Ji-Chuan decoction ameliorates slow transit constipation via regulation of intestinal glial cell apoptosis
Xiu-Min Wang, Li-Xia Lv, Yue-Si Qin, Yu-Zhu Zhang, Ni Yang, Shu Wu, Xiu-Wen Xia, Hong Yang, Hong Xu,Ying Liu, Wei-Jun Ding
Abstract
Key Words: Slow-transit constipation; Ji-Chuan decoction; Taurine and hypotaurine metabolism; AKT;Enteric glial cell; Apoptosis
lNTRODUCTlON
Chronic constipation is a common complaint and can generally be divided into defecatory disorder,mixed constipation, normal transit constipation and slow transit constipation (STC). STC is the major type of chronic constipation characterized by a substantial increase in bowel transit time. STC has become an epidemic that particularly impacts the quality of life of elderly patients[1]. Despite high morbidity worldwide, the etiology of STC is poorly understood. An accumulation of publications indicates that multiple factors have been documented in the pathogenesis of STC[2-4]. Consequently, an interdisciplinary approach is necessary for exploring its pathological characteristics and developing therapies based on multiple components and multiple targets, such as traditional Chinese medicine(TCM)[5,6]. The active components of Ma-Zi-Ren-Wan could safely and effectively relieve the severity of functional constipation. Based on network pharmacology[7], the anti-STC mechanism of Gui-ren-Run-chang granules, another TCM formula, is associated with repairing the SCF/c-kit pathway and reducing aquaporin-4 expression in the colon[8].
Ji-Chuan decoction (JCD) is a representative TCM formula that originated from Jing-Yue-Zhang in the Ming Dynasty. It has been extensively used for STC and other gastroenteric disorders for hundreds of years[9]. JCD, as a typical TCM prescription, is composed of six herbs and includes multiple bioactive ingredients and complex targets. Studies have shown that JCD can effectively shorten colonic transit time, improve anorectal dynamics, regulate gastrointestinal neurotransmitters, alleviate constipation symptoms in STC patients, and improve the quality of life of STC patients[9,10]. After 1 mo of continuous use, only a few patients experienced adverse reactions such as dizziness (1.7%) and dry mouth (1.7%)[10]. Our clinical practice confirmed that JCD was a useful formula for STC therapy.Cistanche deserticola(C. deserticola) is the monarch drug for JCD, and the combination of geniposide and Lactobacillus plantarum KSFY06 has been shown to have anti-montmorillonite-induced constipation effects in Kunming mice[11,12]. However, direct experimental study of the effects of JCD against STC remains to be performed. In this work, we attempt to assess the laxative effect of JCD against STC through a multiomics approach, including metabolomics and TCM network pharmacology, to explore the integrated therapeutic mechanism of JCD.
MATERlALS AND METHODS
Reagents and JCD preparation
Rabbit anti-AKT monoclonal antibody (No: bs-0115R) was provided by Bioss. The biotin secondary antibody anti-rabbit working solution (Goat, SP-9001) was provided by Beijing Zhongshan Jinqiao Biological Technology Co., Ltd. Glial fibrillary acidic protein (GFAP) (mouse, code: ab4648) and Caspase3 (rabbit, code: ab4051) were purchased from Abcam. CY3-labeled goat anti-mouse immunoglobulin G (IgG) (code: 202110) and fluorescein isothiocyanate-labeled goat anti-rabbit IgG (code:GB22303) were purchased from Servicebio.
The JCD solution was prepared according to the protocol described by Cuiet al[13]. A clinical packet of JCD is composed of six Chinese herbs,i.e., Angelica sinensis(15 g),C. deserticola(9 g),Achyranthes bidentata(A. bidentata) (6 g),Fructus aurantii(F. aurantii) (4.5 g),Alisma orientalis(3 g) andCimicifuga heracleifuga(3 g). Six packets of JCD were purchased from Chengdu Hospital of Integrated TCM and Western Medicine and identified by Dr. Wan Lin from Chengdu University of TCM. These herbs were then soaked in 2430 mL ddH2O (w/v 1:10) for 30 min and decocted for 20 min. Combined solutions following three decoctions were filtered by a 0.22-μm filter, concentrated to 0.81 g of crude herb per milliliter by a vapor evaporator, and stored at -20 °C for further use. In the past, scholars have studied the hyphenated to liquid chromatography characteristic fingerprints of JCD substances[14].
