lmpact of adalimumab on disease burden in moderate-to-severe ulcerative colitis patients: The one-year, real-world UCanADA study
Talat Bessissow, Geoffrey C Nguyen, Osman Tarabain, Laurent Peyrin-Biroulet, Nathalie Foucault, Kevin McHugh, Joannie Ruel
Abstract
Key Words: Disease burden; Patient-reported outcome; Depressive symptoms; Ulcerative colitis;Adalimumab; Real-world data
lNTRODUCTlON
Ulcerative colitis (UC)—an intermittent, idiopathic, and chronic disease of the colon—has a worldwide incidence of 1 to 20per100000 individuals and a prevalence of 5 to 500per100000 individuals[1,2]. As of 2018 in Canada, it is estimated that 120000 individuals live with this disease.
The burden of UC has been recognized to extend beyond its clinical signs and physical symptoms such as bloody diarrhea and abdominal pain, including the development of anxiety/depression, a decreased health-related quality of life (HRQoL), an impact on work productivity and social interactions, and impairments in sexual function[3-6]. As such, having access to multidisciplinary,collaborative, chronic disease models of care improves patients’ HRQoL[7].
Around 30% of patients with inflammatory bowel disease (IBD) experience psychiatric disorders, and depression/anxiety in these patients have been shown to be three times greater than in the general population[8]. In UC patients, the prevalence of depression symptoms and disorders have been estimated as 16.7% [95% confidence interval (CI): 12.0%-21.4%][9]. Assessing the severity of depression in over 158000 IBD patients, a Patient Health Questionnaire-9 items (PHQ-9) pooled mean score of 7.6(95%CI: 6.3-8.8, on a 0-27 scale) has been reported, which can be interpreted as a mild depression[9,10].
Among physical symptoms, fatigue, which is not relieved by rest and implies limitations of daily activities[11], has been reported by 42% to 47% of UC patients at diagnosis[12]. Fatigue has been shown to impact IBD patients’ QoL and is experienced by those of all ages, with some studies suggesting a greater burden in women[13-17]. In a recent review on IBD, Nocerinoet al[18] documented strong associations between fatigue and sleep disturbance and inadequate sleep, highlighting a proportion of more than 50% of both active and inactive IBD patients reporting sleep deficiency.
Consistent with the increasing inclusion of patient’s voice in all aspects of health care as in UC therapies[19] and aiming to align with the FDA guidance[20], the use of patient-reported outcome(PRO) questionnaires are more and more used as clinical endpoints in IBD studies[21]. The use of PRO instruments helps understand patients’ preferences, which in turn has been shown to be associated with treatment acceptance and adherence[22-25].
In IBD populations, depression/anxiety has been measured with a wide range of tools, including the PHQ-9[9,26-29]. The Inflammatory Bowel Disease Questionnaire (IBDQ) and its short version (SIBDQ)as well as the EuroQol 5-Dimentions, 5 Levels (EQ-5D-5L) questionnaire have been used to assess HRQoL[30-34], fatigue has been assessed by the Functional Assessment Chronic Illness Therapy-Fatigue (FACIT-F) questionnaire[18,35-37], and work productivity with the Work Productivity and Activity Impairment (WPAI) questionnaire[26,30,38-40].
Developed and validated in 2012 in a population-based cohort, the inflammatory bowel disease disability index (IBD-DI) is specific to assess disability in IBD patients[41-43]. On a 0-100 scale, the mean(interquartile range) value of the IBD-DI was 35.3 (Q1 = 19.6; Q3 = 51.8). Higher IBD-DI values were associated with female gender (P< 0.001), clinical disease activity (P< 0.0001), and disease duration (P=0.02)[42].
To provide real-world data on improvements in daily lives of UC patients, the overall goal of the UCanADA study was to gather evidence on effectiveness, quality of life, disability, and work productivity during an adalimumab treatment. The primary objective was to evaluate psychological distress/depression symptoms using change from baseline in the PHQ-9 after 1 year of a real-world adalimumab treatment in moderate-to-severe UC patients.
MATERlALS AND METHODS
Study design and patients
In this prospective, single arm, 1-year multicenter Canadian post-marketing observational study, adults(≥ 18 years) with a confirmed diagnosis of UC and a moderate-to-severe disease activity-evidenced by either a Mayo endoscopic subscore (MES) of 2 or 3 from endoscopic investigation in the previous 3-mo closest to the baseline visit, or a Mayo rectal bleeding subscore ≥ 2 and a calprotectin value greater than 250 mg/g-were enrolled if they were prescribed adalimumab as part of their treatment by their treating physician. If a patient was previously treated with vedolizumab or any anti-tumor necrosis factor (TNF)agent (except adalimumab), an appropriate washout period took placeperroutine practice, which period varied usually from 2 to 3 mo.
Excluded from the study were patients who either previously received adalimumab, used infliximab or any anti-TNF agent and did not clinically respond at any time unless they experienced a treatment limiting reaction; had a history of subtotal colectomy with ileorectostomy or colectomy with ileoanal pouch, Kock pouch, or ileostomy for UC or planned bowel surgery; had a current diagnosis of indeterminate colitis, ulcerative proctitis only, or with a current diagnosis and/or have a history of Crohn’s disease; had other TNF immune-modulated disease; OR had a significant history of renal,neurologic, psychiatric, endocrinologic, metabolic, immunologic, cardiovascular, or hepatic disease that in the opinion of the investigator would adversely affect his/her participating in this study. Also, we excluded from the study pregnant or breast-feeding female patients and patients currently participating in another prospective study including controlled clinical trials.
Eligible patients were approached to participate in the study after a decision to change the patient’s therapy for adalimumab was already made by the treating physician. To participate in the study and to disclose personal health information, all patients were required to sign a patient authorization form (or written informed consent), which was approved by an Independent Ethics Committee/Institutional Review Board (ClinicalTrials.gov Identifier: NCT02506179). The study was conducted between July 2015 and December 2019 in 23 Canadian sites, with approximately half of the sites being community based and the other half academic based.
Assessments
Patients were followed for 52 wk post initiation of adalimumab treatment (baseline). The assessments were performed during patients’ routine care visits schedule, coinciding approximately to 8 and 52 wk after baseline, in accordance with the Canadian approved label (product monograph) and asperregional requirements. The completers population was defined as patients who received at least one dose of treatment, and at least one follow-up appointment and did not terminate the study early or discontinue.
