Recent progress on thermal conductivity of graphene filled epoxy composites
Ruicong Lv,Yanjuan Ren,Haichang Guo,Shulin Bai
School of Materials Science and Engineering,HEDPS/Center for Applied Physics and Technology,Key Laboratory of Polymer Chemistry and Physics of Ministry of Education,Peking University,Beijing,100871,China
Keywords:Graphene Epoxy Composites Thermal conductivity
ABSTRACT With the rapid development of the electronic industry,the requirements for packaging materials with high thermal conductivity (TC)are getting higher and higher.Epoxy is widely used as package material for electronic package applications.But it's intrinsic TC can't meets the increasing demands.Adding high TC graphene into epoxy matrix is a proper way to reinforce epoxy composites.This review focuses on the filler modification,preparation process and thermal properties of graphene-filled epoxy resin composites.Different ways of covalent and non-covalent modification methods are discussed.The various kinds of graphene coating layer are also summarized.Then we analysis the hybrid filler system in epoxy composite.We hope this review will provide guidance for the development and application of graphene-filled epoxy resin composites.
1.Introduction
With the rapid development of the communication industry,the electronic packaging industry is also undergoing high-speed upgrades[1].While the miniaturization of electronic products brings excellent convenience to people,it also places high requirements on the heat dissipation performance of packaging materials.When using epoxy resin as a packaging material,due to its low intrinsic TC (TC),the generated heat cannot be removed in time,which will bring safety hazards to local overheating[2].It cannot guarantee the regular operation of the circuit,which restricts its applications in the field of packaging.Therefore,the preparation of epoxy-based composite materials with higher TC is essential for developing the electronic industry[3].
Generally speaking,there are two ways to enhance the TC of epoxy resins[4].One is to increase the intrinsic TC of epoxy.The key to prepare intrinsic thermally conductive polymers is to arrange the molecule in a particular direction and increase molecular rigidity.Through the molecular design method,the epoxy molecular chain with a liquid-crystal structure is synthesized to realize the orderly arrangement of mesogenic elements [5].This method can increase the mean free path of phonon transmission and reduce the scattering of phonons in the disordered molecular chain,thereby reducing thermal resistance [6–8].Another improved method is to mix the thermally conductive filler with epoxy [9].The size,shape,filling amount,and intrinsic TC of the filler are essential to the composite material [10].The fillers are evenly dispersed in the matrix by an external force.After casting,coating,and solvent removal,the composite material is cured under appropriate conditions.
Thermally conductive fillers include ceramic,metal and carbon materials.Ceramic fillers include aluminum oxide [11],aluminum nitride[12],boron nitride [13],silicon dioxide [14],etc.These fillers have excellent insulation properties and stability when filled.Metal materials such as copper [15] and silver [16] are also used on certain occasions.These fillers usually have a relatively large density and are generally filled as powder and nanowires.Carbon-based fillers include graphite[17],graphene[18],carbon nanotubes[19],carbon black[20],etc.Most nano-carbon materials have a specific anisotropy and high intrinsic TC.But it's tendency of self-aggregation will decrease the TC[10].Graphene has a high theoretical TC [21].However,when it is directly filled,it cannot obtain a very high TC as described by the law of mixing.There is a specific incompatibility between the graphene and the epoxy during the filling process.There is also an agglomeration phenomenon of the graphene sheets.When its content is too high,the system cannot be uniformly mixed during the curing process,and it is not easy to form[22].Fig.1 Gives a summary of modification methods of graphene.
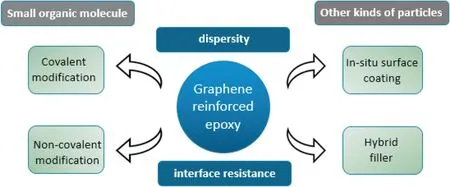
Fig.1.A summary of modification of graphene reinforced epoxy.
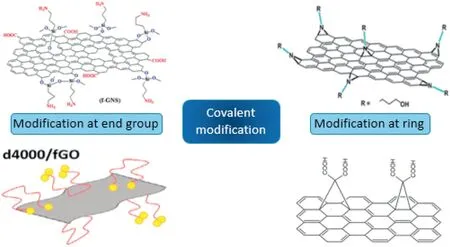
Fig.2.Different type of covalent modification of graphene [29–32].
In this article,we summarize the recent works of thermally conductive graphene-filled epoxy composites.First,the pretreatment method of graphene sheets is summarized and discussed from the perspectives of covalent and non-covalent modification.Then,single graphene and hybrid fillers filled epoxy composites are described.Finally,the challenges and future development trend in this emerging area are analyzed.
2.Surface functionalization of graphene
As a typical two-dimensional material,graphene has excellent thermal conductivity and mechanical properties.However,due to its large specific surface area and complete conjugated system,when used as a filler,it will agglomerate and have poor interfacial strength [23].Many unsuitable filler-matrix interfaces and filler-filler interfaces in graphene-filled composites cause a mismatch of phonons at the interface,resulting in a significant drop in heat transfer efficiency [24].On the other hand,the agglomeration harms the formation of the overall heat conduction path.In order to obtain an ideal filling effect,it is necessary to modify the filler before filling.
The modification treatment of the graphene surface can generally be divided into covalent and non-covalent modification treatment[25].The covalent modification is generally to react on the graphene sheet surface and residues on edge to chemically bond with the modifier[26].One side of the modifier interacts with a small amount of carboxyl,hydroxyl,epoxy and other functional groups on the graphene surface.The other end couples with epoxy matrix during curing.The non-covalent effect is generally to use the conjugated system of the modifier and the delocalized system of graphene to construct a strong π-π effect,which can separate the graphene sheets and improve interaction with the resin matrix [27].In different graphene-epoxy composite material systems,it is essential to select appropriate modification methods to improve the overall performance of the composite material.
2.1.Covalent modification of graphene
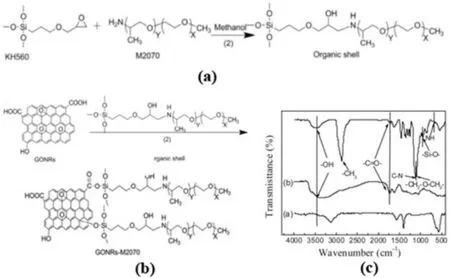
Fig.3.(A) Synthesis KH560-M2070 coupling agent.(b)Preparation GONRs-M2070.(c)FTIR of a)pristine MWCNTs b)GONRs c)GONRs-M2070 [33].