STC model-made, JCD treatment and sample collection
This experiment was approved by the Animal Ethics Committee, Chengdu University of TCM (license 2016-16). Thirty C57BL/6J male mice, aged 7 wk and weight 20.21 ± 2.10 g, were purchased from Chongqing Evansville Laboratory Animal Co., Ltd. (Chongqing, China). The animals were adaptively fed for 7 d in an environment with relative humidity of 45%-55%, a 12-h light/dark cycle and temperature of 22 ± 2 °C. The mice were then randomly divided into six groups, with five animals in each group: Healthy control (HC), STC model (STC), positive drug treatment (mosapride, MSP), low dose of JCD (JCDL), middle dose of JCD (JCDM) and high dose of JCD (JCDH). Mice in the HC group were orally administered normal saline (0.1 mL/10 g/d) as a negative control. The other mice were induced as the STC models by oral administration of compound diphenoxylate (10 mg/kg/d) for 14 d.After model identification, mice in the MSP group were orally administered with MSP (2.5 mg/kg);mice in the JCDL (3.04 g/kg), JCDM (6.08 g/kg) and JCDH (12.16 g/kg) groups were orally administered with JCD; and normal saline (0.1 mL/10 g/d) was gavaged in the HC and STC groups.Each mouse was administered treatments once a day for 14 d. Body weight, food intake and water intake were monitored every week. At the end of experiment, the feces of each mouse were collected under sterile procedures, frozen in liquid nitrogen and stored at -80 °C for further use.
The number of defecation particles within 6 h was counted, and the wet weight of the stool samples was evaluated. Then, the dry weight of the stool was weighed after drying at 60 °C for 12 h in a desiccator, and the moisture content of the stool was calculated. The colonic samples were harvested after euthanasia[15]. The acetylcholine (ACH) concentrations were detected by enzyme-linked immunosorbent assay.
Evaluation of the intestinal propulsive rate
The intestinal propulsive rate was measured after the last administration as follows[16]: All mice were fasted for 12 h and allowed free access to water. Then, mice were fed charcoal powder in 10% acacia gum. After 30 min, the abdomen was opened and the intestines were removed. The length from the pylorus to the ileocecal junction, as well as the charcoal transport distance were measured. The intestinal propulsive rate was calculated by the following formula[17]: Charcoal transit ratio (%) =distance of charcoal transport (cm)/length from pylorus to ileocecal junction (cm) × 100%.
Hematoxylin and eosin and immunofluorescence staining
The collected tissue samples were fixed with 10% formalin, dehydrated with alcohol, and embedded in paraffin wax. The embedded tissues were then sliced into 5-μm slices using a microtome (Leica, Buffalo Grove, United States) and stained with hematoxylin and eosin (HE). The pathological features were imaged by a digital microscope (Xiamen, China), and the optical densities were quantified using Image-Pro Plus 6.0.
Ultrahigh-pressure liquid chromatography coupled with tandem mass spectrometry analysis
Metabolomics was performed with a Vanquish UHPLC (Thermo, Germany) coupled with a Q Exactive™ HF (Thermo, Germany) platform (Novogene, Beijing, China) as previously described. Fecal samples (100 mg) were ground in liquid nitrogen, incubated on ice for 5 min, and centrifuged at 15000 ×g for 20 min at 4 °C. The supernatant was diluted with liquid chromatography-mass spectrometry grade water to a final concentration of 53% methanol. After another centrifugation step, the supernatant was injected into the liquid chromatography-tandem mass spectrometry system for analysis. Samples were injected into a Hypesil Gold column (C18) using a 17-min linear gradient at a flow rate of 0.2 mL/min.The eluents in positive polarity mode were Eluent A (0.1% FA in water) and Eluent B (methanol). The eluents for negative polarity mode were Eluent A (5 mmol/L ammonium acetate, pH 9.0) and Eluent B(methanol). The solvent gradient was set as follows: 2% B, 1.5 min; 2%-100% B, 12.0 min; 100% B, 14.0 min; 100%-2% B, 14.1 min; and 2% B, 17 min. The Q ExactiveTMHF mass spectrometer was operated in positive/negative mode with a spray voltage of 3.2 kV, a capillary temperature of 320 °C, a sheath gas flow of 40 arb, and an auxiliary gas flow of 10 arb. Then, the data were matched to the mzCloud,mzVault, and MassList databases for accurate qualitative and relative quantitative results. The threshold for differential metabolites was set as variable importance in the projection (VIP) > 1.0, fold change (FC) > 1.5 or < 0.667 andPvalue < 0.05. The metabolic functions and relevant metabolic pathways were enriched by MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/).