During these visits, the patients’ medical history and changes in medical conditions, previous and concomitant medications, and disease severity and activity [clinical response defined as simple clinical colitis activity index (SCCAI)[44] decrease from baseline of ≥ 2, clinical remission defined as SCCAI score ≤ 2, endoscopic evaluation (MES), assessment of rectal bleeding (Mayo Rectal bleeding Subscore),and the physician’s global assessment (PGA)] were assessed.
Also, patients were required to fill, on paper at the physician’s office, eight PRO questionnaires for evaluating: The presence and severity of depression (PHQ-9 using a 0-27 scale)[10], the entire spectrum of limitations in functioning in patients (IBD-DI questionnaire evaluating 4 domains of body functions,activity and participation, body structures, and environmental factors)[41], HRQoL (EQ-5D-5L questionnaire comprising mobility, self-care, usual activities, pain/discomfort, and anxiety/depression using a 0-1 scale)[45], SIBDQ questionnaire assessing the social, emotional, bowel, and systemic domains on a 1-7 scale[46], fatigue (FACIT-F questionnaire having a fatigue subscale score with a range from 0 to 52)[47], sleep related outcomes [Medical Outcomes Study Sleep scale (MOS Sleep) 12-item questionnaire including sleep disturbance, sleep awakening short of breath or with headache, sleep adequacy, somnolence, and quantity of sleep/optimal sleep][48], work related outcomes [WPAI: UC V2.0[49] presenting percentages of absenteeism (work time missed), presenteeism (impairment while working), an overall work impairment (overall productivity loss, accounting for both absenteeism and presenteeism), and activity impairment (impairment in activities outside work)], and Valuation of Lost Productivity (VOLP) questionnaire[50], assessing the impact of health conditions on lost productivity in monetary units. The order by which the PRO questionnaires were filled was varied to limit the potential of missing data that would systemically be found for a particular instrument.
Safety assessments included serious adverse events (AEs), any non-serious event of malignancy in patients 30 years of age and younger[51], unusual failure in efficacy, and AEs leading to discontinuation. These were coded using Medical Dictionary for Regulatory Activities version 17.1.
Study size and statistical methods
The sample size was calculated assuming a proportion of 15% of patients would improve their PHQ-9 score compared with baseline and change severity category. Using an alpha of 0.05 with a lower CI of 6%, a sample size of 72 patients would be needed. To account for a potential 25% attrition over the course of one year, the sample size was increased to 100 patients. It was anticipated that up to 30% of the 100 moderate-to-severe UC patients newly treated with adalimumab would have prior experience with biologics.
The primary effectiveness endpoint—the proportion of patients with a change in depressive symptoms using the PHQ-9 score from baseline following initiation of adalimumab and after 1 year of treatment—was calculated, and the 95%CIs were estimated. Changes in PHQ-9 scores from baseline were tested by paired samplet-test. Least-square mean (LS mean) of the changes were also estimated by the mixed effect repeated measures models where baseline values were included as a covariate.Changes in severity categories were tested by Bowker’s test (kxktable wherek> 2) or McNemar’s test (2× 2 table).
To understand the independent effect of clinical effectiveness on the probability of improving in PHQ-9 at week 52, a logistic regression analysis was conducted to examine the effect of clinical response and clinical remission adjusting for the baseline PHQ-9 score and other potential prognostic factors. A similar analysis was conducted to assess the association between clinical effectiveness on changes of PHQ-9 scores from baseline. LS mean of the changes associated with clinical response and clinical remission was estimated by the mixed effect repeated measures models using all follow-up visits.
For secondary outcomes, the IBD-DI, EQ-5D-5L, SIBDQ, FACIT-F, and MOS Sleep, scores at baseline,week 8, and week 52 were summarized, and changes in scores from baseline were tested by paired samplet-tests. LS mean of the changes were also estimated by the mixed effect repeated measures models where the baseline value was included as a covariate. Productivity outcomes (WPAI and VOLP)at baseline, week 52, and changes in outcome from baseline were summarized. The 95%CIs for the changes were estimated by bootstrapped percentile CIs based on samples of 10000.
The sensitivity to change for the IBD-DI was evaluated using the effective size (ES) and the standardized response mean (SRM). For both statistics, values of 0.020, 0.50, and 0.80 or greater were used to represent small, moderate, and large, respectively. The association between the change in PROs(EQ-5D-5L, SIBDQ, FACIT-F, and MOS Sleep) and clinical response/remission (effectiveness) were assessed using a mixed model for repeated measures using observations from all follow-up visits with the baseline value included in the model as a covariate. All models with repeated measures included a random intercept with the effectiveness variable (fixed, forced-in), visit (fixed, forced-in), baseline value of the PRO measure (fixed, forced-in) and other covariates. Cross-sectional regression models included an intercept with the effectiveness variable (forced-in), baseline value of the PRO measure (fixed) and other covariates. Least squares means,Pvalue and 2-sided 95%CI of the difference between the two groups defined by the clinical effectiveness were determined. Additional details on the statistical analysis used to determine the correlation between effectiveness (clinical response and remission) rates and PRO measures are provided in the Supplementary material section.
Missing data were imputed only for the sensitivity analysis of the primary outcome. For missing responses on PRO questionnaire items, missing data were handledperthe imputation solutions provided in the coding of the PRO instruments. To assess the impact of missing data on the primary endpoint estimate, the sensitivity analysis was performed using two imputation methods: Nonresponder imputation (NRI), defined as patients who did not provide week 52 effectiveness data or dropped out of the study prior to week 52 were considered as no improvement; and last observation carried forward (LOCF) defined as the last effectiveness assessment prior to week 52 was used for those missing week 52 assessment.
All calculations and analyses were performed using SAS version 9.4 (Cary, NC: SAS Institute Inc.)under the Windows 10 Enterprise operating system at the Centre for Health Evaluation and Outcome Sciences, in Vancouver, Canada.
RESULTS
Patients
One hundred patients from 23 Canadian sites were included in the study (Figure 1). Respectively, 94, 48,and 98 patients were included in the effectiveness population [intent-to-treat (ITT) population], the completers population, and the safety population. Patients in the ITT population had a mean age (SD) of 42.5 (15.3) years and a mean body mass index of 25.4 (4.5) kg/m2(Table 1). The majority was White(93.6%) and male (59.6%). The mean age at UC diagnosis was 34.5 (15.3) years, and the mean duration of disease was 7.9 (8.0) years. Forty-eight (48%) patients completed the study.
