Analysis of invasiveness and tumor-associated macrophages infiltration in solid pseudopapillary tumors of pancreas
Jie Yang, Chun-Lu Tan, Dan Long, Yan Liang, Li Zhou, Xu-Bao Liu, Yong-Hua Chen
Abstract
Key Words: Pancreatic neoplasms; Solid pseudopapillary tumors; Malignancy; Tumor-associated macrophages; Histological labeling; Immunohistochemistry
lNTRODUCTlON
Solid pseudopapillary tumor (SPT) is a rare pancreatic tumor, first reported by Frantz in 1959[1].Considering its malignant behaviors, including the invasion of peripheral organs and metastasis at diagnosis or recurrence after surgical resection, the World Health Organization (WHO) has classified SPT as a low-grade malignant tumor[2,3]. The overall prognosis of SPT patients is good, with 5-year and 10-year survival rates up to 89.5% and 86.3%, respectively[4]. Reportedly, among all SPT patients, only 9.2% are initially diagnosed as malignant with invasion or metastasis[3]. Thus, one of the challenges in managing SPT patients lies in predicting malignant behavior.
Currently, clinical features are being studied to predict the malignant behaviors of SPT. For instance,patients over 40 years of age have been found to be more likely to develop malignant SPT[5,6]. The computed tomography (CT) imaging features of multiple and geographic uptake type and progressive enhancement during the delayed phase have shown potential predictive power[6,7]. One of the most frequently mentioned predictive factors is capsule or incomplete capsule of SPT, with the latter being significantly correlated with malignancy[1,5,8-10]. However, the capsule or incomplete capsule state is primarily assessed on CT imaging. Assessment of the integrity of a capsule and growth pattern of tumor cells, especially on SPT margins under the microscope, might reflect the real biological behavior of SPT.
Macrophages that infiltrate or surround the tumor microenvironment are termed as tumor-associated macrophages (TAMs)[11]. TAMs, especially those of the M2-like macrophage subtype, could enhance tumor invasion and metastasis[12]. However, only limited studies are available regarding the role of TAMs in the SPT microenvironment and their relationship with malignant behavior of SPT. Herein, we classified SPT patients in either a capsule group or invasion group based on the integrity of capsule and growth pattern of tumor cells determined microscopically, and compared the clinical features and malignant behaviors between the two groups. Furthermore, we used immunohistochemical (IHC)staining to analyze the infiltration of TAMs, especially the M2-like macrophages, in primary SPT and liver metastasis sites.
MATERlALS AND METHODS
Patients and tissues
Twenty-five formalin-fixed paraffin-embedded tissue samples (including 22 tumors and peritumor tissues, and 3 metastatic sites) from 22 patients pathologically diagnosed with an SPT according to WHO 2010 criteria between 2009 and 2019 at West China Hospital were included in this retrospective study. All the samples were obtained by surgery and reviewed by two expert pathologists blinded to the original data. This study was approved by the biomedical ethics committee from our hospital with written informed consent from all subjects (2014 Trial No. 37).
Data collection and definition
Based on the integrity of the capsule and growth pattern of tumor cells, especially on tumor margins,evident in hematoxylin-eosin (HE)-stained sections under a microscope, SPT patients were divided into two groups: the capsule or invasion group. The invasion feature was defined as a tumor with an incomplete fibrous capsule with tumor cells invasively present in normal pancreatic tissue, as determined microscopically. On the other hand, a capsule feature is considered when the tumor has an intact fibrous envelope (Figure 1).
The malignant behaviors of SPT at diagnosis, including deep infiltration into the surrounding tissue and lymph node or distant organ metastasis, were recorded. The Ki-67 index was measured based on a manual visual counting method[13], and 3% was chosen as the cut-off value, according to findings from a recent study[14].
Clinical data was collected, including sex, age, serum tumor marker CA19-9, CA125, carcinoembryonic antigen, and neuron-specific enolase (NSE). Tumor size (largest diameter of the tumor),tumor location (head and neck or body and tail), component of the tumor (solid and cystic or solid),capsule or incomplete capsule, and calcification were also measured based on the CT imaging data and surgical specimens.