Network pharmacology analysis and immunohistochemical validation
TCMSP (https://tcmsp-e.com/) was applied to find the chemical components and corresponding targets of JCD, with OB ≥ 30% and DL ≥ 0.18. The UniProt database (https://www.UniProt.org/) was used to correct potential targets. The chemical composition and corresponding targets of JCD were checked by BAT-MAN (http://bionet.ncpsb.org.cn/batman-tcm/index.php), with a score cutoff over 500. Then, the targets obtained from TCMSP and BAT-MAN were combined and deduplicated. Taking“slow transfer constipation” as the keyword, the target points with a score greater than 5.0 were collected from the GeneCard database (https://www.genecards.org/), and all the targets collected from OMIM (https://omim.org/) were included in the follow-up study. The common genes of drug targets and disease genes were put into the STRING11.0 (https://string-db.org/) database to construct a protein-protein interaction (PPI) network. The relevant data were imported into Cytoscape (3.7.1)software in tsv format, and the cytoHubba plug-in was used to display the top 30 nodes in the betweenness algorithm as Hub nodes and to display them in Excel. The distribution pattern and expression levels of AKT, which is one of the most important hub nodes in colonic tissues, were analyzed by immunohistochemistry. Rabbit anti-AKT antibody (Bioss; 1:100) was applied overnight at 4°C, followed by horseradish peroxidase-conjugated goat anti-rabbit IgG incubation at room temperature for 30 min, and diaminobenzidine was used for staining. The graphic processing software Image-Pro plus 6.0 was used to quantify the expression of AKT. Five fields of each slide were randomly observed,and the optical density values were determined. The components acting on AKT are considered core components of JCD.
Integrated analysis of differentially expressed metabolites and target genes
The HGNC database (https://www.genenames.org/) was applied to transform the obtained JCD target genes into mouse genes. The Joint-Pathway analysis of MetaboAnalyst 5.0 was used to enrich differentially expressed metabolites (DEMs) and target genes associated with JCD. Except for those nominated by other diseases, the top 25 pathways were selected for integrated analysis. The degree of apoptosis of enteric glial cells (EGCs) in colon tissue was analyzed by immunofluorescence. Rabbit anti-caspase3 antibody (Abcam; 1:100) was applied overnight at 4 °C, and mouse anti-GFAP antibody (Abcam; 1:100)was stained at 37 °C for 30 min followed by 4’,6-diamidino-2-phenylindole for 10 min at room temperature. ImageJ graphics processing software was used to calculate the apoptosis of EGCs. Five fields of each slide were randomly observed, and densitometric values were estimated.
Statistical analysis
Data processing and statistical analyses were performed by Graph Pad Prism 7 (GraphPad, La Jolla, CA,United States). Data are expressed as the mean ± SD. One-way analysis of variance was used to compare the data of six groups. Student’sttest was used for the pairwise comparison of data. The results were considered significant whenP< 0.05.
RESULTS
JCD promoted intestinal motility, increased excitatory neurotransmitters and reduced intestinal inflammation in STC mice
General restoration of STC symptoms was observed after JCD treatment. The food intake, water intake and body weight in the JCDH and JCDM groups were similar to those in the HC group (Supplementary Table 1). Compared with HC mice, the average number of stool particles collected from STC mice was markedly reduced. After JCD administration, the average number of stool particles collected from the JCDL, JCDM and JCDH groups was significantly increased and was similar to the level of the positive control drug MSP (Figure 1A). Compared with the HC group, the dry weight, wet weight, and water content of the feces in the STC group were significantly decreased, and the dry weight, wet weight, and water content of the feces in the MSP, JCDM, and JCDH groups were significantly increased(Table 1). Compared with the HC group, the intestinal propulsion rate was significantly decreased in the STC mice, whereas the intestinal propulsion rate was significantly increased in the JCDM, JCDH,and MSP groups (Figure 1B). Finally, the concentrations of excitatory neurotransmitters (colon ACH) in STC mice were significantly reduced, while those in the three JCD groups were significantly increased(Figure 1C).
In addition, pathological observations demonstrated that JCD significantly reduced constipationassociated intestinal inflammation. Pathological observation demonstrated typical pathology of intestinal inflammation in STC mice, with inflammatory infiltration and necrosis in the distal colon. JCD repaired the colonic injuries in a dose-dependent manner (Figure 2). Briefly, the results showed that JCD exerts its effects by enhancing intestinal motility, promoting excitatory neurotransmitters and inhibiting intestinal inflammation in STC mice. Therefore, it is considered that the modeling was successful.