Impact on psychological distress/depression symptoms
Following routine care treatment with adalimumab, the proportion of patients who improved in psychological distress/depressive symptoms using the PHQ-9 total score at week 52—defined as a change in PHQ-9 total score from baseline, the study primary endpoint—was 61.5% (40/65) (95%CI:49.7%-73.4%) for the ITT population and 65.9% (29/44) (95%CI: 51.9%-79.9%) for the completers population (Figure 2A). To assess the impact of missing data, the sensitivity analyses conducted on the primary endpoint showed that the proportions of patients who improved in psychological distress/depressive symptoms, using the NRI and LOCF imputation methods, were similar to the proportion obtained using the original non-imputed data analysis, with the ITT population(Supplementary Table 1).
Overall, changes from baseline in the PHQ-9 total score were significant at weeks 8 and 52 (P= 0.010),with changes slightly higher for the completers population [-2.5 (6.1) and -3.4 (6.8) at weeks 8 and 52]than in the ITT population [-2.2 (6.1) -2.4 (7.1)] on a 0-27 scale (Table 2). The proportion of patients with a PHQ-9 total score 10 (yellow flag category,i.e., moderate or more severe depression) was 25.6%(21/82) at week 8, which slightly increased to 29.2% (19/65) at week 52 for the ITT population. These proportions were lower and stable over time (18%-19%) for the completers population. For patients with severe depressive symptoms (red flag category), the proportions improved from 19.1% (18/94) and 16.7% (8/48) at baseline to 12.3% (8/65) and 2.3% (1/44) at week 52 for the ITT population and completers population, respectively.
The PHQ-9 questionnaire items that showed the highest improvement from baseline were ‘Poor appetite or overeating’ (response ‘Not at all’ increased by 23.3% from baseline to week 52, and response‘Nearly every day’ decreased by 20.3% from baseline to week 52), ‘Little interest or pleasure in doing things’ (response ‘Not at all’ increased by 15.3% from baseline to week 52, and response ‘Nearly every day’ decreased by 12.1% from baseline to week 52), and ‘Feeling tired or having little energy’ (response‘Not at all’ increased by 8.2% from baseline to week 52, and response ‘Nearly every day’ decreased by 20.3% from baseline to week 52) (Supplementary Table 2).
Clinical endpoints
The proportions of patients who achieved a clinical response (decrease from baseline ≥ 2 in SCCAI score) remained similar throughout the study [64.2% (52/81) at week 8 and 65.7% (44/67) at week 52],and the proportion who achieved clinical remission (SCCAI score ≤ 2) slightly increased [41.5% (34/82)at week 8 and 47.8% (32/67) at week 52] in the ITT population (Figure 2B). For the completers population, these proportions increased during the study for both the clinical response and clinical remission, reaching 85.4% (35/41) and 73.2% (30/41), respectively, at week 52 (Supplementary Table 3).
Similarly, the proportions of patients who achieved endoscopic healing remained constant between weeks 8 and 52 [42.9% (6/14) had a Mayo endoscopic score of 0 or 1 and 11.5% (3/26), had a fecal calprotectin concentration < 50 μg/g] in the ITT population (Figure 2C), whereas in the completers population, the proportions increased over time [71.4% (5/7) and 80.0% (8/10), measured with the Mayo endoscopic score and 7.7% (1/13) and 11.8% (2/17) measured with the fecal calprotectin concentration, at weeks 8 and 52, respectively] (Supplementary Table 3).
No major changes were observed over time in the extracolonic feature, with the majority of patients not having arthritis at both baseline and follow-up visit [81.5% (66/81) at week 8 and 82.1% (55/67) at week 52] (Figure 2D). The proportion of patients who had arthritis at baseline and none at follow-up was 8.6% (7/81) at week 8 and 9.0% (6/67) at week 52. Similar proportions were observed in the completers population (Supplementary Table 3).

Methotrexate 5 (5.1)Cyclosporine 1 (1.0)Medication use:Corticosteroids 9861 (62.2)Since prior 6 mo to current Imuran (azathioprine)39 (39.8)6-MP 5 (5.1)5-ASA 67 (68.4)Methotrexate 8 (8.2)Cyclosporine 0 (0.0)Montreal classification of extent of UC: E2 = left sided (distal) ulcerative colitis, E3 = extensive (pancolitis) ulcerative colitis; Mayo endoscopic subscore: 0 =normal or inactive disease, 1 = mild disease, 2 = moderate disease, 3 = severe disease; 5-ASA: 5-aminosalicylic acid; 6-MP: Mercaptopurine; BMI: Body mass index; ED: Emergency department; UC: Ulcerative colitis.

Figure 1 UCanADA study flow diagram. The intent-to-treat population was defined as patients who received at least one dose of treatment and had at least one follow-up appointment. The completers population was defined as patients who received at least one dose of treatment, and at least one follow-up appointment and did not terminate the study early or discontinue. The safety population was defined as patients who received at least one dose of treatment. FV: Final visit; ITT:Intent-to-treat.
The proportion of patients who were PGA responders, defined as a decrease from baseline of ≥ 1 point, varied from 66.7% (50/75) to 61.5% (40/65) for the ITT population (Figure 2E) and from 73.2%(30/41) to 83.7% (36/43) for the completers population, from week 8 to week 52, respectively(Supplementary Table 3). While at baseline the majority of patients had moderate disease [73.9%(68/92)], at week 8 the highest proportion of patients had mild disease [35.5% (27/76)], and at week 52,50.0% (33/66) of patients were assessed as normal (Figure 2E). The proportion of patients assessed with severe disease remained comparable between week 8 [5.3% (4/76)] and week 52 [6.1% (4/66)]. Similar results were reported for the completers population (Supplementary Table 3).
A proportion of 5.5% (4/73) of patients reported complications including hospitalization and surgery at week 52 in the ITT population (Supplementary Table 4), and none were reported among the completers population (Supplementary Table 3). The proportion of patients with current steroid usedecreased between week 8 and week 52 from 29.4% (25/85) to 19.2% (14/73) in the ITT population and from 22.2% (10/45) to 12.8% (6/47) in the completers population.