Graphene oxide(GO)is exfoliated from graphite in concentrated acid and the oxidizing environment by the Hummers' method [28].Then reduced graphene oxide (RGO) is obtained by high temperature or chemical reduction with hydrazine hydrate or other reducing reagent.The graphene obtained in this way contains more oxygen-containing functional groups and is accessible to graft small molecule modifiers.The contact thermal resistance between the filler and substrate can be effectively reduced due to the chemical connection.Covalent modification can be divided into two types: the interaction with the functional group of the graphene end group.The other is the ring-opening modification with the conjugated ring of the graphene itself[25],as shown in Fig.2.
The most common covalent bond modification is to use a silane coupling agent.The molecular structure of the silane coupling agent is generally YR-Si(OR)3,where Y is a nucleophilic organic functional group,such as epoxy group and amino group,which can form a chemical bond with the matrix.–OR is an alkoxy group,such as methoxy group,oxethyl group and chlorine.These hydrolyzable groups can interact with the hydroxyl and epoxy groups on the surface and edge of the graphene to achieve a coupling effect.Wang et al.[29]directly used 3-aminopropyltriethoxysilane(APTES)to treat graphene.Graphene nanosheets with APTES are ultrasonically obtained under the catalysis of dicyclohexylcarbodiimide (DCC).The end-group-grafted graphene nanosheets of silane increased the tensile strength and elongation at the break by 45%and 133% respectively when filled with 1 wt% fillers.Li et al.[33]pretreated γ-glycidoxypropyltrimethoxysilane (KH560) and polyetheramine M2070 before using the coupling agent with graphene,as shown in Fig.3(a).The epoxy group on KH560 reacts with terminal amine and opens the ring.The alkoxy group is coupled with the epoxy group and the carboxyl group on the GO surface in Fig.3(b).The existence of -CH3,C–N,–CH2-CH2-,-Si-O-and other bonds can be observed from the infrared spectrum,in Fig.3(c),which proves the exists of covalent bonding with graphene and coupling agent.Because the long molecular chain has good compatibility with the epoxy matrix,the filler has a better dispersion in epoxy matrix.The flexural strength,impact strength and glass transition temperature are increased by 16.2%,83.9 and 32.5%respectively under 0.6 wt%filling.Oh et al.[34]synthesized 3-methacryloxypropyltriethoxysilane (MPTES) functionally rGO by sol-gel method.They introduced a methacrylate group with a carbon-carbon double bond and prepared polyglycidyl methacrylate grafted rGO by free-radical copolymerization.At 7 wt% filler content,the TC of the graphene-composite material reaches 0.75 W m-1K-1.Compared with the pure graphene-filled composite material,the filler-matrix voids are also much reduced.
Using surfactants such as polyether amines as the covalent modification is also a feasible strategy[35].Polyether amine(PEA)is a type of polymer whose main chain is a polyether,and end groups are amine groups.The general chemical formula is C3n+3H6n+10OnN2,as shown in Fig.4(a).By choosing different main-chain structures,a series of properties such as reactivity,toughness,viscosity and hydrophilicity of polyether amine can be adjusted.Different types of polyether amines are distinguished according to the different molecular weights.The advantage of polyether amine as a modifier is that it can be used as a curing agent to participate in curing epoxy resin.After modifying the graphene surface,it can participate in constructing the thermosetting system,reducing the gap and contact thermal resistance between the filler and the matrix [36].Vryonis et al.[30] studied the dispersion of polyether amine D230,D4000 and T440 functionalized graphene oxide in the epoxy resin matrix.It can be seen from the low magnification photo of the cross-section of the composite material that the higher content of the original graphene appears obvious agglomeration in the matrix dispersion,as shown in Fig.4(c).The long-chain T440 and D4000 showed good dispersion after treatment,indicating that long-chain surfactants with lrage molecular weight have an improved effect on the dispersion of fillers in composite materials.Tang et al.[37] used D230 polyether amine to modify GO.The composites' Young's modulus and tensile strength were increased by 536% and 269% respectively at 2.7 vol%.Before polyether amine modification,GO was dispersed in the water phase.After modification,it was well dispersed in dichloromethane.By the FTIR spectra in Fig.4(b),after refluxing of GO with D230,two new peaks appear at 2854 and 2958 cm-1due to the presence of–CH2and–CH stretching vibration.1099 cm-1corresponds to aliphatic ether,implying the presence of D230.Peaks at 1172 cm-1(C–N stretching vibration)indicates the covalent bonding between D230 and GO.

Fig.4.(A) Molecular structure of A) d230 (~n2),d4000 (~n68) and B) t440 (~n2).(b)FTIR spectra of GO,D230,GO-D230-80 [37].(c)Optical microscopy images of samples filled with different type of modified graphene at 0.4 vol% and 0.6 vol% [30].
There are other ways to modify graphene terminal functional groups by different modifier.Zhang et al.[38] used 4,4-methylene diphenyl diisocyanate(MDI)to couple expanded graphite(EG).Amine-terminated macromolecular chain (ATBN) is grafted on the surface of EG.ATBN enhances the interface connection between epoxy resin and filler.It can be seen from XPS that the N element content increases and the N1s peak confirms the presence of isocyanate groups.In the case of 25 wt% filler filling,the TC reaches 3.827 W m-1K-1.Feng et al.[39] covalently grafted polyphosphoramide oligomer PDMPD on the surface of graphene and filled it with alpha alumina particles.Compared with alumina-epoxy composites,the addition of 1 wt%PFR-RGO increased 11.2%TC.Adding PDMPD effectively reduces the sedimentation of particles,enhances the interface effect,and has a better flame retardant effect.By covalently grafting polyphosphoramide oligomers,a high heat-resistant protective layer is formed on the surface of graphene to block the heat of combustion and unstable degradation products.These grafting methods mainly use the functional groups of the graphene end groups to graft small molecules and some oligomers.Graphene participates in the polymerization process of the epoxy resin during the filling process,reduces the interface misfit,and improves the mechanical properties and thermal conductivity.
