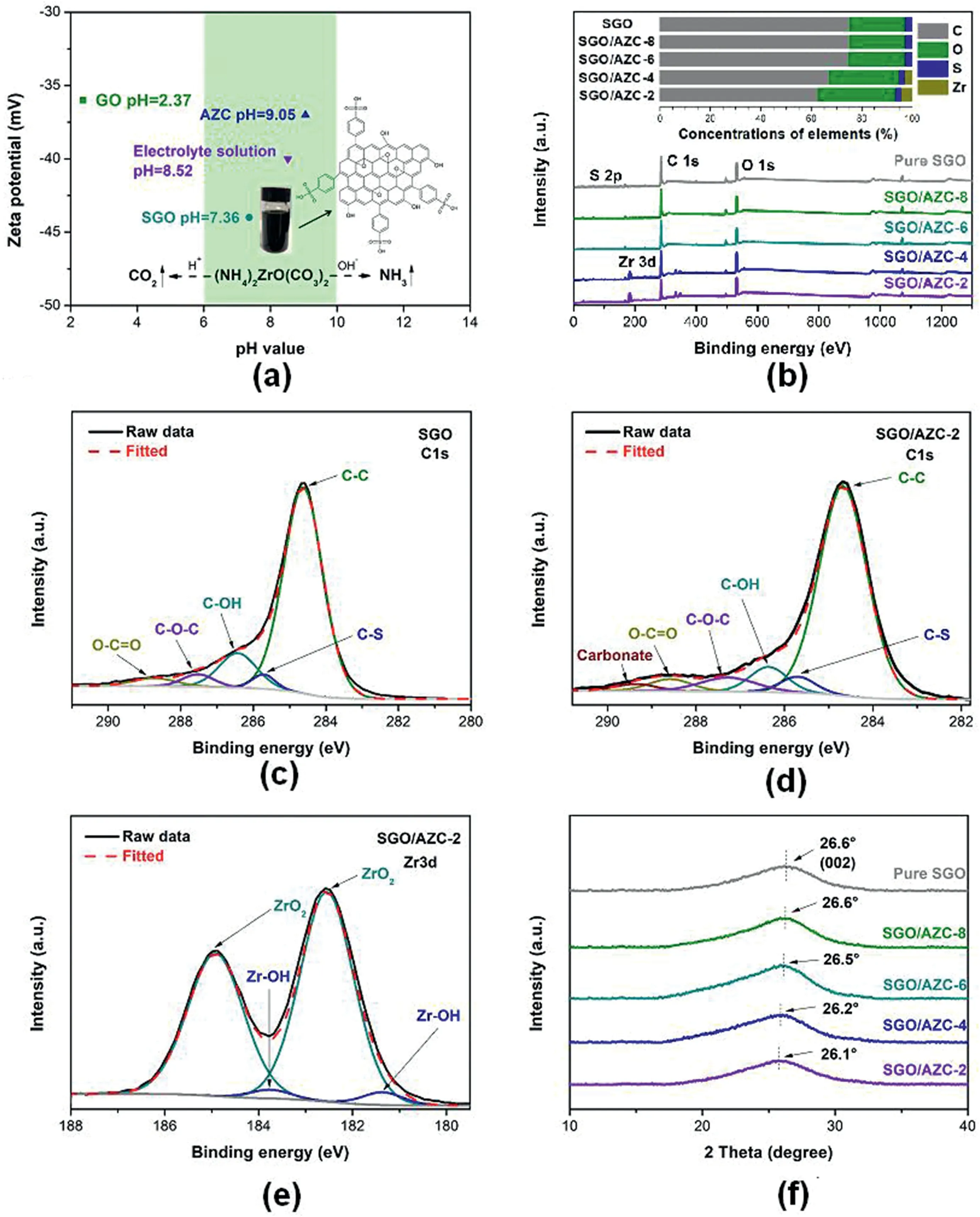
Fig.2.(a)The pH and Zeta potential of the aqueous dispersion of GO,SGO,AZC and the electrolyte solution of SGO/AZC;(b)XPS spectra of SGO,SGO/AZC-8,SGO/AZC-6,SGO/AZC-4 and SGO/AZC-2 coatings;C1s curve fitting of(c)SGO and(d)SGO/AZC-2 coatings;(e)Zr3d curve fitting of SGO/AZC-2 coatings;(f)XRD patterns of SGO and composite coatings.