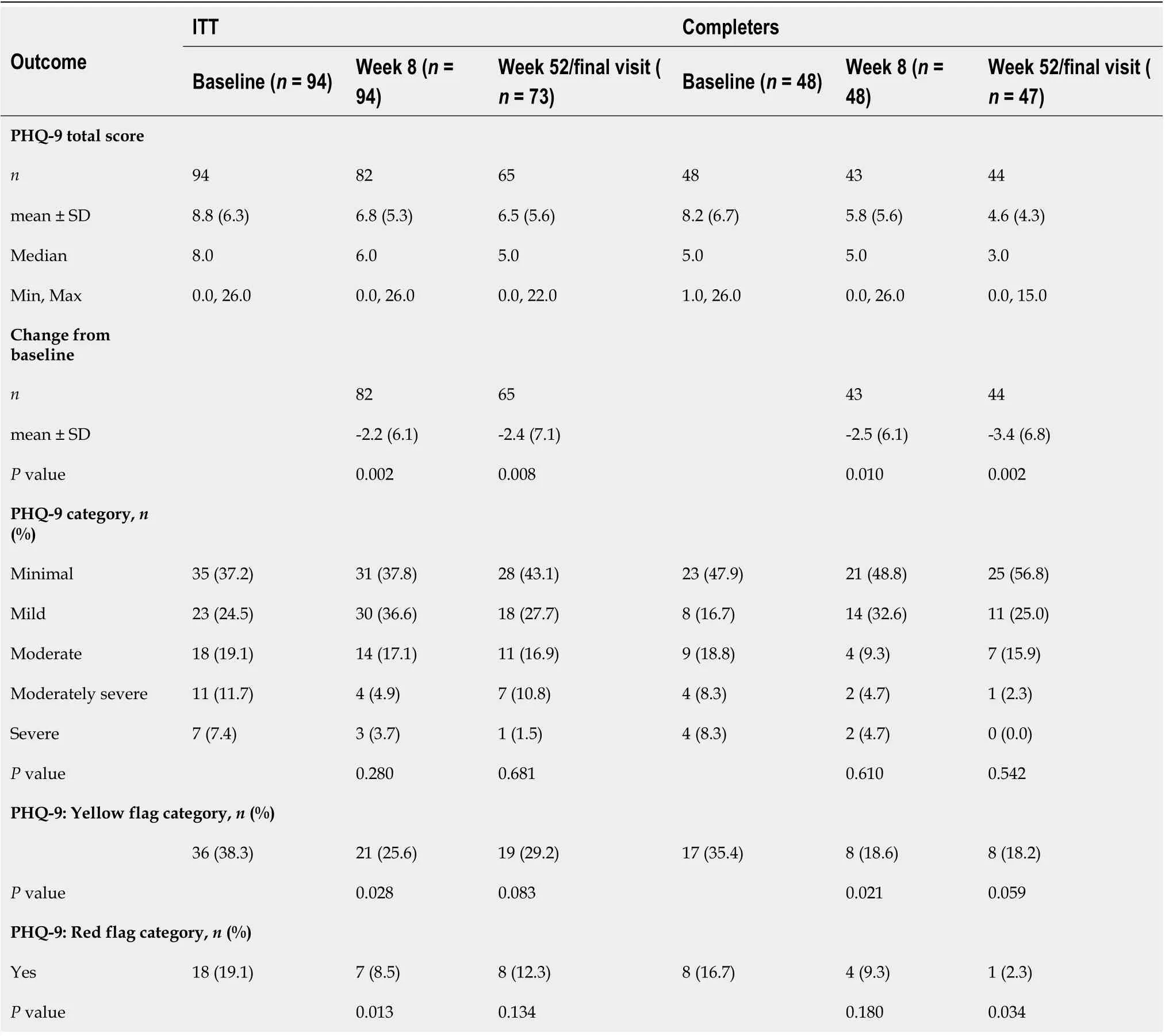
Table 2 Patient Health Questionnaire-9 items at week 8 and week 52/final visit
Improvement in IBD-DI
The mean change from baseline in IBD-DI increased from -10.7 (17.21) at week 8 to -13.8 (22.24) at week 52 (P< 0.001), with proportions of patients who improved disability increasing from 72.6% (53/73) to 74.1% (40/54) over time in the ITT population (Figure 2F). Results from the completers population were slightly higher [mean change from baseline = -14.69 (17.99) and -20.09 (17.74) and improved disability =81.4% (35/43) and 88.9% (32/36) at weeks 8 and 52, respectively] (Supplementary Table 5).
The IBD-DI was moderately sensitive to change for the ITT population, varying from -0.61 to -0.77 for ES and had a SRM of -0.62 (Supplementary Table 6). For the completers population, the IBD-DI was greatly sensitive to change. The ES varied from -0.75 to 1.08 and the SRM varied from -0.82 to -1.13
Correlations between PHQ-9 and clinical outcomes
A correlation analysis showed that at week 52 an improvement in PHQ-9 total score was associated with the baseline PHQ-9 score, with a higher baseline score predicting a greater improvement on the PHQ-9(Table 3). No associations were detected between an improvement in PHQ-9 and clinical response;however, an association was measured with clinical remission in the ITT analysis (OR: 7.94; 95%CI: 1.42-44.41;P= 0.018), though this was not statistically significant in the completer analysis.