Another type of covalent modification is to directly weaken part of the conjugated structure and use some highly reactive small molecules to form rings on the surface of graphene.Naebe et al.[31] covalently functionalized the graphene obtained by thermal reduction through the Bingel reaction to improve the interfacial bonding between graphene and epoxy resins.1,8-Diazabicycloundecene (DBU)is added to graphene for 2,1-cycloaddition reaction,and carboxyl groups are introduced on the surface of graphene.When the added amount is only 0.1 wt%,the flexural strength increases by 22%,and the storage modulus increases by 18%.Compared with the covalent modification on the end group,the treatment on the ring is more suitable for graphene with few functional groups.The larger delocalization area also provides abundant grafting sites.Depaifve et al.[32]treated graphene with a one-step preparation of azide methanol at 150°C in a nitrogen atmosphere to form a nitrogen three-membered ring on the graphene plane,as in Fig.5(a) Similar to oxidation treatment,nitration treatment also interacts with conjugation group on the graphene surface.In the cm-GNP (nitrene precursor and thermally decomposed) spectrum (Fig.5(c)),C–O andC–N is observed,which confirms the covalent modification of GNP with nitrene precursor.But when using oxidation methods to modify graphene (Fig.5(d)),the content of sp3carbon dramatically increases,which directly influence the Integrity of conjugate structure.It can be seen from the CT images of fillers and voids that compared to the filling of GO and original graphene,the proportion of voids in the graphene-filled composite material after nitration treatment is significantly reduced.The filler is dispersed uniformly in the matrix.In the case of 2 wt% filling,the TC of the original graphene filling is 0.43 W m-1K-1,the GO filling is 0.27 W m-1K-1,and the TC of the equal content filling after nitration treatment reaches 0.49 W m-1K-1.It shows that nitration treatment is more reliable for the surface modification of graphene.
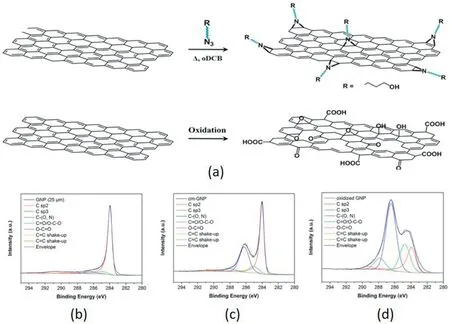
Fig.5.(a)Synthesis of functionalized graphene via nitrene.(b)XPS of a) pristine graphene,b)cm-graphene,c)oxidized graphene [32].

Fig.6.Basic type of non-covalent modification.
2.2.Non-covalent modification of graphene
The mechanically exfoliated and thermally reduced graphene nanosheets have a large output and low cost,which can be purchased in large quantities on the market.However,this kind of graphene has a high carbon content and few oxygen-containing functional groups on the surface or end groups.It is not easy to covalently carry out grafting and processing.On the other hand,covalent treatment will cause inevitable damage to the graphene structure and reduce the filler's intrinsic TC.Non-covalent modification mainly involves the adsorption process of modifier molecules on the graphene surface.Mainly useπ-πinteraction,cation-π interaction,hydrogen bond,hydrophilic and hydrophobic interaction to fix small molecules on the surface of graphene nanosheets[25],as shown in Fig.6.It aims to weaken the van der Waals force between the sheets and increase the interaction between graphene and epoxy matrix[40].
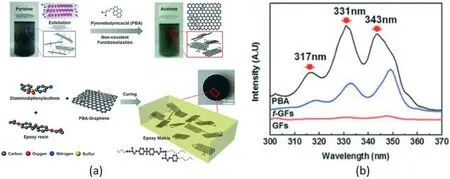
Fig.7.(A) PBA non-covalent modify graphene surface.(b)UV–vis spectra results for PBA,GFs and f-GFs [41].
In order to construct a non-covalent modification system forππinteraction,pyrene derivatives with larger delocalization systems are generally used as modifiers for treatment.Song et al.[41]used 1-pyrenebutyric acid(PBA)to non-covalently modify graphene,which reached a TC of 1.53 W m-1K-1when filled with 10 wt%,as shown in Fig.7(a).It was observed from the ultraviolet–visible spectrum (Fig.7(b)) that the characteristic peaks of graphene shifted after modification,indicating that theπ-πeffect affected the electron cloud distribution.Teng et al.[42]prepared polyglyceryl methacrylate Py-PGMA containing localized pyrene groups to modify graphene sheets,and the TC of the composite material reached 1.91 W m-1K-1at a content of 4 phr.The conjugated part of Py-PGMA can be adsorbed on the graphene surface.The epoxy-functional group of the end group can form a cross-linked structure with the matrix during the curing process,thereby reducing the contact thermal resistance between the filler and matrix without destroying the graphene.There are also reports on the use of block copolymers containing pyrene groups as modifiers.Yeom et al.[43] prepared a biphenyl and pyrene functionalized block copolymer modified graphene sheet through the reversible addition-fragmentation chain transfer polymerization (RAFT) reaction.The surface is covered with a layer of oligomer layer containing a large amount of oxygen-containing functional groups through the π-π effect,which can be connected with the epoxy resin matrix to enhance the interface compatibility.The TC is increased by 47.7 times under the condition of 13.6 vol%filling.
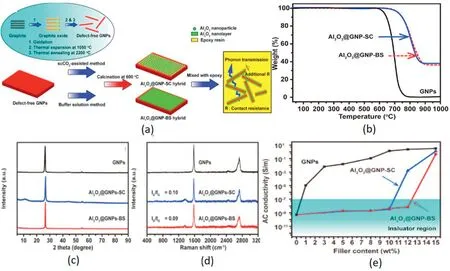
Fig.8.(A)Buffer solution method and scCO2 assisted in-situ coating Al2O3.(b)TGA of different type of Al2O3-graphene.(c)XRD patterns and (d)Raman spectra of GNPs and Al2O3@GNP hybrid.(e)electrical conductivities of epoxy composites fillers with GNPs and hybrids.