IHC analysis
IHC staining was used to evaluate the intratumoral and peritumoral presence of TAMs (CD68-positive)and M2-like macrophages (CD163-positive), a subtype of TAM, in primary and metastasis site tissues.Five micrometer-thick sequential tissue sections were obtained from paraffin-embedded tissue samples and used for the IHC analysis as described in previous studies[15,16]. The primary antibodies used in IHC staining were antibodies against CD68 (rabbit anti-human, D4B9C, dilution 1:400; Cell Signaling Technology, Danvers, MA, United States) and CD163 (rabbit anti-human, D6U1J, dilution 1:500; Cell Signaling Technology). Anti-rabbit IgG, horseradish peroxidase-linked antibody was used as the secondary antibody. For the assessment of expression of CD68- and CD163-positive cells, the tissue sections were screened using each immunohistochemistry slide at the low power fields (× 4), and the hot spots were selected. Immune cell staining was scored by counting the number of stained immune cells in three high power fields (× 400) in the hot spots. IHC sections were independently assessed by three authors (Yang J, Chen YH, and Zhou L).
Statistical methods
All statistical analyses were carried out using Statistical Package for Social Sciences (SPSS) Version 24(IBM Corp., Armonk, NY, United States). Categorical variables are expressed as percentages (%), and continuous variables are expressed as medians. Normality and homogeneity of the data were assessed by Shapiro-Wilk’s and Levene’s tests, respectively. Fisher’s exact test and Mann-Whitney test were used to detect differences in categorical and continuous variables between groups of patients. APvalue< 0.05 was considered statistically significant.
RESULTS
Patient characteristics
Among the 22 SPT patients, 11 were identified for each group based on the capsule or invasion histopathologic feature, respectively. The clinical and pathological characteristics between the capsule and invasion groups are shown in Table 1. The patients included 4 males and 18 females, ranging in age from 12 years to 65 years (median age of 35 years) and showed no difference between the two groups (P= 0.586 andP= 0.146, respectively). Only 3 patients showed CA19-9 or CA125 elevation in the invasion group. Interestingly, 2 patients in the capsule group and 5 in the invasion group showed positivity for NSE, although the difference did not reach statistical significance (P= 0.361). All 22 patients received surgical treatment, which included 4 pancreaticoduodenectomies, 10 distal pancreatectomies, 7 centralpancreatectomies, and 1 total pancreatectomy. All 3 patients with liver metastasis received combined segmental hepatectomy.

Table 1 Clinical and pathological characteristics between capsule and invasion solid pseudopapillary tumor patients
No difference was evident in tumor size, location, component, nor calcification between the two groups. However, compared with the capsule group, malignant behavior of SPTs was more frequent in the invasion group, including in 2 patients with peripheral organ invasion, 3 with liver metastasis, and 1 with lymph node and spleen metastases (P= 0.045). Furthermore, Ki-67 index more than 3% was also more frequent in the invasion group (P= 0.045).
TAM infiltration in primary tumor site
Since the invasion group had increased malignant behavior compared with the capsule group, we compared the infiltration of TAMs in the SPT microenvironment between the two groups. All primary tumor samples of the 22 patients showed positivity for macrophage and M2-like macrophage infiltration in IHC analysis. Although the intratumoral macrophage infiltration was increased in both groups compared with normal pancreatic tissue, the invasion group had a significant increase of intratumoral macrophage infiltration compared with the capsule group (allP< 0.0001; Figure 2A-C). A similar trend was observed for infiltrated peritumor macrophages. The TAM infiltration in peritumoral tissue,especially in the tumor margin, was markedly increased in the invasion group compared with capsule group (P< 0.0001; Figure 2D-F).

Figure 1 Computed tomography imaging, specimen and histopathologic features of capsulized and invasive solid pseudopapillary tumors. A, C and E: For the capsulized solid pseudopapillary tumors (SPTs), computed tomography (CT) imaging (A) and specimen (C) showed a clear distinction from surrounding pancreatic tissue, and an intact fibrous envelope on the tumor margin on hematoxylin and eosin (HE)-stained section (E); B, D and F: Invasive SPT was indistinguishable from surrounding normal pancreatic tissue on CT imaging (B) and in the specimen (D), and tumor cells showed infiltrative growth into normal pancreatic tissue on HE-stained section (F). N: Normal pancreas tissue; T: Tumor. *Tumor margin.
As a subtype of TAMs, the expression of CD163-positive M2-like macrophages was relatively low compared with CD68-positive TAMs. Elevation in both intratumor and peritumor sites in both the invasion group and capsule group compared with the normal pancreas tissue was evident by IHC (allP< 0.0001; Figure 3). Similarly, the expression of CD163-positive M2-like macrophages was also markedly increased in the intratumor and peritumor sites in the invasion group in comparison to the capsule group (allP< 0.0001; Figure 3).