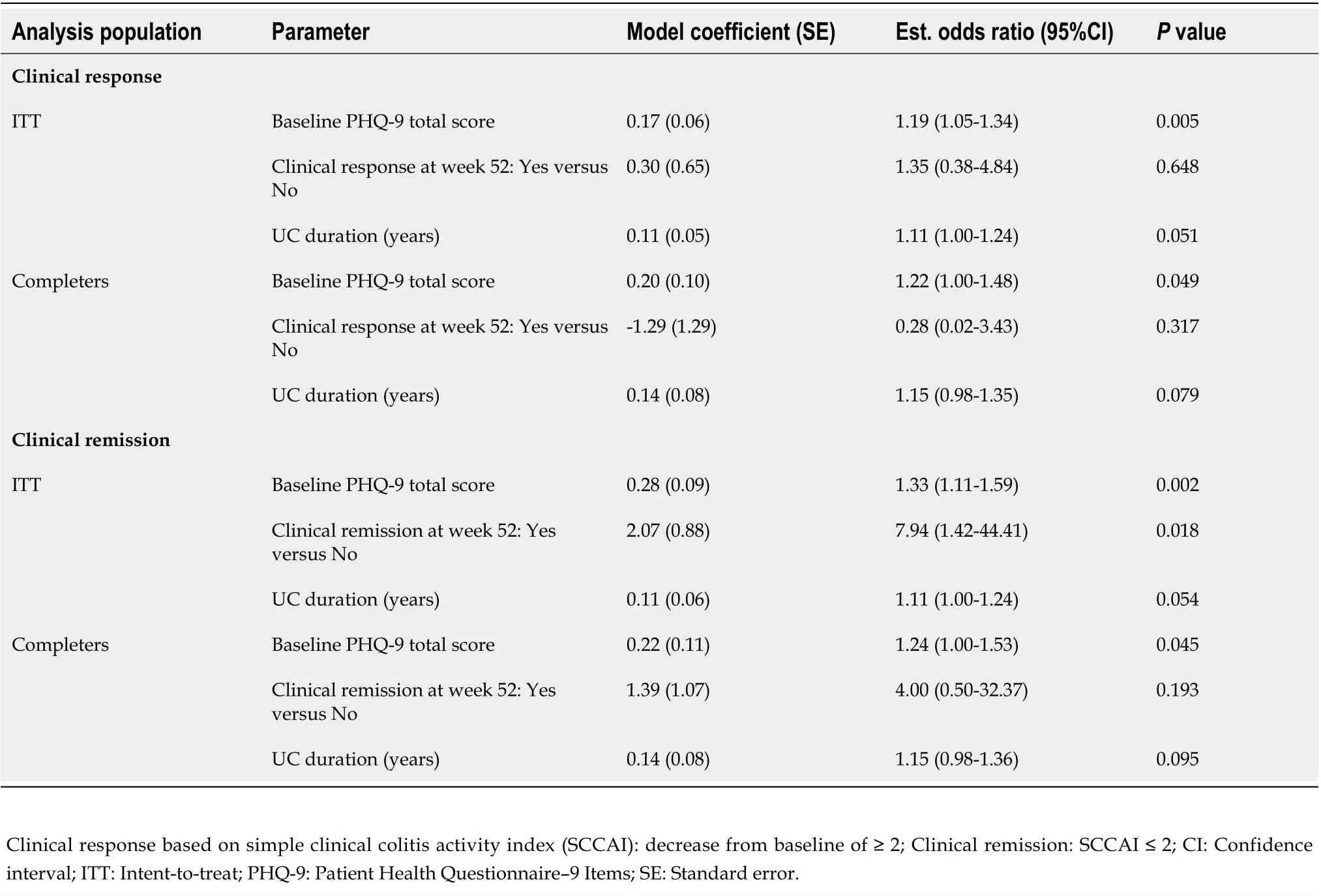
Table 3 Association between clinical response/remission and improvement in Patient Health Questionnaire-9 items total score at week 52/final visit-intent-to-treat and completers populations
A regression analysis between the PHQ-9 total score and clinical response/remission at week 52 showed an association at week 52 using the ITT population (P< 0.001), but not in the completer population (P≥ 0.098) (Supplementary Table 7).
Impact on other PROs and correlation with clinical outcomes
For the other PRO tools used to assess the impact of the adalimumab treatment, changes from baseline at weeks 8 and 52 were significant for the EQ-5D-5L utility score, SIBDQ total score, FACIT-F total score,MOS Sleep Problems Index I and Sleep Problems Index II (P= 0.049) measured in the ITT population(Table 4) and the completers population (Supplementary Tables 8 and 9). A regression analysis showed that changes in the PRO measures between baseline and week 52 were all significantly associated with clinical outcomes (P< 0.001), except for the MOS Sleep measures (P≥ 0.064) (Supplementary Table 10).
The SIBDQ items of social function and bowel symptoms improved the most from baseline [mean change at week 52, 1.09 (2.11) and 0.93 (1.66), respectively on a 1-7 scale] (P< 0.001) (Table 4). Observing the FACIT-F measures, patients reported gaining more over time from the physical fatigue [3.86 (7.11)]than from the functional fatigue [2.58 (6.36)], emotional fatigue [2.06 (5.26)], and social impact of fatigue[1.32 (4.60)], at week 52 (P= 0.024). For the MOS Sleep subscales, only the sleep adequacy subscale significantly improved over time, with a mean change from baseline of 11.69 (5.26) at week 52 (P<0.001).
All the WPAI scores improved from baseline, and the ones that improved the most were activity impairment [-16.9% (29.8) at week 8 and -16.7% (33.6) at week 52],i.e., activities performed outside of work, and overall work impairment [-16.2% (30.2) at week 8 and -14.5% (34.4) at week 52], which combines absenteeism and presenteeism at work (Table 4). Similar results were reported for the completers (Supplementary Table 11).
For the VOLP, the most important changes were reported at week 52 and were related to paid work[paid work productivity loss in the past x months = -42.2 (115.7) hours and any paid work productivity loss in the past x months = -17% (-16.7%)] and lost productivity [any costs of lost productivity in the past x month = -15% (11.6%) hours and total costs of lost productivity in the past x months ($) = -1998 (-7299.7) $]. Study completers reported slightly higher results (Supplementary Table 11).
Safety
The safety profile was consistent with the known safety profile of adalimumab. During the study, 18(18.4%) patients experienced at least one AE (Table 5). The AEs reported by more than 1% of patientswere: Colitis ulcerative [6 (6.1%) patients], drug ineffective [6 (6.1%) patients], haematochezia [2 (2.0%)patients], and arthralgia [2 (2.0%) patients]. Each of the severe AEs was experienced by only one (1.0%)patient, which included one event each of anal fissure, colitis, dysphagia, and mouth ulceration. One patient experienced two events of severe oesophagitis.