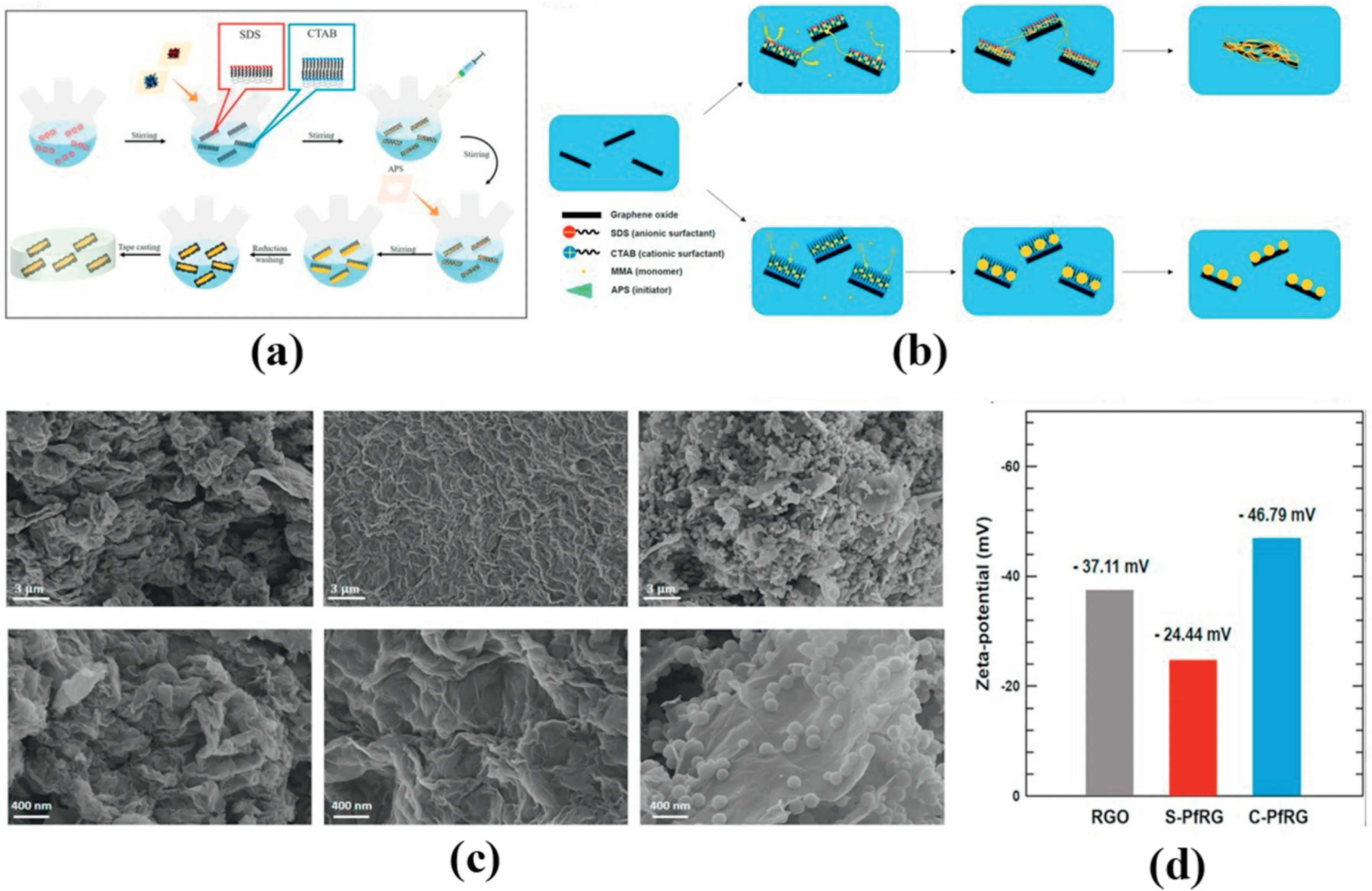
Fig.9.(A)Illustration of admicellar polymerization process for preparing S-PfRG and C-PfRG.(b)Mechanism foe formation of S-PfRG and C-PfRG.(c)FE-SEM images of a,d) RGO,b,e)S-PfRG,c,f)C-PfRG.(d)Zeta-potential of rGO and modified rGO [57].
In addition to using the delocalized group of the modifier molecule to treat the graphene surface,there are other ways to improve the filling effect without destroying the graphene structure.Wan et al.[44] used polyethylene glycol octyl phenyl ether (Triton X-100) as a surfactant to modify graphene.The graphene is modified with amphiphilic nonionic surfactants to improve the compatibility and wettability of the graphene sheets in the matrix.Tian et al.[45] used carboxyl-terminated hyperbranched polyester (HBP) to treat graphene oxide nanosheets.Hyperbranched polyester is embedded in the graphene oxide layer due to its highly branched structure,forming a steric hindrance effect,effectively preventing the agglomeration of flakes.The addition of modifiers also reduces the contact angle between HBP-GO and epoxy resin and improves the wetting performance.The tensile strength and elastic modulus of the composite filled with 0.5 phr increased by 41.4% and 49.9%respectively.
Non-covalent modification mainly takes advantage of the larger specific surface area and higher intrinsic TC of the two-dimensional material [46].Regardless of whether it is covalent or non-covalent treatment,essentially,it is modified from the interaction between the graphene and epoxy resin matrix.Choosing an appropriate modification method according to the system requirements is an effective strategy to improve graphene-filled epoxy resin's comprehensive performance.
3.Insulation coating on graphene surface
Not only are requirements placed on composite materials in terms of TC and mechanical properties,but there are also specific requirements in terms of electric insulation,flame retardancy,and formability of the composites.Graphene has a high intrinsic TC,and when used as a single filler,it does not necessarily meet the needs of a multi-scale industry.As we all know,graphene,as the two-dimensional carbon material with the highest TC,is determined by a complete conjugate structure and smooth phonon transmission path.However,this surface also allows high-speed migration of electrons,posing a potential safety hazard for the electrical breakdown on special occasions.In-situ deposition of an appropriate thickness of an insulating layer on the surface of graphene,such as oxides or polymers can better meet this demand.