TAM infiltration in liver metastasis site
Tumors in the liver metastasis sites also showed invasion features. Tumor cells were observed as invasively grown into normal hepatic cells, and no complete fibrous capsule was found on HE-stained sections (Figure 4A and B). Liver metastasis of 3 SPT patients showed positivity for macrophage and M2-like macrophage infiltration. Like the primary tumor site of the invasion group, both intratumor and peritumor areas of the metastasis site had relatively high-level CD68-positive TAM infiltration.However, there was no difference between the intratumor, peritumor and normal hepatic tissues. The CD163-positive M2-like macrophages had relatively high expression in both the intratumor and peritumor metastasis sites. No statistically significant difference was found upon comparison of the CD163-positive M2-like macrophage infiltration of intratumor, peritumor and normal hepatic tissues.

Figure 2 lnvasion group showed increased tumor-associated CD68-positive macrophage infiltration compared with capsule group by immunohistochemical analysis. A-F: Immunohistochemical-stained imaging and analysis of tumor (A-C) and peritumor (D-F) infiltrated with CD68-positive macrophages in representative patients from the capsule (A, D) and invasion (B, E) groups. N: Normal pancreas tissue. bP< 0.01; dP < 0.0001.

Figure 3 lnvasion group showed increased tumor-associated CD163-positive M2-like macrophage infiltration compared with capsule group by immunohistochemical analysis. A-F: Immunohistochemical-stained images and analysis of tumor (A-C) and peritumor (D-F) tissues infiltrated with CD163-positive M2-like macrophages in representative patients from the capsule (A, D) and invasion (B, E) groups. N: Normal pancreas tissue. dP < 0.0001.
DlSCUSSlON
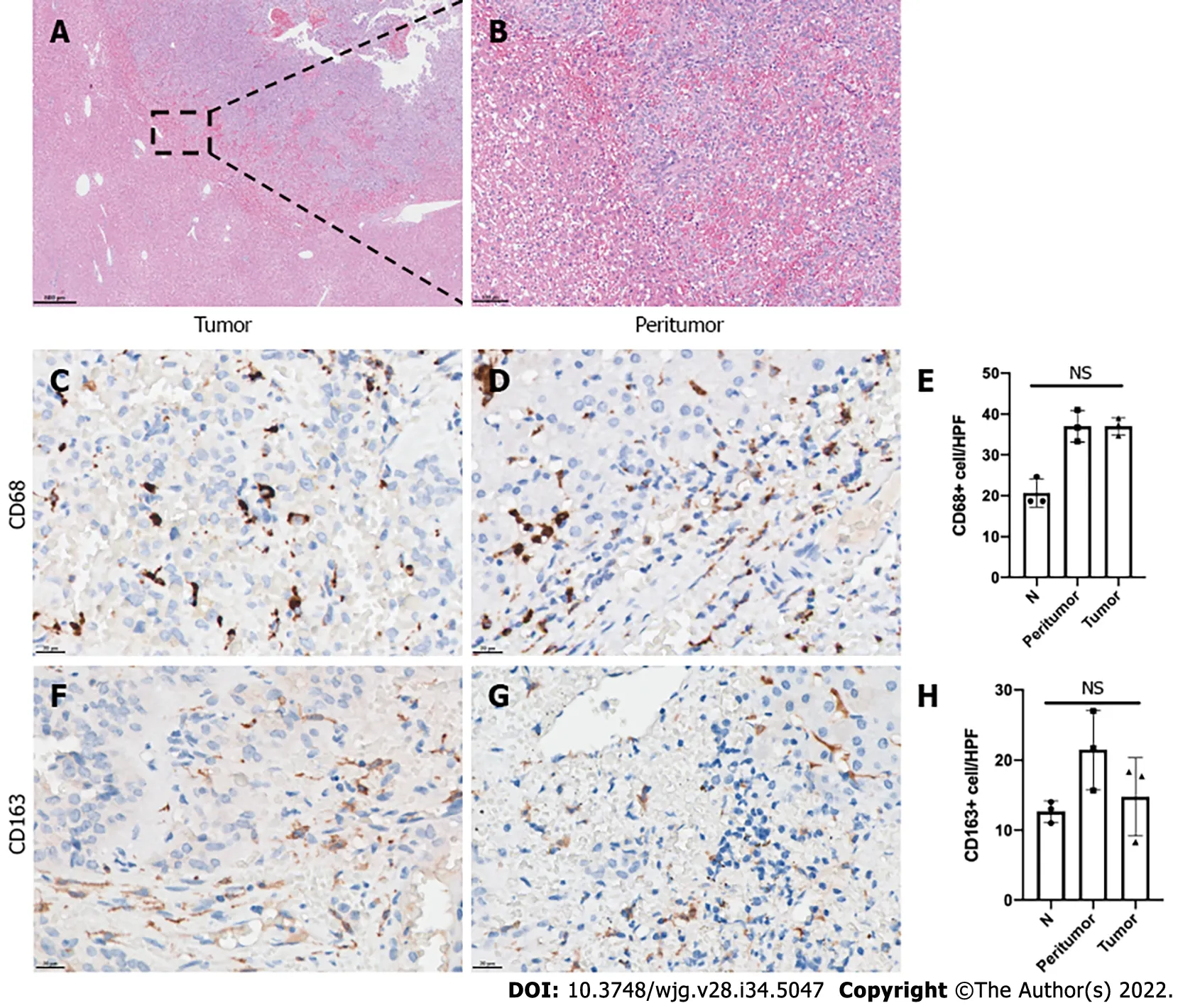
Figure 4 Tumor-associated macrophage infiltration in liver metastasis sites of solid pseudopapillary tumor patients. A and B: Hematoxylineosin staining of the liver metastasis site of representative solid pseudopapillary tumor (SPT) patients; C-H: Immunohistochemical-stained imaging and analysis of intratumor and peritumor CD68 (C-E) and CD163 (F-H) expression in the liver metastasis site of representative SPT patients. N: Normal hepatic tissue; NS: No significance.
In the present study, we found that the invasion feature evident under a microscope was associated with malignant behavior of SPT. Additionally, we elaborated the spatial distribution of TAMs in the SPT tumor microenvironment and showed distinct expression of TAMs and M2-like macrophages between tissues with invasion and capsule features. Furthermore, we also described the intratumor and peritumor distribution of TAMs and M2-like macrophages in the liver metastases site. Considering the malignant behavior of SPTs, this study may provide a histopathological basis for identification of malignant SPTs. The phenomenon of increased infiltration of TAMs, especially M2-like macrophages, in the invasion feature might help in subsequent investigations of the underlying mechanism of TAMmediated SPT malignant behavior, as well as to potentially treat recurrent and metastatic SPT by targeting TAMs.