JCD beneficially regulated taurine and hypotaurine metabolism
Forty-two DEMs (Supplementary Table 2) (negative ion mode) between the HC and STC groups and 86 DEMs (Supplementary Table 3) between the STC and JCDH groups were identified (the sample chromatogram in negative ion mode is shown in Supplementary Figure 1). Among the DEMs, eighteen were altered by the modeling process but recovered after JCDH treatment (Supplementary Table 4). As shown in Figure 3A, the closely focused cluster of quality control (QC) samples indicated the reproducibility of the experiments. Figures 3B and C display the fecal DEMs observed after modeling and JCDH treatment. Compared with the STC group, the R2 (evaluation of the modeling ability) and Q2(description of the predictive ability) of the HC and JCDH groups indicated that the orthogonal partial least squares-discrimination analysis model had high predictability and reliability. Compared with the STC group, the R2 intercepts of the HC and JCDH groups were 0.94 and 0.92, respectively, and the Q2 intercepts were 0.72 and 0.88, respectively (Figures 3D and E). Figures 3F and G show the DEMs by volcano maps. Furthermore, taurine and hypotaurine metabolism were the main pathways impacted by JCDH treatment (Figure 3H).
The core target of JCD is AKT, and the core component is quercetin
To further determine the therapeutic mechanism of JCD in STC, we performed a network pharmacology analysis. After data screening, 45 active pharmaceutical ingredients (Supplementary Table 2), 280 related targets, and 1372 disease targets were obtained. Comparing the goals related to JCD and STC, 89 common goals were identified and registered in the STRING database. Cytoscape 3.7.2 software was used to construct a PPI network, including 132 nodes and 2492 interactive edges (Figure 4A). According to the betweenness centrality value, the top 30 hub nodes were identified (Figures 4B and C), and AKT was considered one of the important targets. Immunohistochemistry confirmed that colonic AKT expression was significantly suppressed in STC mice compared with the HC group, whereas JCD significantly reversed this effect (Figures 4D and E). The chemical that acts on AKT is considered to be the core component of JCD,i.e.,the quercetin fromA. bidentataandC. deserticola,baicalein, kaempferol and wogonin fromA. bidentata, and naringenin fromF. aurantii(Table 2).
JCD inhibited EGC apoptosis
To comprehensively probe the pharmacological mechanism of JCD, a joint-pathway analysis was performed based on DEMs and common genes from network pharmacology. Twenty-five remarkable pathways are presented in Figure 5A. Apoptosis may be an important signaling pathway in the treatment of STC by JCD. GFAP is an EGC marker. Immunofluorescence double-labeling of GFAP and caspase3 showed that compared with the HC group, the apoptotic rate of EGCs in the STC group was significantly increased, while those in the JCDH and JCDM groups were significantly decreased(Figures 5B and C), confirming the predicted results.

Table 1 Dry weight, wet weight and moisture content of feces of mice in each group

Table 2 The core ingredients of Ji-Chuan decoction

Figure 1 Results of the constipation-related index. A: Particle counts of stool; B: Intestinal propulsive rate; C: The expression levels of colonic acetylcholine.ACH: Acetylcholine; HC: Healthy control; STC: Slow transit constipation; JCD: Ji-Chuan decoction; JCDL: Low-dose Ji-Chuan decoction treatment; JCDM: Middledose Ji-Chuan decoction treatment; JCDH: High-dose Ji-Chuan decoction treatment; MSP: Mosapride treatment. Compared with the healthy control group: aP < 0.05,bP < 0.01. Compared with the slow transit constipation group: cP < 0.05, dP < 0.01.
DlSCUSSlON
JCD is an established TCM formula that is a particularly effective therapy for STC differentiated by TCM as Spleen and Kidney Yang deficiency syndrome[9], yet its pharmaceutical mechanism remains unclear. We explored the therapeutic mechanism of JCD in a dose-dependent manner and observed that the best effect was achieved by JCDH. The compound diphenoxylate is widely used to induce STC in animal models and can inhibit the peristaltic reflex of intestinal mucosa. It is easier to induce constipation than the commonly used loperamide and is more available than morphine[17]. It is easier to induce constipation than other loperamides and easier to obtain than morphine[17]. The holistic view is shared between systems biology and TCM[18]; therefore, we used key disciplines of systems biology,such as metabolomics and network pharmacology, to effectively reveal the integrated mechanism of JCD for STC.