3.1.Oxide coating on graphene surface
As a material with good insulation and specific TC,the oxide can be used for coating of two-dimensional materials [50].Unlike the direct mixing and filling of particles,the in-situ coating allows the insulating layer to be directly deposited on the surface of the graphene.Graphene has large specific surface area to provide nucleation sites for oxide deposition.On the other hand,the graphene surface is negatively charged [51].It has a particular attraction to positively charged metal particles,confirmed in the Zeta potential measurement of graphene and precipitant.
Sun et al.[52]used aluminum sulfate octahydrate on the surface of graphene in formic acid-ammonium formate buffer solution for in-situ deposition and calcination into an aluminum oxide layer,as shown in Fig.8(a).The TGA curve(Fig.8(b))in the air can show that the amount of deposition occupies 60 wt%of the filler.When the mixed filler content is less than 12 wt%,the resistivity of the system remains above 107Ωm,which meets the requirements for electrical insulation.In the case of 12 wt%filling,the TC reaches 1.49 W m-1K-1.The content of the alumina coating layer and graphene body can also be adjusted according to requirements.Heo et al.[53]deposited a 50–150 nm thick alumina layer on the graphene oxide surface,where the alumina content is 80 wt%and the GO content is 5 wt% when the TC is up to 3.5 W m-1K-1.Alumina itself has good lubricity and can be filled with high content.The in-situ coating layer formed by aluminum isopropoxide can effectively block the contact between graphene sheets.

In addition to the alumina layer,the silica coating layer is also commonly used to modify graphene in composite materials.Compared to aluminum,the graphene surface is more easily grafted with silane,and then the silica coating layer is deposited by the sol-gel method.Li et al.[54] prepared silica-coated flake graphite-filled epoxy resin composites by the sol-gel method under the action of surfactants and acid-base catalysis.In the case of 50 wt% filling,the volume resistivity is 3.11 ×109Ωm,and the TC is 3.08 W m-1K-1.The volume resistance increased by 33.7 times after modification,indicating that the surface silica coating layer has a practical effect on improving the high conductivity of the filler.The graphite is treated with surfactant cetyltrimethylammonium bromide(CTAB)and polyoxyethylene sorbitol ester(Tween 20)and then hydrolyzed with ethyl orthosilicate (TEOS) in a neutral environment to obtain dioxide Silicon modified filler.Pu et al.[55]used 3-aminopropyl triethoxy silane(APTES)to graft the end groups of graphene oxide.Then they used TEOS as the precursor to deposit silica on the nanosheets.The grafted APTES enhanced graphene oxide and nano-silica compatibility.In the case of a filling amount of 8 wt%,the TC increases by 72%relative to the matrix and electrical insulation are maintained.It can be seen from the SEM image of the cross-section that the roughness is significantly reduced after treatment,indicating that the coating has improved the originally incompatible graphene surface.Hsiao et al.[56]heated at 700°C in Ar environment after deposition with ethyl orthosilicate.Under 1 wt% filling,the TC increased by 61%,while the TC of the unburned composite material only increased by 35% under the same filling amount.From the perspective of thermal stability,the filler has almost no heat loss before 450°after ignition.Compared with other coating layers,the silica coating layer is easier to interact with the groups on the graphene side chain.
3.2.Polymer coating on graphene surface
Using insulating oxide as the in-situ coating layer is an effective surface modification measure,and graphene with polymer is also a feasible strategy.As an inorganic material,the oxide itself does not have sufficient interaction with epoxy resin.However,the coated polymer layer has unique advantages compared to the original graphene-polymer interface.It also allows the two-dimensional material to have a good dispersion ability in the matrix.
Oh et al.[57]used single micelle polymerization to coat polymethyl methacrylate(PMMA)on the surface of graphene and studied the effect of surfactants on the coating,as in Fig.9(a).The TC of the graphene treated with CTAB reached 0.67 W m-1K-1after being coated with 3 wt% filling.After the graphene oxide nanosheets and the surfactant are subjected to agitation treatment,methyl methacrylate monomer(MMA)is injected.Then reflow polymerization is initiated with ammonium persulfate (APS) to obtain a PMMA-coated filler.Because the zeta potential (Fig.9(d)) of the graphene surface is negative,the positively charged CTAB pretreatment has a better effect in the test,and the mechanism is shown in Fig.9(b).The deposition of PMMA has an excellent isolation effect for graphene powder with a strong tendency to agglomerate.As in SEM images(Fig.9(c)),we can find that when using positive surfactant,PMMA tends to be sphere.Luo et al.[58]introduced polyethylene glycol (PEG) into the liquid crystal epoxy and graphene interface,and the TC was 10.17 W m-1K-1at 53.33 wt%.The liquid crystal polymer has highly oriented domains,which effectively suppresses phonon scattering and has a higher TC than conventional bisphenol A epoxy resins.The introduction of polyethylene glycol improves the interface compatibility between graphene and epoxy resin to facilitate formability and heat conduction paths under high fillingconditions.At the same time,it also improves the electromagnetic shielding performance of the composite material,up to 50 dB.
3.3.Other coating methods on graphene surface
In addition to directly coating on the surface of graphene,some work about coating graphene on larger fillers’surfaces can build a unique filler system.As a two-dimensional material with high TC,graphene itself is easy to electrostatically adsorb on the surface of large-sized fillers with positive electrical properties.So we can build the thermal pathway at the condition of low graphene loading.