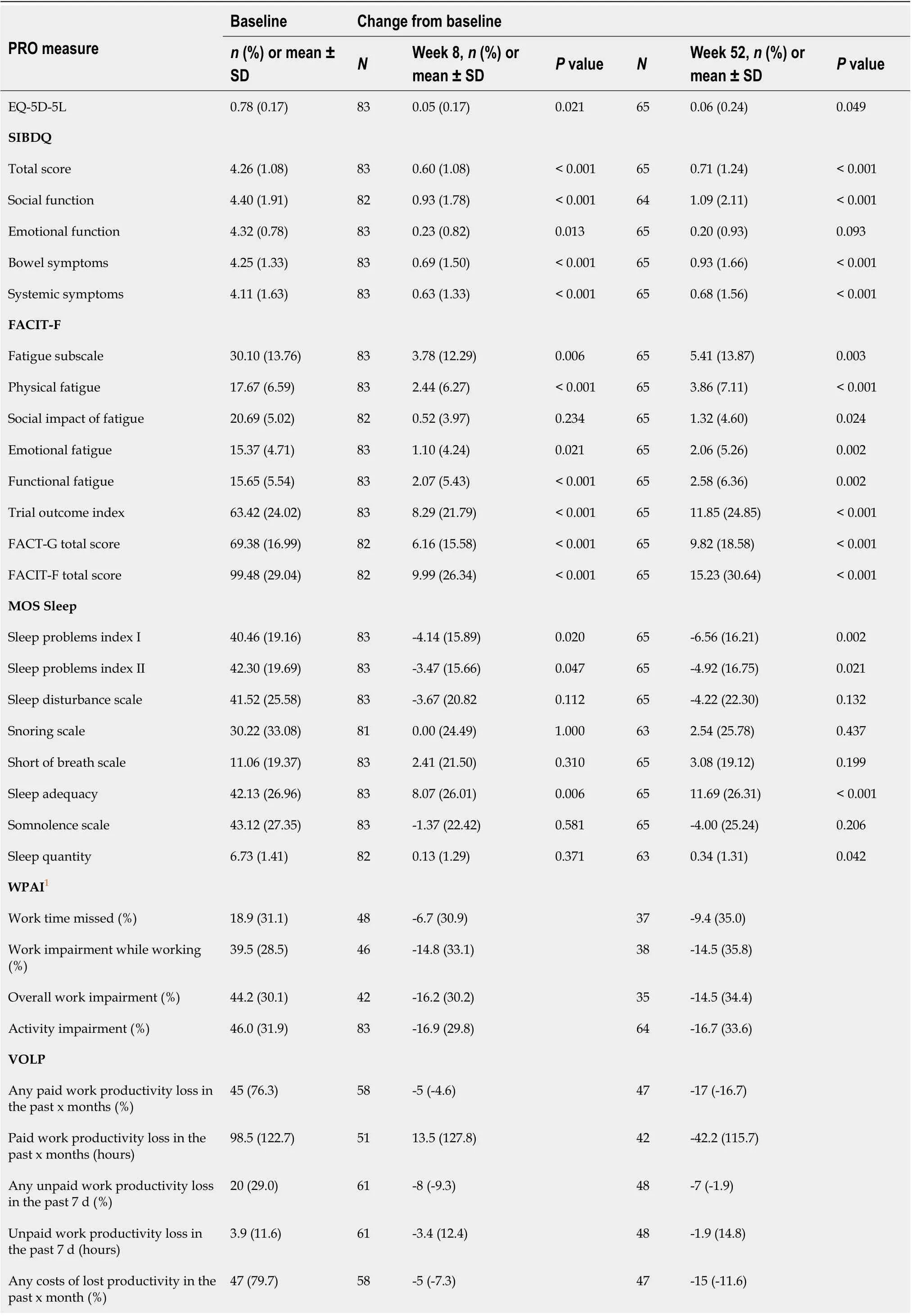
Table 4 Change from baseline in other patient-reported outcomes at weeks 8 and 52-intent-to-treat population
Two (2.0%) patients experienced serious treatment-related AEs that were assessed by the investigator to be reasonably possibly related to adalimumab: 1 (1.0%) patient experienced two events of severe oesophagitis that led to hospitalization and prolongation of hospitalization, and one event of severe aggravated colitis that led to hospitalization; 1 (1.0%) patient experienced severe injection site pain.There was one report of cutaneous basal cell cancer in a 63-year-old male. Monitored asperprotocol safety variable, there were no reports of malignancy in patients 30 years of age and younger. No death was reported during the study, and no new signal or unexpected trend was identified for the patient population.
DlSCUSSlON
To fill an information gap on Canadian real-world data on the effectiveness of adalimumab on PRO measures in moderate-to-severe UC patients, and consistent with the FDA guidelines on the use of PRO measures to support labelling claims[20], the UCanADA study enrolled 100 patients from 23 centres using as a primary endpoint the change from baseline in depressive symptoms at week 52, measured by the PHQ-9 questionnaire.
The PHQ-9 measures in study UCanADA showed that over 60% of the study population improved in psychological distress/depression symptoms during the real-world adalimumab treatment, with most gains observed at week 52 in the completers population (65.9%). Significant changes from baseline were observed at week 8, which were maintained at week 52 and were slightly higher for the completers population (P= 0.010). Despite this improvement, these scores may be interpreted as a remaining mild depression in patients[10] and not necessarily a clinically meaningful change[52-54], which indicates a potential relevance to offer psychological support to this population[7,55]. In the present observational study, while patients who had a preliminary failure to biologics were excluded, patients with secondary failures were included, which may have led to the inclusion of patients with greater psychological burden than biologic-naïve patients.

Figure 2 Effect of a real-world adalimumab treatment on effectiveness and patient-reported outcomes in moderate-to-severe ulcerative colitis patients. A: Proportion of patients who improved in Patient Health Questionnaire-9 items total score at week 52 in the intent-to-treat and completers populations (%), improvement defined as change from baseline, bars show 95% confidence intervals; B: Proportion of patients achieving clinical response/remission at weeks 8 and 52 (%), measured by the simple clinical colitis activity index (SCCAI) ≤ 2; C: Proportion of patients with endoscopic healing at weeks 8 and 52 (%),healing measured as a Mayo endoscopic subscore of 0 or 1 or a fecal calprotectin concentration < 50 μg/g; D: Proportion of patients with extracolonic feature at weeks 8 and 52 (%), measured by the SCCAI; E: Proportion of physician’s global assessment responders and severity at weeks 8 and 52 (%), responders defined as with a decrease from baseline of ≥ 1 point; F: inflammatory bowel disease index mean change from baseline and proportion of patients who improved (%). Bars represent standard deviations. PRO: Patient-reported outcome; UC: Ulcerative colitis; PHQ-9: Patient Health Questionnaire-9; IBD: Inflammatory bowel disease;SCCAI: Simple clinical colitis activity index; ITT: Intent-to-treat; PGA: Physician’s global assessment.
Our results show a significant change from baseline in PHQ-9 score earlier during treatment than those of a recently reported cohort of 1804 UC outpatients, who were included regardless of treatment assignment or disease activity[26]. However, a similar decrease in PHQ-9 score was measured in UC patients at least 30 d after being initiated on an anti-TNF therapy (including infliximab, adalimumab, or certolizumab) and/or immunomodulator therapy (methotrexate or azathioprine)[29].
At week 52, clinical response and clinical remission were achieved respectively by 65.7% and 47.8% of the ITT population, and 85.4% and 73.2% of the completers population. These results are comparable to those from the InspirADA study at week 8, in which 463 moderate-to-severe UC patients from 92 international sites were treated with adalimumab following usual clinical practice[40].
To our knowledge, UCanADA is the first study reporting associations between PHQ-9 scores and clinical response/remission in UC patients in a real-world setting. A regression analysis showed that in the ITT population, the odds of improving depressive symptoms for those achieving a clinical remission at week 52 was 7.94 higher compared to those not achieving a clinical remission (OR: 7.94; 95%CI: 1.42-44.41;P= 0.018).