2.2.Characterization
The surface and cross section of the SGO/AZC composite coatings were observed by field emission scanning electron microscope(SEM,FEI Quanta FEG) with energy dispersion spectrometer (EDS).Fourier transform infrared (FTIR,Spectrum 100 PerkinElmer) spectra,X-ray photoelectron spectroscopy(XPS,PHI5300 ESCA)and X-ray diffraction(XRD,Bruker D8 ADVANCE) was employed to characterize the structure of SGO/AZC coatings.
The corrosion resistance of the SGO/AZC composite coatings was investigated by an electrochemical workstation (PARSTAT 4000A,Princeton Applied Research),using a classical three-electrode cell.The working electrode was the coated specimens with a sampling area of 1 cm2.The reference electrode was Ag/AgCl (0.205 V/SHE) and the counter electrode was a platinum net.Before the measurement,the working electrodes were exposed to 3.5%NaCl solution for about 20 min to obtain a steady open circuit potential.Tafel polarization tests were repeated for 20 times with 24 h intervals for each sample in the range of-0.1 V–0.1 V (OCP) at a scanning rate of 1 mV s-1.The electrical conductivity of the SGO/AZC composite coatings was characterized by a four-point probe tester(RTS-9).
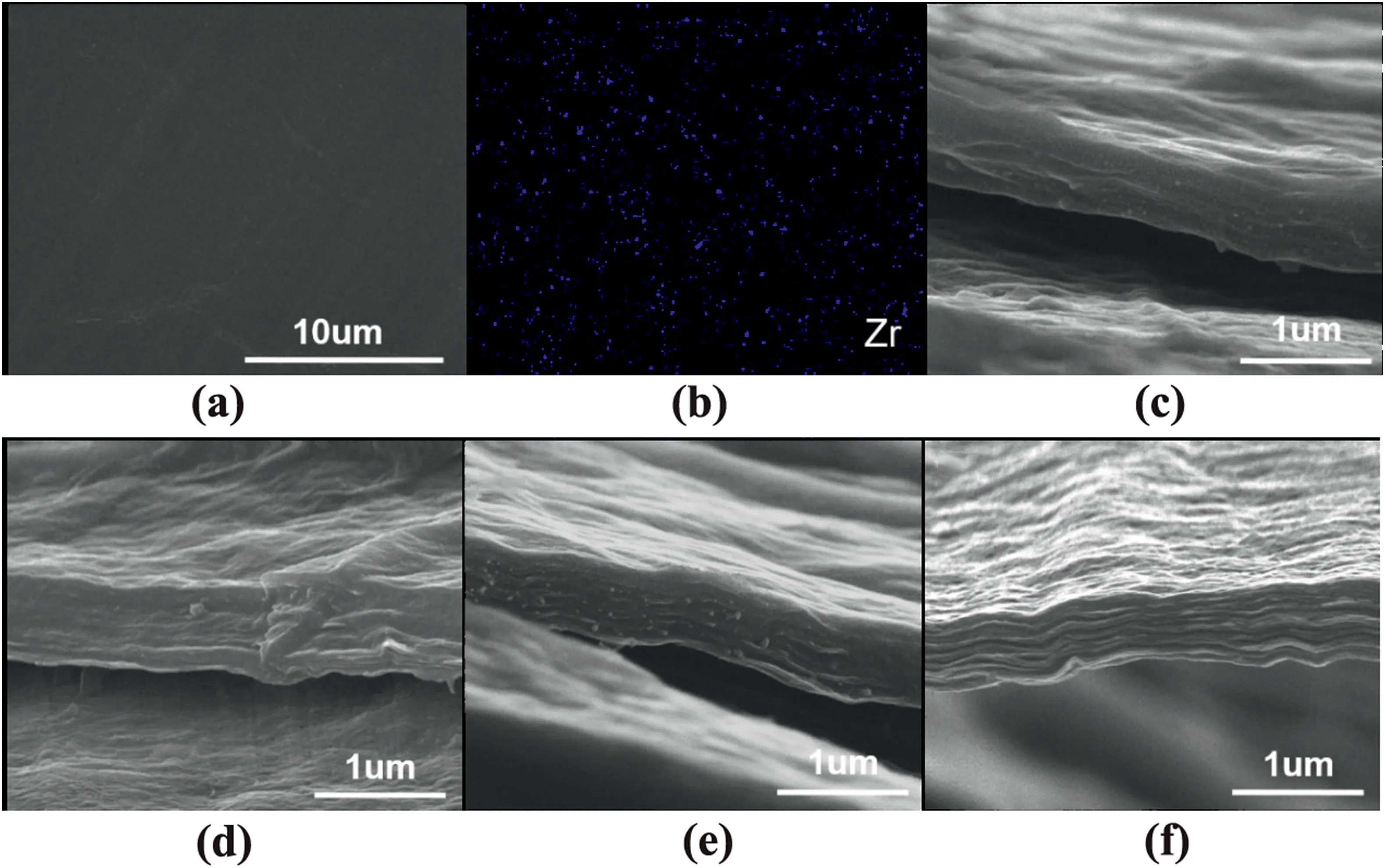
Fig.3.(a)SEM micrograph and(b)Zr element mapping of SGO/AZC-2 coating surface;cross-section morphologies of(c)SGO/AZC-8,(d)SGO/AZC-6,(e)SGO/AZC-4 and (f) SGO/AZC-2 composite coatings.