Eksik et al.[59] wrapped polymethacrylic resin (PMMA) balls with rGO,as shown in Fig.10(a).When the net graphene content reaches 1 wt%,the TC reaches 1.4 W m-1K-1.The positively charged PMMA microspheres are polymerized in deoxidized deionized water,and GO is adsorbed by electrostatic effect.It is reduced with hydrazine monohydrate at 80°C and ground into powder to prepare composite material.In SEM images (Fig.10(b)),we can find when the amount of graphene increase,PMMA balls will connect by the bridge of graphene,which can significant increase the TC of epoxy composites.A clear core-shell structure can be observed in TEM image.Unlike the work mentioned above,graphene with high TC is used as a thermal network here,and PMMA microspheres are used to support constructing the network.Ye et al.[60] heated GO coated with Al(OH)3 in hydrogen and argon atmosphere to obtain Cu@rGO@Al2O3 core-shell structure filler.It has the advantages of high TC,oxidation resistance and electrical insulation.The TC reaches 3.41 W m-1K-1when filled with a total mass fraction of 70 wt%.The surface of copper microspheres was modified with 3-aminopropyltriethoxysilane (APTES),and Cu@GO powder was prepared in suspension.Al(OH)3 was precipitated in situ in ammonia water and heated in an oxygen-free environment to obtain a 3-layer core-shell Cu@r-GO@Al2O3 filler.Due to the aluminum oxide layer on the surface,the system still maintains a certain degree of insulation despite the highly conductive Cu particles and graphene layer inside.Graphene acts as an intermediate phase connecting the high TC metal core and the insulating protective layer,which plays a role in the system's stability.Similarly,the graphene coating structure design mainly depends on the requirements for TC,electricity,mechanics,and other properties.Designing a suitable filler system according to the needs of the application scenario is a unique advantage compared with the traditional single filler filling,and it also satisfies.The original intention of customizable composite material design(Table 1).

Table 1 Summary of the thermal conductive performance of modified graphene reinforced epoxy composites in recent literatures.
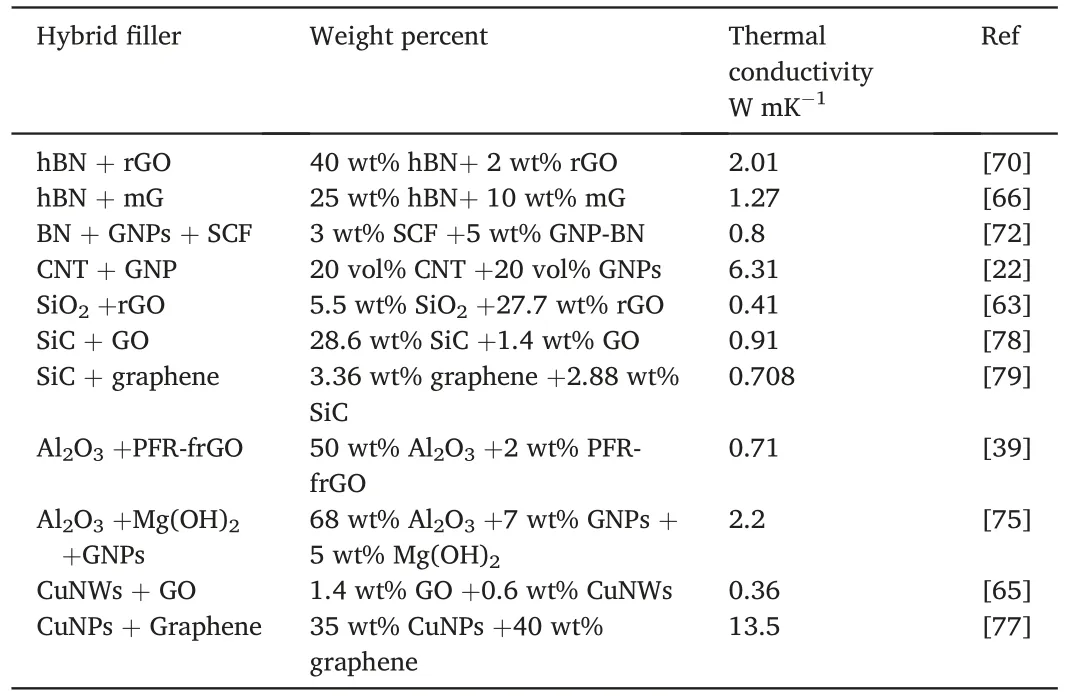
Table 2 Summary of the thermal conductive performance of hybrid fillers reinforced epoxy composites in recent literatures.
4.Hybrid fillers for epoxy composites
As a composite material,it has different performance requirements in various aspects,and a single filler sometimes cannot meet the application requirements.When graphene is filled with low content,the mechanical properties of the composite material are improved.However,when the content is increased,the system's viscosity increases sharply,and the decrease in formability also leads to a decrease in mechanical properties[61].Correspondingly,the overall TC is not significantly improved in the low-content filling because the heat conduction path is not formed.If choosing to blend with other particles,we can appropriately improve the material's overall performance under the premise of ensuring normal curing[62].
Fig.11.Graphene and co-fillers [22,65–67].
Besides,many studies have confirmed a synergistic effect when hybrid fillers are mixed and filled.The phenomenon of 1 +1>2 occurs when filling,which is particularly significant in improving TC.When fillers of different sizes and morphologies or types are filled together,the packing density will be higher than that of a single particle.Furthermore,the agglomeration phenomenon is not as evident as that of a single particle.The small-sized particles are filled in the gaps of the large-sized fillers [63].During the formation of the heat conduction path,phonons can be more effectively transmitted along with the filler network [64](Fig.11).
4.1.as hybrid filler
As a two-dimensional material with higher TC,boron nitride(BN)has excellent advantages in electrical insulation.In the case of co-filling with graphene nanosheets,it will block the electric conductive path between graphene,but it can still build a thermal network for phonon transmission[68].Choosing different size BN to fit graphene can obtain high TC composite materials with good insulation properties.