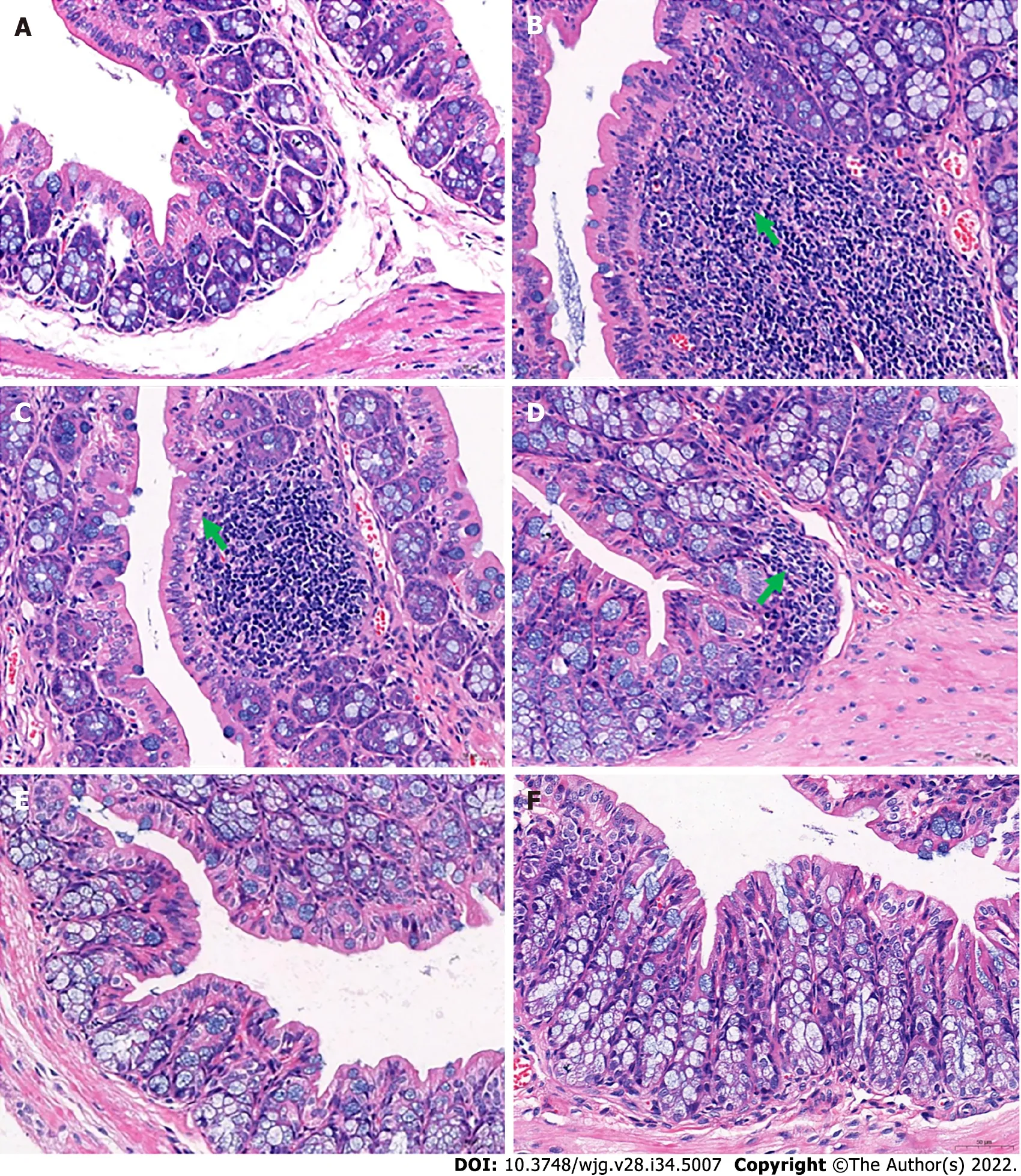
Figure 2 Histological changes in the distal colon. A: Distal colon of the healthy control group; B: In the distal colons of mice in the slow transit constipation group, more severe inflammatory infiltration was observed; C: In the distal colons of mice in the low-dose Ji-Chuan decoction (JCD) treatment group, more severe inflammatory infiltration was observed; D: In the distal colon of mice in the middle-dose JCD group, there was reduced inflammation; E: High-dose JCD treatment restored the normal tissue morphology of the distal colon of constipated mice; F: Mosapride treatment restored the normal tissue morphology of the distal colon of constipated mice. Sections were observed at 400 × using a light microscope.
JCD substantially improved the manifestation of STC in mice
Constipation refers to laborious defecation, dry and hard stools and low stool volume. Effective drugs will increase key indices of constipation, such as the quantity and water content of the feces and the intestinal propulsive rate[19,20]. In this study, we observed that JCD substantially improved almost all indices tested in this work (Figures 1A and B, Table 1). The intestinal propulsive rates, extensively used to evaluate the pathological degree and curative effect of constipation, were significantly decreased after diphenoxylate treatment. Similar anti-constipation effects were obtained among the JCDM, JCDH and the positive reagent MSP groups (Figure 1B). In addition, the three JCD doses could statistically increase the concentration of excitatory neurotransmitter (ACH) (Figure 1C), suggesting that the increase in excitatory neurotransmitter is a critical approach for anti-constipation by JCD[3]. Our colonic pathological observation showed that JCD significantly reduced inflammatory infiltration and repaired pathological damage caused by the compound diphenoxylate in a dose-dependent manner (Figure 2),confirming previous studies[21]. Therefore, our work indicates an integrated effect of JCD on STC by promoting excitatory neurotransmitters, inhibiting gastroenteric inflammation and accelerating intestinal motility.