3.Results and discussion
3.1.Film-formation of the SGO/AZC conversion coatings
SGO sheets moved from electrolyte to the surface of 304SS under the electric field.The appearance of the coated specimens was shown in Fig.1.The SGO coatings were easily peeled off due to the weak metal/coating adhesion.The film-formation of SGO/AZC coatings was excellent because AZC,as an inorganic crosslinking agent,can nail the coatings on the metal substrates.
Fig.2a showed the zeta potential values of aqueous dispersion of GO,SGO,AZC and electrolyte solutions.The aqueous solution of AZC can be stably stored at the pH range of 6–10.When the pH value was lower than 6,AZC was hydrolyzed to zirconium oxide/hydroxide,while carbon dioxide was released and precipitation was observed due to the low solubility of zirconium oxide/hydroxide[33].When the pH value was higher than 10,ammonia was rapidly released from the aqueous solution of AZC.The aqueous dispersion of GO is acidic due to the ionization of carboxyl groups.To prepare a stable electrolyte with appropriate pH value and good dispersion stability of graphene layers,sulfonation of GO was carried out.
The chemical structure of SGO was inserted in Fig.2a,as we have presented previously[32].The sulphur and oxygen atomic concentration of SGO was 2.71%and 21.87%,as shown in the XPS spectrum(Fig.2b).The C1s peak of SGO was deconvoluted into the components at 284.6,285.8,286.4,287.2 and 288.6eV,corresponded to C–C(77.42%in total area),C–S (3.15%),C–OH (12.34%),C–O–C (3.76%) and O–C–O(3.34%),respectively,as shown in Fig.2c.The grafting of phenylsulfonic acid groups on SGO was proven by the presence of C–S bonds in XPS results,as well as the vibration peaks of S–O and S-phenyl groups shown in FTIR spectrum (Fig.S1).Besides,the SGO had much lower oxygen atomic concentration than GO due to the reduction by NaBH4.The XPS spectrum and C1s fitting of GO were shown in Fig.S2.The oxygen atomic concentration of GO was around 31.08%and the percentage of C–C bond counted as only 52.5% in total area of C1s.The aqueous dispersion of SGO had a pH value of 7.36 and a zeta potential value of-44 mV,which favoured the dispersion stability of SGO due to the strong electrostatic repulsion between neighbouring SGO sheets.The electrolyte had a pH value of 8.52,where the aqueous dispersion of AZC was stable.In addition,the ionization of phenylsulfonic acid groups benefited the migration of SGO to the anodic metal substrate during electrodeposition.
3.2.The crosslinking effects of AZC
The XPS spectra of SGO/AZC composite coatings were shown in Fig.2b.The S element was detected due to the present of SGO.The content of Zr element was increased with the AZC/SGO ratio in the electrolyte,and reached the highest atomic concentration of 4.34% for SGO/AZC-2.The atomic ratio of AZC in the coatings was much lower than that in the electrolyte (11.42% for SGO/AZC-2),indicating that deposition rate of SGO was higher than that of AZC.The composition of the coatings was dominated by SGO,and a small amount of AZC was distributed in the coatings.
The C1s XPS spectrum of the SGO/AZC-2 coating was deconvoluted by fitting curves and shown in Fig.2d.Compared with SGO,the SGO/AZC coatings showed a new peak at 289.4 eV,which was assigned to carbonates (2.05%).Meanwhile,the amount of hydroxyl groups was decreased from 12.34% to 6.37%.These were resulted from the condensation reaction between zirconium hydroxyl groups of hydrolyzed AZC and the hydroxyl groups of SGO,which produced covalent Zr–O–C bonds,as shown in Fig.1.