These results-as well as the significant associations measured between the PHQ-9 total score and clinical response/remission at week 52 (P< 0.001) and between clinical response/remission and IBD-DI,EQ-5D-5L, SIBDQ total score, and FACIT-F fatigue subscale (P= 0.002)-are consistent with the other findings showing a relationship between disease activity and HRQoL[34,35,56]. In a 6-mo study including 199 UC patients, a consistent and almost linear relationship was demonstrated between SCCAI values and the EQ-5D-5L index values (correlation: ρ: -0.53;P< 0.001)[34].
Other studies conducted in UC patients reported correlations between disease activity indexes and HRQoL measures. Aniwanet al[35] reported a good correlation between the SIBDQ and the combination of self-rated rectal bleeding and stool frequency using the 6-point partial Mayo score(ClinPRO2) and MES, from a study on 90 UC patients (r= -0.70;P< 0.01). Assessed on 110 UC patients,a significant correlation has been reported between SIBDQ and SCCAI and MES alone (r= -0.79 andr=-0.58, respectively)[56]. Consistent with our findings, these support an association between clinical remission and improved HRQoL.
To the PHQ-9 item ‘Feeling tired or having little energy’ between baseline and week 52, the proportion of patients feeling it ‘Nearly every day’ decreased by 20%, and those feeling it ‘Not at all’increased by 8%. These results are in line with the significant decrease in fatigue shown in the FACIT-F total score and fatigue subscale (P= 0.006).
As fatigue has been reported to be strongly associated with sleep disturbances in IBD patients[18], not surprisingly our scores from the MOS sleep problems indexes I and II also significantly improved during the study, as well as sleep adequacy and sleep quantity subscores (P= 0.042 at week 52). Using the NIH PROMIS questionnaire in 160 patients with IBD using either and anti-TNF or vedolizumab,Stevenset al[57] reported significant and meaningful improvement in sleep quality by week 6 (P=0.009), which was paralleled by a significant reduction in depression (P< 0.05), as measured in the UCanADA study population. These results reinforce the need to assess sleep disorders a part of an algorithmic approach for the systemic workup of fatigue[18].
Also related to fatigue, in a study including 1185 IBD patients (462 with UC), Willietet al[58] reported a strong correlation between FACIT-F score and IBD-DI measure (r= -0.78) as well as overall work impairment (r= -0.70). Similar trends were observed in UCanADA,i.e., a significant improvement in fatigue (FACIT-F total score;P< 0.001) mirrored by a significant improvement in IBD-DI measures (P<0.001) as well as a meaningful improvement in overall work impairment during the study. Our IBD-DI measures are similar to those reported from other UC populations[43]. In a meta-analysis including 3167 IBD patients, Loet al[43] reported a significant lower disease disability rates in patients on biological treatment than those on corticosteroids (P< 0.01).
At week 8 and week 52, there was a gain between 15% and 17% in work impairment, overall work impairment, and activity impairment in the UCanADA study population. These represent less gains than those reported from the InspirADA population at week 26; however, they were twice as high as the minimal clinically important difference of 7% in WPAI outcome[59].
Limitations of the research methods used in this study are related to, but may not have been limited to, the observational nature of the study with regards to missing data, which has been alleviated with the use of sensitivity analyses for the primary endpoint. This study consisted of a small cohort of patients, and only 48 (48%) patients completed the study. However, the results between the ITT population and completers population were fairly consistent. The PRO questionnaires being selfadministered provide subjective data as opposed to objective data. The collection of secondary PROs data may be subject to a recall bias.
CONCLUSlON
At week 52 in a real-world setting, adalimumab was effective in reducing depressive symptoms in patients with UC, with more than 60% of the patients achieving an improvement the PHQ-9 with a mean improvement of 2.4 points. A broad range of PROs including HRQoL and work productivity also significantly improved during the study. The safety profile was consistent with the known safety profile of adalimumab, and no new signal or unexpected trend was identified for the patient population.
ARTlCLE HlGHLlGHTS
Research background
The efficacy and safety of adalimumab have been demonstrated in pivotal trials, but there remained a need to assess more holistically how the clinical results translate into concrete improvements in key aspects of the daily lives of ulcerative colitis (UC) patients, such as symptoms, health-related quality of life (HRQoL), and disability.
Research motivation
Although some patient-reported outcomes (PROs) from existing studies may have items capturing some of these aspects, limited data was available for adalimumab in UC, specifically on psychological distress/depression, disability, fatigue, and pain or sleep quality in real-life setting.
Research objectives
The overarching goal for the UCanADA study was to assess the real-life effectiveness of adalimumab on PRO measures, while taking the opportunity to use the inflammatory bowel disease disability index to assess the impact of adalimumab on key components of patients’ functioning when affected with moderate-to-severe UC.
Research methods
UCanADA was a single arm, prospective, 1-year multicenter Canadian post-marketing observational study in which multiple PRO questionnaires were completed—with psychologic distress/depression symptoms as the primary endpoint—by patients with moderate-to-severe UC. Assessments were performed during patients’ routine care visit schedule, which was at the initiation of adalimumab(baseline), after induction (approximately 8 wk), and 52 wk after baseline. Additional optional assessments between weeks 8 and 52 were collected at least once but no more than two times during this period. Serious safety events and per-protocol adverse events were collected.
Research results
One hundred patients were included in this final analysis, with 94 (94%) patients included in the efficacy population (identified as the intent-to-treat (ITT) population), 48 (48%) patients included in the completers’ population, and 98 (98%) patients included in the safety population. The primary endpoint-the proportion of patients who achieved a change from baseline, defined as an improvement in total severity score relative to baseline, in the Patient Health Questionnaire-9 items (PHQ-9) measure at week 52-was 61.5% [40/65 patients; 95% confidence interval (CI): 49.7%-73.4%] for the ITT population and 65.9% (29/44 patients; 95%CI: 51.9%-79.9%) for completers. The safety profile was consistent with the known safety profile of adalimumab, and no new signal or unexpected trend was identified for the patient population.
Research conclusions
At week 52, adalimumab, used in a real-life study, was effective in reducing depressive symptoms in patients with UC, with more than 60% of the patients achieving an improvement the PHQ-9 with a mean improvement of 2.4 points. Thus, the treatment with adalimumab contributed to reducing the depressive symptoms frequently experienced in patients with UC as well as improving a broad range of PROs such as HRQoL and work productivity, as assessed with PRO instruments. The safety profile was consistent with the known safety profile of adalimumab, and no new signal or unexpected trend was identified for the patient population.