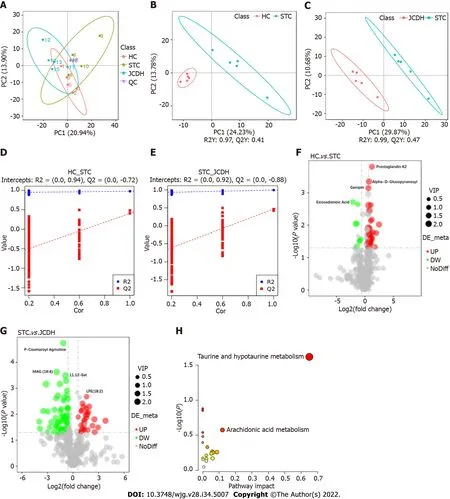
Figure 3 Different intestinal metabolites detected by ultrahigh-pressure liquid chromatography coupled with tandem mass spectrometry.
JCD altered critical enteric metabolites, especially taurine and hypotaurine
The metabolome results showed that the pathways involving taurine and hypotaurine metabolism were important for the anti-constipation effect of JCD. Taurine is a sulfur-containing α-amino acid with antiinflammatory properties that can be sequentially converted into homocysteine, cystathionine, cysteine and/or hypotaurine[22,23]. The concentration of taurine was significantly reduced in loperamideinduced constipation rats, and red liriope ameliorated constipation and increased the level of taurine[24,25]. Our work also showed that the contents of taurine and its metabolite hypotaurine were negatively associated with the manifestation of STC and positively associated with intestinal transit rates[26].However, the molecular mechanism of taurine on gastrointestinal motility and the regulatory mechanisms of JCD remain to be further explored.

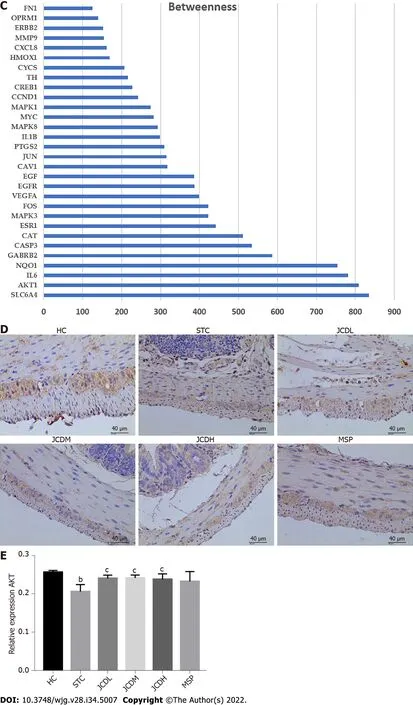
Figure 4 Network construction, screening and validation of the core targets for Ji-Chuan decoction treatment of slow transit constipation. A: Protein-protein interaction (PPI) network; B: Top 30 protein targets with the largest betweenness in the PPI network; C: Betweenness analysis of the top 30 protein targets; D and E: The relative expression of AKT as assessed by immunofluorescent staining. AKT: Serine/threonine-protein kinase; HC: Healthy control;STC: Slow transit constipation; JCD: Ji-Chuan decoction; JCDL: Low-dose Ji-Chuan decoction treatment; JCDM: Middle-dose Ji-Chuan decoction treatment; JCDH:High-dose Ji-Chuan decoction treatment; MSP: Mosapride treatment; SLC6A4: Sodium-dependent serotonin transporter; AKT1: RAC-alpha serine/threonine-protein kinase; IL: Interleukin; NQO1: NAD(P)H dehydrogenase (quinone)1; GABRB2: Gammaaminobutyric acid receptor subunit beta-2; CASP3: Caspase-3; CAT:Catalase; ESR1: Estrogen receptor; MAPK3: Mitogen-activated protein kinase 3; FOS: Proto-oncogene c-Fos; VEGFA: Vascular endothelial growth factor A; EGFR:Epidermal growth factor receptor; EGF: Pro-epidermal growth factor; CAV1: Caveolin-1; JUN: Transcription factor AP-1; PTGS2: Prostaglandin G/H synthase 2;MAPK8: Mitogen-activated protein kinase 8; MYC: Myc proto-oncogene protein; MAPK1: Mitogen-activated protein kinase 1; CCND1: G1/S-specific cyclin-D1;CREB1: Cyclic AMP-responsive element-binding protein 1; TH: Thyroid hormone; CYCS: Cytochrome c; HMOX1: Heme oxygenase 1; MMP9: Matrix metalloproteinase-9; ERBB2: Receptor tyrosine-protein kinase erbB-2; FN1: Fibronectin. Data are expressed as the mean ± SD. Compared with the healthy control group: bP < 0.01. Compared with the slow transit constipation group: cP < 0.05, dP < 0.01.