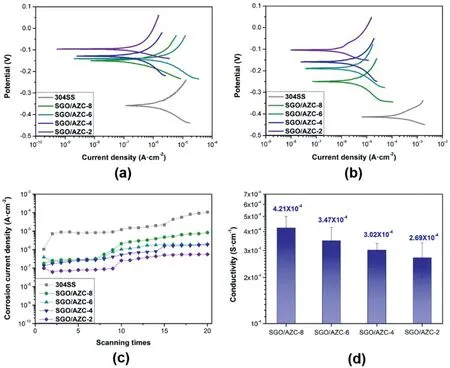
Fig.4.Potentiodynamic polarization curves of 304SS,SGO/AZC-8,SGO/AZC-6,SGO/AZC-4 and SGO/AZC-2 coatings on(a)the first scan and(b)the 20th scan;(c)the Icorr of composite coatings as a function of scanning times;(d) the electrical conductivity of the composite coatings.
The Zr3d XPS spectrum of SGO/AZC-2 was fitted as the superimposing of two sets of doublets.The doublet located at 182.5 and 184.9 eV was corresponded to the zirconia,while the one at the lower binding energy was associated with the Zr–OH[34].The fraction of the zirconia was dominated as 97.6%,resulted from the dehydrating reactions.The scheme of dehydration reaction was presented in Fig.1.The hydroxyl bridging groups connected adjacent zirconium ions of AZC.Upon the emitting of NH3,CO2and bound water,a large amount of Zr–OH were presented.The condensation reaction between Zr–OH and hydroxyl groups on SGO and oxidized 304SS surfaces,leading to formation of Zr–O–C and Zr–O-metal.The network structure was formed upon the dehydration,resulting from the crosslinking effects of AZC,which improved the cohesion of the coatings and the coating/metal interfacial bonding.The hydroxyl bridging groups between adjacent zirconium ions also dehydrated upon heating.
XRD patterns of SGO and SGO/AZC composite coatings were shown in Fig.2f.SGO showed a broaden peak at 26.6°,corresponding to an interplanar distance of 0.33 nm,which was close to the characteristic peak of graphite due to the reduction of GO.The diffraction peaks of SGO/AZC coatings shifted gradually from 26.6°to 26.1°with increasing the AZC content.The addition of AZC increased the interlayer spacing of SGO,indicating the intercalation of AZC between SGO sheets.Combining the results of XPS and XRD analysis,the SGO/AZC coatings was consisted of SGO layers,intercalated with small amount of amorphous zirconia.
3.3.Alignment of SGO in the composite coatings
The surface of the SGO/AZC coatings was very smooth as shown Fig.3a.The Zr element was uniformly distributed as presented in blue color(Fig.3b).Fig.3(c~f)showed the cross-section morphology of the composite coatings with different SGO/AZC mass ratios.The coating thickness was about 500 nm.The SGO sheets were parallelly aligned on the 304SS substrates,which was driven by the electric field and the attraction between the phenylsulfonic acid groups on SGO and the anodic 304SS substrates.With a high magnification of 8 × 104,the coatings seemed to be highly compacted without showing micro-defects.The layered structure of the SGO/AZC-2 coatings become clearer with the increasing content of AZC,which was probably resulted from the increased interlayer spacing of SGO,as shown in Fig.2f.The aligned SGO sheets were intercalated by small amount of AZC as crosslinker,which assembled the nacre structure,usually featured compactness,good mechanical properties and appearance [35].Electrodeposition was beneficial to the uniform thickness,the preferred orientation and the close-packing of the coatings.
3.4.Properties of the SGO/AZC composite coatings
Fig.4 (a,b) showed the polarization curves of the SGO/AZC composite coatings for the first and the 20th scan.The corrosion potential(Ecorr) and Icorrwere listed in Table S1.The Ecorrvalue of composite coatings increased with the AZC content.The bared 304SS showed the lowest Ecorrof -0.36 V.The higher Ecorrvalue represented the lower corrosion tendency and the higher Icorrvalue was associated with the higher corrosion rate [36].The bared 304SS substrate showed the Icorrvalue of 1.04 μA cm-2,higher than the specimens coated with SGO/AZC composite coatings.The Icorrvalue decreased with increasing the AZC content in the composite coatings for the first scan.The SGO/AZC-2 specimen had the Icorrvalue of 0.098 μA cm-2.Considering the 500 nm thickness of the composite coatings,the corrosion protection properties of SGO/AZC-2 coatings was outstanding.The Icorrvalue of the chromate conversion coating was reported to be 0.14 μA cm-2with a thickness of 1.1 μm on 304SS substrates[37].The Icorrvalue of the AZC conversion coatings with 33.75%CAP was reported to be 0.20 μA cm-2with a thickness of 1.0 μm [31].