Shtein et al.[69]mixed BN and graphene with different particle sizes.The TC can be 1.84 W m-1K-1when filled with 15 vol%μm-BN and 2 vol%nm-BN.When graphene and nm-BN are mixed at 16 vol%and 1 vol%,the TC reaches 4.72 W m-1K-1and keeps insulation.The large-sized micron graphene sheet layer and the small-sized nano BN are filled together to construct a two-dimensional filler lapped thermal conduction network.Su et al.[66]modified hBN with dopamine and then co-filled it with multi-layer graphene mG,as shown Fig.12(a).In the case of 25 wt%BN and 10 wt%graphene,the external TC is 1.27 W m-1K-1.Pure hBN has fewer surface functional groups and poor interfacial adhesion,and the force between it and the resin matrix is enhanced after dopamine modification.The uniform dispersion also blocks the direct contact of graphene and hinders the formation of a conductive network.In the presence of 25 wt%hBN,the resistivity can still be maintained above 108Ω when filled with 10 wt% graphene,which meets the insulation requirements of the electrical field.In the cross-section SEM image(Fig.12(b)),As the filler content increases,the overlapping heat conduction paths can gradually be observed.Feng et al.[70] used the synergistic effect of hexagonal boron nitride (h-BN) and functionalized graphene to achieve a TC of 2.01 W m-1K-1at a total loading of 40 wt%.A hydrophilic graphene hybrid filler containing Ni(OH)2 nanobelts and rGO was prepared by a two-step hydrothermal method.It was co-filled with nano-BN in the epoxy matrix.The formation and self-assembly of Ni(OH)2 in the first hydrothermal process formed the structure of nanoribbons.The second step is assembled with graphene sheets with negative charges on the surface during hydrothermal.The ribbon-like Ni(OH)2 nanoribbons form a bridge between the graphene nanosheets and the small-sized nano-BN,which also hinders the accumulation of the same filler due to static electricity.On the other hand,due to the effects of catalytic carbonization and endothermic decomposition,it has a flame retardant effect.There is also work to pre-modify the mixed-filled boron nitride and graphene to achieve a higher synergistic effect.
4.2.One-dimensional carbon materials as hybrid filler
One-dimensional carbon materials such as chopped carbon fibers and carbon nanotubes also have particular applications in the field of cofilling [71].One-dimensional materials are more likely to be used as bridges to increase the TC of the system when they are co-filled with two-dimensional graphene sheets.
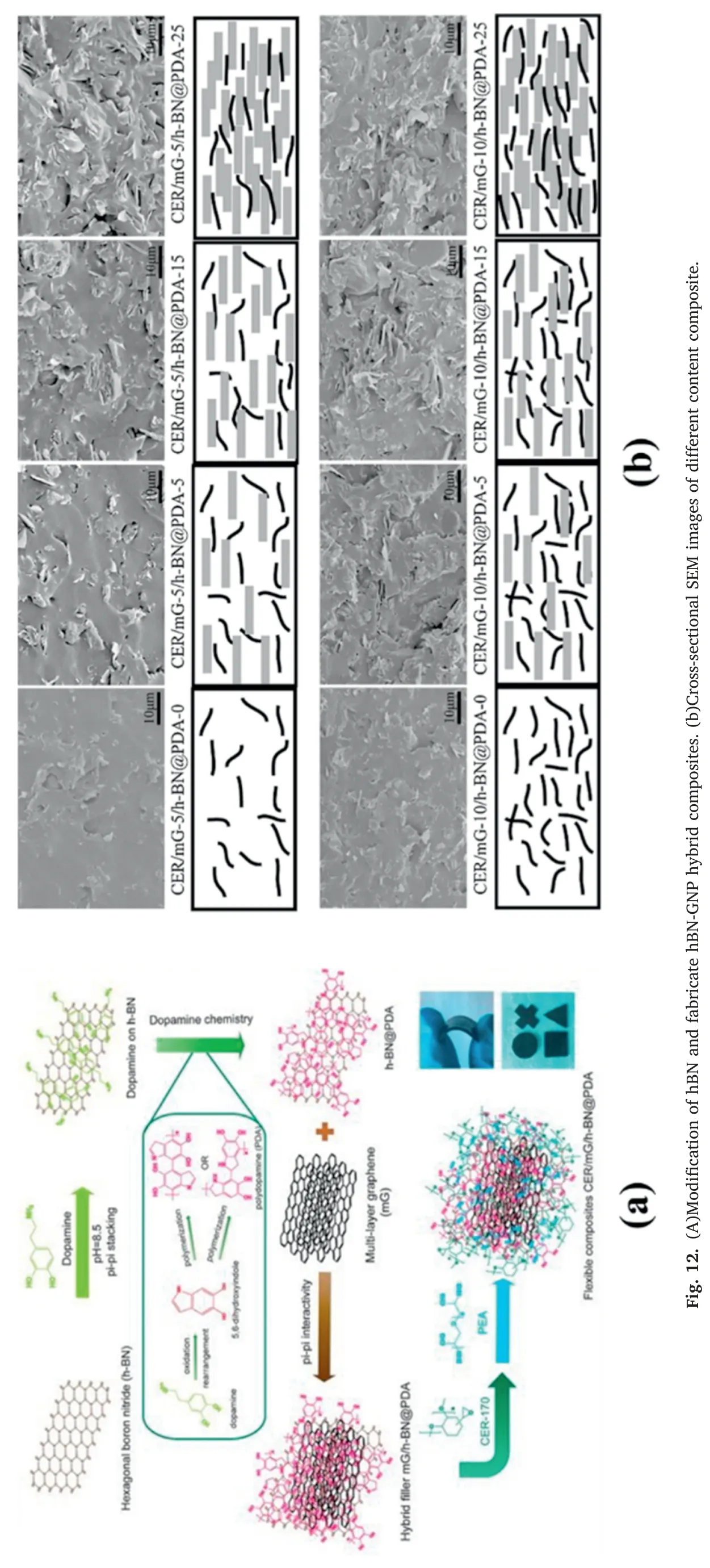

Fig.13.(A)Prepare the GO-SiC self-assembled three-dimensional structure and epoxy composites.(b)Zeta potential of GO and SiC particles.(c)SEM images of ER/GOSiC composites.A)ER/GO-SiC25,b)ER/GO-SiC50,c)ER/GO-SC100,d)ER/GO-SiC150 [58].
Chopped carbon fiber has high TC and low electrical conductivity.The diameter is about 5–8 μm.The interlayer connection is not a slidable structure similar to graphite and has a low thermal expansion coefficient.Owais et al.[72] dispersed graphene and BN nanosheets in a chopped carbon fiber SCF-based epoxy composite material.Under the co-filling of 3 wt% SCF and 5 wt% modified GNP-BN,the TC is 0.8 W m-1K-1,and the resistivity remains at 6.41×1013Ωm.The graphene is modified with 3-mercaptopropionic acid (MPA) in advance.The mercapto group is attached to the end group of the graphene,and the amino group participates in the curing process of the epoxy.With the participation of 3%SCF,the TC of the 5% 1:1 graphene-boron nitride filled composite material increased from 0.56 W m-1K-1to 0.8 W m-1K-1,showing a significant synergistic enhancement effect.Due to the rigidity of chopped carbon fibers,the overlap of carbon fibers between different layers can also be observed in the cross-sectional SEM photos.