JCD restored the levels of AKT, a key protein involved in apoptosis regulation
PPI network prediction showed that AKT is the core target of JCD for STC therapy. In our experiments,STC mice showed downregulated expression of AKT, while all three doses of JCD treatment reversed the abnormally reduced expression levels. AKT plays an important role in cell survival and apoptosis.Previous studies have shown that high glucose levels can induce EGC apoptosis through the AKT pathway, which is closely related to intestinal motility[27]. Another study showed that EGCs could protect the nervous system from hyperglycemia-induced damage by activating the Akt/GSK-3β pathway[28]. Therefore, we speculate that the treatment of STC with JCD may be related to EGC apoptosis.
JCD rescued excessive EGC apoptosis
Experimental evidence in humans and animals suggests that EGCs play a key role in regulating gastrointestinal motility and transit[29-32]. A cohort study of twenty-six STC patients showed reduced EGCs compared with ten healthy volunteers[33]. EGC can increase the expression of Akt and ZO-1 by releasing glial cell-derived neurotrophic factor, indirectly regulating the integrity of the intestinal epithelial barrier, reducing intestinal inflammation and improving delayed colonic transit[34,35].Another study showed that enteric glial LPAR1 signaling regulates gastrointestinal motility through EGCs and may contribute to chronic intestinal pseudo-obstruction in humans[36]. Activation of opioid receptors in EGCs may be associated with morphine-induced constipation[37]. Previous studies have shown that the key components of JCD are associated with neural apoptosis, and many of them also involve changes in AKT protein. For example, wogonin, a key component of JCD identified in this paper, can prevent hippocampal injury after brain trauma through antioxidation and anti-apoptosis,which has been shown to occur through the PI3K/Akt/nuclear factor E2-related factor 2 (Nrf2)/HO-1 pathway[38]. Baicalein reduces sevoflurane-induced neurodegeneration, improves learning and memory retention in rats, and modulates the PI3/Akt/GSK-3β and JNK/ERK signaling pathways[39].Kaempferol prevents cerebral ischemia-reperfusion injury by interfering with oxidative and inflammatory stress-induced apoptosis[40]. Quercetin is involved in neuroprotection by regulating Nrf2,paraoxonase 2, JNK, tumour necrosis factor alpha, PGC-1α, MAPKs, CREB and PI3K/Akt[41].Naringenin can effectively inhibit Aβ25-35-induced neuronal injury in PC12 cells by regulating the ER and PI3K/Akt pathways[42]. To our knowledge, the current work is the first to demonstrate that JCD can improve constipation by reducing EGC apoptosis. Our work applied a multiomics strategy to explore the therapeutic mechanism of JCD in the treatment of STC and found some interesting evidence that remains to be elucidated in more detail in the future. The JCDH group of mice exhibited a better effect, suggesting that a suitable dose needs to be further evaluated.
CONCLUSlON
This work demonstrated that reduced enteric EGC apoptosis may be the critical mechanism of JCD in STC therapy. These findings call for further molecular research to facilitate the clinical application of JCD.
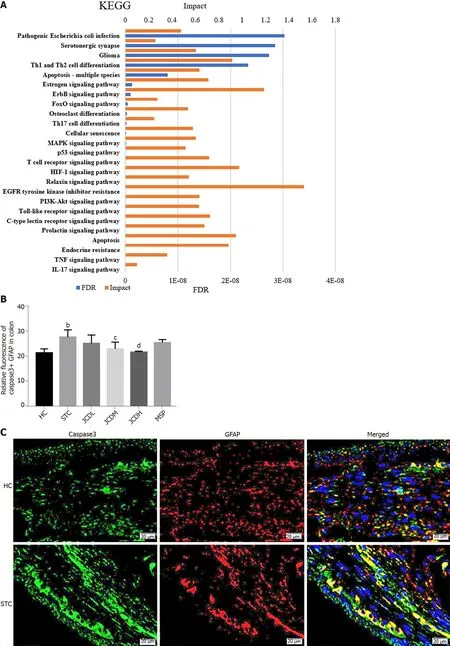

Figure 5 lntegrative analysis and experimental validation of improved network pharmacology and metabolites. A: Integration analysis between the modified network pharmacology and metabolites. The ordinate stands for pathways, the primary abscissa stands for minus false discovery rates, and the secondary abscissa stands for impact; B and C: The relative expression of Caspase3+ Glial fibrillary acidic protein was assessed by immunofluorescent staining.GFAP: Glial fibrillary acidic protein; HC: Healthy control; STC: Slow transit constipation; JCD: Ji-Chuan decoction; JCDL: Low-dose Ji-Chuan decoction treatment;JCDM: Middle-dose Ji-Chuan decoction treatment; JCDH: High-dose Ji-Chuan decoction treatment; MSP: Mosapride treatment; FDR: False discovery rates; KEGG:Kyoto Encyclopedia of Genes and Genomes; Th: Thyroid hormone; EGFR: Epidermal growth factor receptor; MAPK: Mitogen-activated protein kinase; IL: Interleukin;TNF: Tumor necrosis factor; HIF: Hypoxia-inducible factor. Data are expressed as the mean ± SD. Compared with the healthy control group: aP < 0.05, bP < 0.01.Compared with the slow transit constipation group: cP < 0.05, dP < 0.01.