The incomplete capsule feature assessed by CT imaging has been widely used and is considered able to predict malignant behavior of SPT[4]. Yeet al[1] showed this incomplete capsule group had a larger tumor size, an exogenous growth pattern, a malignant tendency during intraoperative frozen biopsy,and more likely to have invasion into the vasculature or organs. Indeed, in our study, the capsule or incomplete capsule measured by CT imaging was consistent with the capsule or invasion feature detected upon evaluation under a microscope. However, 2 patients in each group had distinct results.Considering the increased malignant behaviors of these SPT patients, we are concerned that assessment by CT imaging might miss patients with invasion features that could be detected with a microscope.
Other tumor types, such as small renal cell carcinoma, colorectal cancer and urinary bladder carcinoma, also have histopathological features of capsule or invasion as growth patterns, and the invasive growth feature has been shown as associated with worse prognosis of patients[17-19].
The intact fibrous envelope in extracellular matrix (ECM) might serve as a barrier for tumor cell invasion[20]. In our study, none of the 11 SPT patients with a capsule feature showed malignant behavior, such as invasion to surrounding tissues and distant metastasis. TAMs can secrete several proteolytic enzymes, including matrix metalloproteinases (MMPs, such as MMP7, MMP2, and MMP9),which can mediate the ECM degradation[21]. Particularly, M2-like macrophages could produce chitinase 3-like protein 1 to upregulate MMP expression and promote the invasiveness of gastric and breast cancer cells[22]. The expression of TAMs and M2-like macrophages in peritumor tissues of patients in the invasion group were significantly higher than those in the capsule group, which may be related to the pathological characteristics of incomplete fibrous capsule and tumor cells’ invasive growth pattern, and thus the malignant behaviors. Furthermore, the epithelial-mesenchymal transition hotpot, another mechanism associated with tumor cell invasiveness that mainly occurred at the peritumor site, was also accompanied by TAM infiltration in abundance in hepatocellular carcinoma patients[23]. Of interest, a recent study found that lipid-loaded TAMs could also sustain invasiveness in prostate cancer[24].