Carbon nanotubes are coaxial round tubes formed from a single layer to several layers arranged in hexagons[73].They have high intrinsic TC,but they are prone to agglomeration and non-axial orientation when individually filled [74].Huang et al.[22] used high-content graphene and multi-walled carbon tubes (WCNTs) to fill together,and the TC reached 6.31 W m-1K-1in the case of 20 vol%CNTs and 20 vol%GNPs.The mixed filler vaporizes most of the solvent during rotary mixing and then vaporizes the residual solvent in a vacuum and solidifies under a certain pressure to prepare a composite material with a high filling content.When filling with higher content,mixing and filling have better stacking efficiency,and there will be no excessive filler self-aggregation during removing the solvent.The use of one-dimensional carbon materials and two-dimensional graphene for mixed filling has a more significant synergistic effect than two-dimensional-two-dimensional co-filled systems.However,the pretreatment and selection of one-dimensional fillers are also challenges faced by institute one.
4.3.Ceramic nanoparticles as hybrid filler
Except for these thermally conductive fillers with similar structures for mixing and filling,spherical ceramic powders are also commonly used as hybrid fillers.When two-dimensional graphene is filled at high content,the system's viscosity is too high and it's not easy to cure.Besides,if the electric insulation is required,high content graphene will cause electrical breakdown.Adding ceramic fillers with good lubricity and insulation properties can improve the TC of the composite material without reducing the formability and mechanical properties.
Guan et al.[75]filled epoxy resin with spherical alumina,magnesium hydroxide and graphene nanosheets,and the TC reached 2.2 W m-1K-1under the condition of 68 wt% Al2O3,7%GNP and 5%Mg(OH)2filling,and the epoxy composite remains electrical insulation.The surface of graphene was pretreated with silane coupling agent A187.The study found that increasing the amount of alumina did not affect the system's viscosity,indicating that the spherical particles would not cause loss of molding performance during the molding process and could contribute to a certain extent TC.Alumina is filled with a mixture of 40 μm and 5 μm,and the adaptation of different particles can increase the filling density ofthe system.It can also overlap with the two-dimensional graphene sheet.Magnesium hydroxide,as a flame retardant,can releases crystal water to absorb latent heat when heated and decomposes to form a dense and uniform thermal insulation layer.He et al.[58] used the electrostatic self-assembly of graphene oxide and SiC to form an effective heat conduction path in an epoxy resin matrix,as shown in Fig.13(a).In the case of 30 wt%mixed filler,it reaches 0.91 W m-1K-1,and the conductivity remains 2×10-10Sm-1.The SiC surface was treated with the coupling agent TPEDA to increase the positive Zeta potential(Fig.13(b))and then subjected to electrostatic self-assembly treatment with negative graphene oxide.When adjusting the content of SiC and graphene powder,we can find that when the GO content is too high,it will show uneven distribution due to agglomeration.When the GO content is low,the TC of the system has reached the insulation requirement.The results can be shown obviously in cross-section SEM images(Fig.13(c)).
4.4.Metal nanomaterials as hybrid filler
Metal filler can also be used as co-fillers.The metal nanoparticles have high intrinsic TC.But due to its high density,easy sedimentation and poor interface compatibility,There are certain constraints in the use process.Metal filler can be nanowires or nanoparticles [76],which can fill the voids between large size graphene platelets.
Barani et al.[77] mixed graphene and nano-copper to prepare epoxy-based composites.Spherical nano-copper is embedded between graphene sheets,which increases the packing density of the system.When 40 wt%graphene and 35 wt%copper are mixed and filled,the TC of composites reaches 13.5 W m-1K-1.Graphene uses phonons to conduct heat,while the spherical nano-copper conducts electricity through electrons.The two fillers work together to improve the TC of the system significantly.Because the nanoparticles are easy to oxidize,the nano-copper needs to be mixed with the resin in an argon atmosphere,and graphene powder is added step by step.Each step requires a vacuum degassing to protect the metal filler.Not only spherical nanoparticles participate in the filling,but two-dimensional nanowires can also be filled.Li et al.[65] uniformly coated polydopamine (PDA) on copper nanowires and GO flakes,with TC of 0.36 W m-1K-1when the total filler content is 7 wt%,and electrical insulation is maintained.The copper nanowires could also be observed as bridges on the cross-section.
Compared with complex chemical modification and coating layers,the adding of hybrid fillers is a more straightforward and effective reinforcement method.Adjusting the proportion and surface properties of the filler,choosing the appropriate ratio and processing technology will have more significant synergistic effects and additional benefits(Table 2).
5.Conclusion
In this review,the research progress on graphene reinforced epoxy composites is discussed from preparation,modification,coating treatment and hybrid filling.As a filler with ultra-high TC,graphene has a unique two-dimensional structure,and it is easy to form a heat conduction path in the matrix.Furthermore,there are still many other ways to improve its dispersibility and interface compatibility.Choosing the appropriate kind and size of the filler is crucial to control and design the properties of composite materials.Compared with the internal structure design of composite materials,dispersed phase filling is easier to realize industrialization and mass production.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgment
The work is supported by The National Key Research and Development Program of China(2020YFA0210704).
- Namo Materials Science的其它文章
- A comparative study of polymer nanocomposites containing multi-walled carbon nanotubes and graphene nanoplatelets
- Tensile properties of functionalized carbon nanothreads
- Strain effects on the interfacial thermal conductance of graphene/h-BN heterostructure
- Atomic insights into synergistic effect of pillared graphene by carbon nanotube on the mechanical properties of polymer nanocomposites
- Anti-corrosion and electrically conductive inorganic conversion coatings based on aligned graphene derivatives by electrodeposition
- Fracture behavior of hybrid epoxy nanocomposites based on multi-walled carbon nanotube and core-shell rubber