ARTlCLE HlGHLlGHTS
Research background
Slow transit constipation (STC) is a common intestinal disorder without an effective therapeutic regimen. Ji-Chuan Decoction (JCD) is an established formula for STC. However, its pharmacological mechanism is still unclear.
Research motivation
To determine the ingredients and mechanism of JCD for STC treatment.
Research objectives
To explore the integrated regulatory pattern of JCD against STC through hyphenated techniques from metabolism, network pharmacology and molecular methods.
Research methods
STC model mice were generated by gavage of diphenoxylate for 14 d. STC mice in the low- (3.04 g/kg),medium- (6.08 g/kg) and high-dosage (12.16 g/kg) JCD groups were orally administered. The acetylcholine (ACH) level was detected by enzyme-linked immunosorbent assay. AKT expression and enteric glial cell (EGC) apoptosis were demonstrated by immunofluorescence. The differentially expressed metabolites were tested by nontargeted metabolomics. The targets and core ingredients were identified by network pharmacology.
Research results
JCD significantly promotes intestinal motility, increases colonic ACH content and reduces inflammation in STC mice. It markedly restores the misaligned metabolites, including taurine/hypotaurine, and rescues AKT expression with quercetin. Inhibition of EGC apoptosis is a potential mechanism by which JCD relieves constipation.
Research conclusions
Regulating gut metabolites and reducing EGC apoptosis in STC mice may be the key mechanism of JCD for STC treatment.
Research perspectives
Further investigation into the molecular interactions among the JCD ingredients and metabolites,intestinal microbiota and host response in STC mice is necessary.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Yao Zheng-Hong (Novogene, Beijing, Master of Agriculture),for skillful technical assistance.
FOOTNOTES
Author contributions:Wang XM, Xu H, and Ding WJ designed and coordinated the study; Wang XM, Lv LX, Qin YS,Zhang YZ, Yang N, Wu S, Xia XW, and Yang H performed the experiments and acquired and analyzed the data; Liu Y interpreted the data; Ding WJ contributed to critical revision of the manuscript; and all authors read and approved the final manuscript.
Supported bythe National Natural Science Foundation of China, No. 82074151; and the Experimental Formulary Sichuan Youth Science and Technology Innovation Research Team, No. 2020JDTD0022.
lnstitutional animal care and use committee statement:This experiment was approved by the Animal Ethics Committee, Chengdu University of TCM (license 2016-16).
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
ARRlVE guidelines statement:The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Xiu-Min Wang 0000-0002-3088-8579; Li-Xia Lv 0000-0001-5216-1605; Yue-Si Qin 0000-0002-5327-9554;Yu-Zhu Zhang 0000-0001-6779-0711; Ni Yang 0000-0002-5613-6041; Shu Wu 0000-0002-8729-8801; Xiu-Wen Xia 0000-0003-1962-1221; Hong Yang 0000-0002-5145-4243; Hong Xu 0000-0002-3905-7835; Ying Liu 0000-0002-3318-3486; Wei-Jun Ding 0000-0002-4933-7347.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Wang JJ
 World Journal of Gastroenterology2022年34期
World Journal of Gastroenterology2022年34期
- World Journal of Gastroenterology的其它文章
- Therapeutic strategies for post-transplant recurrence of hepatocellular carcinoma
- Pregnancy and fetal outcomes of chronic hepatitis C mothers with viremia in China
- Spontaneous expulsion of a duodenal lipoma after endoscopic biopsy: A case report
- Trends in hospitalization for alcoholic hepatitis from 2011 to 2017: A USA nationwide study
- Analysis of invasiveness and tumor-associated macrophages infiltration in solid pseudopapillary tumors of pancreas
- lmpact of adalimumab on disease burden in moderate-to-severe ulcerative colitis patients: The one-year, real-world UCanADA study