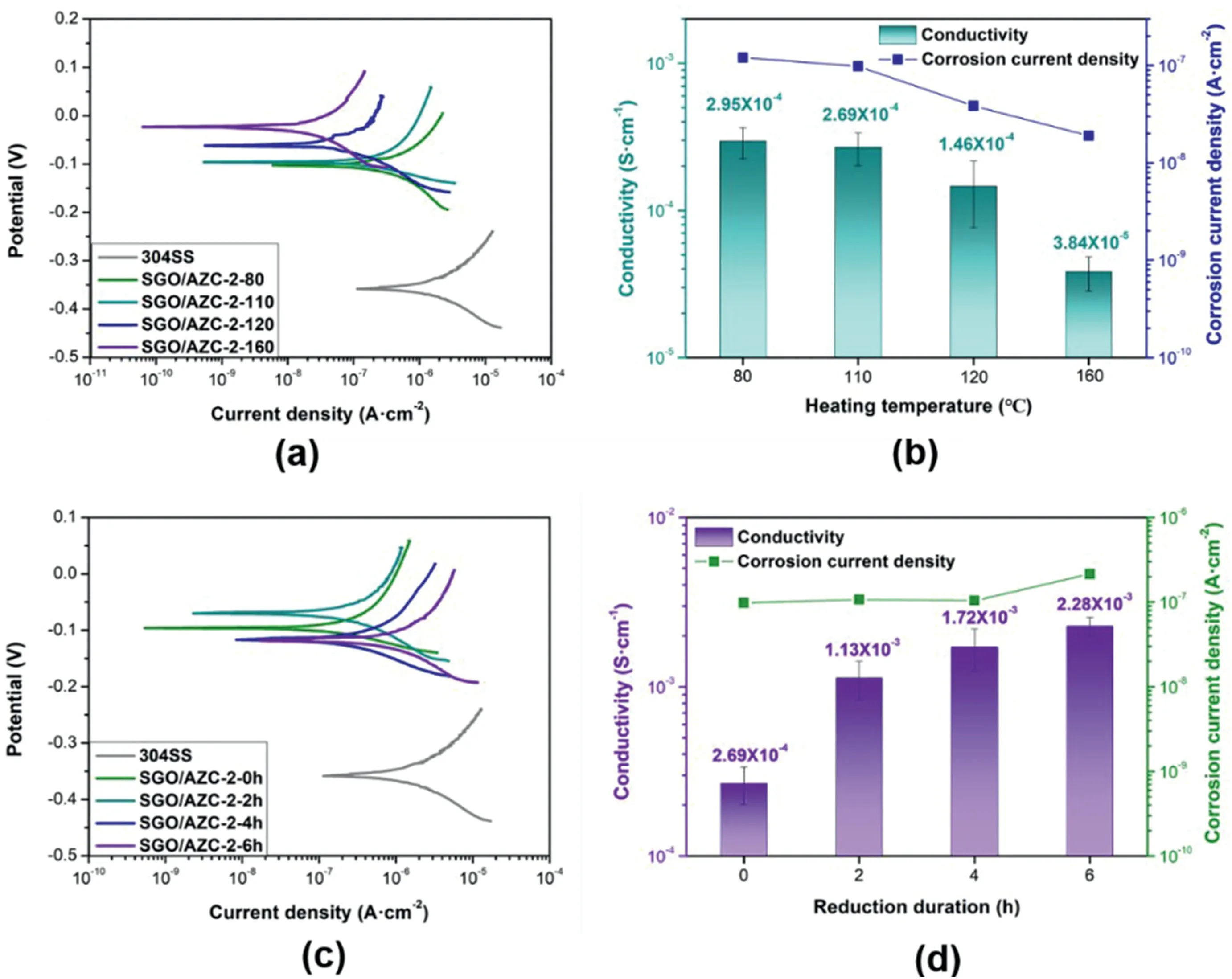
Fig.5.(a,b)Potentiodynamic polarization curves,electrical conductivity and Icorr of the coatings as a function of heating temperature,and(c,d)reduction duration.