Research perspectives
Improvements in PHQ-9 were associated with clinical remission. Beyond the PHQ-9, significant improvements in several PROs were observed suggesting an improvement in HRQoL and work productivity as well. The population in the study, as well as the inclusion and exclusion criteria, was representative of the target population. In addition, coinciding the study visits with the patient’s routine care visit schedule helped increase generalizability of the PRO instruments by decreasing the impact on real life.
ACKNOWLEDGEMENTS
AbbVie and the authors thank all study investigators (Dr. Kenneth Atkinson, Columbia Gastroenterology Management Ltd.; Dr. Guy Aumais, CHUM-Hôpital Maisonneuve-Rosemont; Dr. Rahman Bacchus, Dr. Rahman Bacchus Medicine Corporation; Dr. Andrew Bellini, Bellini Medicine Professional Corporation; Dr. Edmond-Jean Bernard, Le Centre des Maladies Inflammatoires et Intestinales de Montréal (CMIIM); Dr. Ian Bookman, Kensington Cancer Screening Clinic; Dr. Raymond Bourdages,CHAU - Hôpital Hotel-Dieu de Levis; Dr. Brian Bressler, Gastrointestinal Research Institute (GIRI); Dr.Sharyle Fowler, Royal University Hospital; Dr. Alexandre Gougeon, Centre Hospitalier de l'Université Laval (CHUL); Dr. Daniel Green, Oshawa Clinic; Dr. Vivian Huang, Zeidler Ledcor Centre; Dr. Mark MacMillan, Dr. Everett Chalmers Regional Hospital; Dr. Remo Panaccione, University of Calgary Gastrointestinal Research Group; Dr. Denis Petrunia, Discovery Clinical Services; Dr. Ranjit Singh,PerCuro Clinical Research Ltd.; Dr. Richmond Sy, Ottawa Hospital - General Campus; Dr. Audrey Weber, CHUM - Hôpital St-Luc; and Dr. Chadwick Williams, Dr. Chadwick Ian Williams Professional)for their contribution and the patients for their participation in this study. Thank you also to Daphne Guh, MSc., from the Centre for Health Evaluation and Outcome Sciences, and Nick Bansback, Ph.D.from University of British Columbia, for the statistical analyses. Medical writing support was provided by Judy Chiu, MPH, from the Centre for Health Evaluation and Outcome Sciences, and Nathalie Ross,PhD, MWC, from McDougall Scientific Ltd. All of these contributors were funded by AbbVie, Inc.AbbVie sponsored the study and medical writing support for this manuscript; contributed to the design;participated in the collection, analysis, and interpretation of data as well as in writing, reviewing, and approving the final version of this manuscript. No honoraria or payments were made for authorship.
FOOTNOTES
Author contributions:Bessissow T was involved in study design, coordinating the data collection, interpretation of the results, and review and revision of the manuscript; Ruel J, Nguyen GC, and Tarabain O were involved in coordinating the data collection, interpretation of the results, and review and revision of the manuscript; Foucault N and McHugh K were involved in study design, interpretation of the results, and review and revision of the manuscript.
lnstitutional review board statement:All sites had the study reviewed and approved by an ethics committee. While CEC Advarra IRB Services (Aurora, Ontario, Canada) reviewed and approved for some sites, other sites were approved through local/multi centres ethics committees (Dr. Everett Chalmers Regional Hospital, MUHC-Royal Victoria Hospital, CHUM-Hôpital Maisonneuve-Rosemont, CHAU-Hôpital Hôtel-Dieu de Levis, Royal University Hospital, Centre Hospitalier de l'Université de Sherbrooke, CHUM-Hôpital St-Luc, Zeidler Ledcor Centre, Ottawa Hospital-General Campus, Mount Sinai Hospital, London Health Sciences Centre, Centre Hospitalier de l'Université Laval (CHUL), University of Calgary Gastrointestinal Research Group).
lnformed consent statement:All patients were required to sign a patient authorization form (or written informed consent) to participate in the study, and to disclose personal health information.
Conflict-of-interest statement:The authors have either received research support from, served as consultant, speakers bureau, scientific officer, participants of steering committees and advisory boards, and/or received honoraria from the following sponsors: T.B.: AbbVie, Janssen, Takeda, Merck, Pfizer, Roche, Bristol Myers Squibb, Gilead, Sandoz,Ferring, Alimentiv Inc. (formerly Robarts Clinical Trials); J.R.: AbbVie, Janssen, Takeda, Pfizer, Sandoz, Ferring,Alimentiv Inc. (formerly Robarts Clinical Trials); G.N.: AbbVie and Takeda; O.T.: AbbVie; N.F. and K.M.: are employees of AbbVie and may own AbbVie stock; L.P.B.: Dr. Peyrin-Biroulet reports personal fees from Galapagos,AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index Pharmaceuticals, Sandoz, Celgene,Biogen, Samsung Bioepis, Inotrem, Allergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Lilly,Fresenius Kabi, OSE Immunotherapeutics, Enthera, Theravance, Pandion Therapeutics, Gossamer Bio, Viatris,Thermo Fisher. Grants from AbbVie, MSD, Takeda, Fresenius Kabi. Stock options: CTMA.
Data sharing statement:AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor.This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 mo, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-informationsharing/data-and-information-sharing-with-qualified-researchers.html.
STROBE statement:The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Canada
ORClD number:Talat Bessissow 0000-0003-2610-1910; Geoffrey C Nguyen 0000-0001-7083-7429; Laurent Peyrin-Biroulet 0000-0003-2536-6618; Kevin McHugh 0000-0001-9331-1614; Joannie Ruel 0000-0001-6996-0120.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Gastroenterology2022年34期
World Journal of Gastroenterology2022年34期
- World Journal of Gastroenterology的其它文章
- Therapeutic strategies for post-transplant recurrence of hepatocellular carcinoma
- Pregnancy and fetal outcomes of chronic hepatitis C mothers with viremia in China
- Spontaneous expulsion of a duodenal lipoma after endoscopic biopsy: A case report
- Trends in hospitalization for alcoholic hepatitis from 2011 to 2017: A USA nationwide study
- Analysis of invasiveness and tumor-associated macrophages infiltration in solid pseudopapillary tumors of pancreas
- Diagnosis, treatment, and current concepts in the endoscopic management of gastroenteropancreatic neuroendocrine neoplasms