Metastasis to the liver through the portal venous system is a common route for the metastasis of pancreatic tumors. Indeed, 3 SPT patients in our study had liver metastasis. TAMs were involved in vascularization in a tumor environment and intravasation of tumor cells, two major mechanisms for tumor metastasis[11,25]. Vascular endothelial growth factor-A (VEGFA) is one of the primary factors driving expansion of the tumor vascular vascularization[26]. Infiltration of TAMs was associated with increased VEGFA expression and vascular density in tumor microenvironment[27,28]. This is probably because the TAMs could promote the expression of VEGFA in vascular endothelial cells by producing WNT7B[29]. Indeed, elevated expressions of fibroblast growth factor, placental growth factor, and antiinflammatory cytokines, such as transforming growth factor-beta (TGF-β) and IL-10 in M2-like macrophages, could potentially enhance the vascularization[30,31]. Furthermore, tumor-associated M2-like macrophages could directly facilitate tumor metastasis by binding tumor cells and mediating their intravasation[32]. Therefore, when an SPT has an invasion feature, the increase of TAMs, especially M2-like macrophages, might facilitate metastasis compared with an SPT that has a capsule feature.
In addition to the vascularization and intravasation in primary tumor sites, the metastasis process includes the formation of a pre-metastatic niche followed by the growth phase[33]. TAMs in the premetastatic niche show an immunosuppressive phenotype characterized by elevated programmed death ligand-1 and nitric oxide synthase expression[34]. Macrophages are recruited during expansion of the metastatic site in the liver, through the CCL2-CCR2 signaling pathway[35]. Blocking the CCL2-CCR2 signaling axis was shown to reduce macrophage infiltration and metastatic expansion[36]. Few studies have reported the polarization of macrophages within or around liver metastasis sites[37]. In our study,we observed relatively more infiltration of TAMs, especially M2-like macrophages, in both intratumor and peritumor sites after the formation of SPT liver metastases. More importantly, to understand the role of TAMs in the formation of liver metastases, dynamic observation of the temporal and spatial changes of TAMs from a pre-metastatic niche to a pro-metastasis site is required.
Recently, Yanget al[14] classified SPT patients by the Ki-67 index based on their similar biological behavior in the 2017 WHO’s pancreatic neuroendocrine tumor grade. In that study, the Ki-67 index was significantly associated with recurrence of SPT[14]. Regarding the Ki-67 index in the malignant behavior of SPT, in our research, a Ki-67 index more than 3% was observed in 4 patients in the invasion group,including 1 patient with peripheral organ invasion, 1 with both lymph node and spleen metastases, and 2 with liver metastasis. However, the Ki-67 index was only 1% in the remaining patient with peripheral organ invasion and liver metastasis. Indeed, previous meta-analyses that evaluated the Ki-67 index to predict malignant behavior of SPTs demonstrated distinct results, which might be due to the limited number of patients included and differences in the methods used to assess the Ki-67 index[38,39]. A multicenter study is needed to reveal the role of Ki-67 index in SPT patients. TAMs might be involved in the regulation of tumor cell Ki-67 expression. Lindstenet al[40] showed that increased infiltration of macrophages was associated with decreased progesterone receptor (commonly known as PR)expression and increased Ki-67 expression in breast tumor cells. Considering the regular expression of PR in SPT[41], the potential influence of TAMs on Ki-67 index in SPT needs further investigation.
Limitations of the present study should be noted. One of the limitations of this study was that the retrospective design and the paucity of the number of patients did not allow us to investigate causal associations between the TAMs and the invasiveness of SPT. In addition, a single immunohistochemical marker to identify target cells might not reflect the real proportion of M2-like macrophages in TAMs, so we did not compare this proportion between invasion and capsule groups in our study. The dichotomy of M1 and M2 classification of human TAM polarizations was not as obvious as in mouse, so we used M2-like macrophages instead. Further analysis of the specific distribution and function of TAMs in SPT patients using flow cytometry and a single-cell sequencing method might be used.
CONCLUSlON
SPT patients with the invasion feature were more likely to demonstrate malignant behavior. These patients also showed increased infiltration of TAMs, especially M2-like macrophages, in both intratumor and peritumor sites compared with patients with the capsule feature.