Since the Tafel test is a destructive experiment,the Icorrincreased with the scanning times,as shown in Fig.4c.Given the interval of 24 h immersion in 3.5%NaCl solution between each scan,the results can be interpreted as the long-term corrosion protection properties[9].The Icorrof the bared stainless steel increased by two orders of magnitude for the 20th scans,while that of the SGO/AZC-2 increased by less than one order of magnitude.The lowest Icorrwas 0.56 μA cm-2,achieved by SGO/AZC-2,for the 20th scan.
The SGO/AZC composite coatings were graphene derivatives-based inorganic conversion coatings,where aligned SGO offered physical barriers and dehydrated AZC generated a passivation layer at metal/coating interface,as shown in Fig.1.Compared with randomly distributed graphene sheets,the aligned graphene sheets were more effective on preventing the penetration of corrosion medium [38].The passivated interface can break the galvanic contact between the SGO and metal surfaces.Meanwhile,AZC promoted the compactness of the coating by filling the defects and crosslinking the SGO layers.The synergy effects between SGO and AZC were expected from the nacre-like structure.
The electrical conductivity of the SGO/AZC composite coatings decreased from 4.21 × 10-4S cm-1to 2.69 × 10-4S cm-1with increasing the AZC content,which was marginal if the scale bars were taken into consideration.The conductivity of compacted SGO powder was about 1.90 × 10-3S cm-1.Zirconia is an electrical insulator.As discussed in Section 3.2,the Zr atomic content in the coatings was less than 4.34%,so the electrical conductivity of the composite coatings was increased by only one order of magnitude by the intercalated of zirconia.
3.5.Effects of post-treatments
The effects of heating temperature were studied on the properties of SGO/AZC-2 composite coatings,as shown in Fig.5a,b.The Icorrdecreased with increasing the heating temperature.The heating temperature affected the dehydrating extent of AZC[30].Dehydration was beneficial to the corrosion resistance of the composite coatings.However,the electrical conductivity of SGO/AZC composite coatings was decreased with increasing the heating temperature because the residual zirconium ions turned into zirconia.
A post-reduction of the SGO/AZC composite coatings was carried out to increase the electrical conductivity by further eliminating the oxygencontaining functional groups of SGO.Fig.5c,d showed the corrosion resistance and electrical conductivity of composite coatings after different reduction duration by HI vapor.The electrical conductivity of the SGO/AZC composite coatings was improved for one order of magnitude by 6 h HI treatment.It has been reported that HI can effectively reduce the oxygen-containing functional groups on graphene derivatives and improve their electrical conductivity [39].The corrosion resistance of the composite coatings did not change much with the reduction duration.
4.Conclusions
Electrically conductive inorganic conversion coatings were fabricated on 304SS by electrodeposition.The coatings were composed of highly aligned SGO layers,intercalated with small amount of amorphous zirconia.AZC,an inorganic crosslinking agent,was the precursor of zirconia.When the mass ratio of SGO to AZC was 2:1 in the deposition electrolyte,the Icorrof the composite coatings reached 0.098 μA cm-2with a coating thickness of only 500 nm,which outperformed the toxic chromate conversion coatings.The electrical conductivity of SGO/AZC composite coatings were 2.69 × 10-4S·cm-1and 2.28 × 10-3S·cm-1before and after a mild reduction by HI vapor.The high electrical conductivity and corrosion protection properties were benefitted from the nacre-like structure of the coatings,where the SGO provided electron pathways and physical barriers for corrosive medias,the thin layers of intercalated zirconia improved the cohesion between SGO layers and compactness of the coatings.We hope the graphene-based inorganic composite coatings can find their unique applications where corrosion protection and electrical conductivity were required.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China U1560108,Science and Technology Commission of Shanghai Municipality (17511101603,18ZR1426300,19JC1410400) and Shanghai Municipal Education Commission(2019-01-07-00-07-E00015).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nanoms.2021.07.011.
- Namo Materials Science的其它文章
- A comparative study of polymer nanocomposites containing multi-walled carbon nanotubes and graphene nanoplatelets
- Recent progress on thermal conductivity of graphene filled epoxy composites
- Tensile properties of functionalized carbon nanothreads
- Strain effects on the interfacial thermal conductance of graphene/h-BN heterostructure
- Atomic insights into synergistic effect of pillared graphene by carbon nanotube on the mechanical properties of polymer nanocomposites
- Fracture behavior of hybrid epoxy nanocomposites based on multi-walled carbon nanotube and core-shell rubber