ARTlCLE HlGHLlGHTS
Research background
Solid pseudopapillary tumor (SPT) has been classified as a low-grade malignant tumor, and indeed only 9.2% patients of all SPT patients are initially diagnosed as malignant with invasion or metastasis. Thus,one of the challenges in managing SPT patients is predicting malignant behavior. One of the most frequently mentioned predictive factors is whether the tumor had a capsule or incomplete capsule as assessed by computed tomography imaging. However, assessing the integrity of the capsule and growth pattern of tumor cells, especially on SPT margins, using a microscope might reflect the real biological behavior of SPT. Tumor-associated macrophages (TAMs), especially the M2-like macrophages subtype, could enhance tumor invasion and metastasis; however, only limited studies are available regarding the role of the TAMs in the SPT microenvironment and their relationship with malignant behaviors of SPT.
Research motivation
The present study suggests that an invasiveness feature, as determined using a microscope is a good predictive factor for the malignant behavior of an SPT and illustrates the macrophage infiltration in the tumor microenvironment of an SPT.
Research objectives
Instead of assessing the integrity of the capsule on computed tomographic imaging, we divided SPT patients into two groups, either with invasion or capsule, based on the integrity of capsule and growth pattern of the tumor cells, especially on the tumor margins, under the microscope; we then investigated differences in the malignant behavior and TAM infiltration between these two groups.
Research methods
Hematoxylin-eosin-stained sections of SPT patients were used to assess the integrity of the capsule and growth pattern of tumor cells, especially on tumor margins. Immunohistochemical staining was used to evaluate the intratumoral and peritumoral presence of TAMs (CD68-positive) and M2-like macrophages(CD163-positive).
Research results
Malignant behavior was present more frequently in the invasion group, including in 2 patients with peripheral organ invasion, 3 with liver metastasis, and 1 with both lymph node and spleen metastases.Immunohistochemical analysis found that the invasion group had a significant increase of CD68-positive TAMs and CD163-positive M2-like macrophages in intratumoral and peritumoral sites in comparison with the capsule group.
Research conclusions
SPT patients with invasion detected using a microscope were more likely to have a tumor that demonstrated malignant behavior and TAM infiltration, especially of M2-like macrophages.
Research perspectives
This study may provide a histopathological basis for identification of malignant SPT. The phenomenon of increased infiltration of TAMs, especially M2-like macrophages in the invasion feature, might help us to investigate the underlying mechanism of TAM-mediated SPT malignant behavior, as well as to potentially treat recurrent and metastatic SPT by targeting TAMs.
FOOTNOTES
Author contributions:Yang J, Tan CL, Long D, Liang Y, Zhou Li, Liu XB, and Chen YH designed the research study;Yang J, Tan CL, Liu XB, and Chen YH performed the research; Yang J, Long D, Liang Y, Zhou Li, and Chen YH analyzed the data and wrote the manuscript; all authors have read and approved the final manuscript.
Supported byNatural Science Foundation of China, No. 82071746; Key Research and Development Projects in Sichuan Province, No. 2019YFS0043; and 1·3·5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University, No. ZY2017302.
lnstitutional review board statement:The study was reviewed and approved by the Medical Ethics Committee of West China Hospital at Sichuan University (2014 Trial No. 37).
lnformed consent statement:All study participants or their legal guardians provided informed written consent about personal and medical data collection prior to study enrollment.
Conflict-of-interest statement:All authors report no relevant conflict of interest for this article.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Jie Yang 0000-0001-9352-162X; Chun-Lu Tan 0000-0002-7315-1964; Yong-Hua Chen 0000-0001-8485-0755.
S-Editor:Wu YXJ
L-Editor:A
P-Editor:Wu YXJ
 World Journal of Gastroenterology2022年34期
World Journal of Gastroenterology2022年34期
- World Journal of Gastroenterology的其它文章
- Therapeutic strategies for post-transplant recurrence of hepatocellular carcinoma
- Pregnancy and fetal outcomes of chronic hepatitis C mothers with viremia in China
- Spontaneous expulsion of a duodenal lipoma after endoscopic biopsy: A case report
- Trends in hospitalization for alcoholic hepatitis from 2011 to 2017: A USA nationwide study
- lmpact of adalimumab on disease burden in moderate-to-severe ulcerative colitis patients: The one-year, real-world UCanADA study
- Diagnosis, treatment, and current concepts in the endoscopic management of gastroenteropancreatic neuroendocrine neoplasms
