Long noncoding RNA ZNFX1-AS1 promotes the invasion and proliferation of gastric cancer cells by regulating LlN28 and CAPR1N1
Zhong-Ling Zhuo, Hai-Peng Xian, Yu-Jing Sun, Yan Long, Chang Liu, Bin Liang, Xiao-Tao Zhao
Abstract
Key Words: Long noncoding RNA; ZNFX1-AS1; Gastric cancer; Biomarker; Invasion; Proliferation
lNTRODUCTlON
Gastric carcinoma is the 2ndprimary cause of death due to carcinoma and the 6thmost common cancer worldwide. Approximately 1000000 new cases of gastric cancer are diagnosed each year, and approximately 800000 patients die from the disease, accounting for approximately 8.2% of global cancer-related mortality[1]. The progression-free survival (PFS) rate and prognosis of gastric carcinoma highly depend on the TNM stage of the disease. The high mortality is associated with the faultiness of standard screening protocols and lack of overt early symptoms[1]. Therefore, early detection and optimal treatment are necessary to improve the prognosis of gastric carcinoma. However, current biologic markers, such as carbohydrate antigen 199 (CA-199) and carcinoembryonic antigen (CEA), have low sensitivity and specificity[2]. Therefore, there is an urgent need to find novel biomarkers for early diagnosis of gastric cancer.
Long noncoding RNAs (lncRNAs) are a class of non-protein-coding RNA molecules > 200 nucleotides in length[3]. Recently, some studies have reported that lncRNAs are involved in protein modification and gene expression and that their dysregulation leads to a variety of genetic diseases[4-6]. Published articles have also shown that lncRNAs exhibit significant regulatory effects on transcription patterns in malignant tumors, some of which involve tumor cell invasion and metastasis, with a poor prognosis[6,7]. For example, H19 and SUMO1 pseudogene 3 (SUMO1P3) are upregulated in gastric cancer, while gastric cancer-associated transcript 1 is downregulated[7-11]. Additionally, HOTAIR overexpression may be associated with tumor escape mechanisms[12]. These studies strongly suggest that lncRNAs underlie the molecular etiology of gastric carcinoma. It is worth noting that the detection of circulating lncRNAs provides a new gastric biomarker for gastric cancer that is expected to be useful for monitoring and screening patients with gastric cancer[13].
Here, we selected 15 candidate cancer-associated lncRNAs. Among these lncRNAs, lncRNA ZNFX1-AS1 (ZFAS1) was found to be upregulated in the plasma of preoperative gastric cancer patients compared with healthy controls, and expression of ZFAS1 was significantly associated with lymphatic invasion, advanced TNM stage, and poor prognosis. We then investigated the biological effects of ZFAS1 on the survival, proliferation, and migration of gastric cancer cells. The underlying mechanism of ZFAS1 and the relationship between ZFAS1 and tumorigenesis were identified. These results showed that lncRNA ZFAS1 is a potential biomarker for gastric cancer.
MATERlALS AND METHODS
Sample collection and ethics statement
All samples were obtained from Peking University People’s Hospital between July 2015 and June 2016.Seventy-five matched (pre- and postoperative) whole blood and serum samples were collected from patients who underwent gastrointestinal surgery for gastric resection. In addition, intraperitoneal free cancer cell (IFCC) samples were collected from 60 of these patients. Other whole blood and serum specimens were collected from 60 gastric stromal tumor patients (before surgery), 60 gastritis or ulcer patients (before treatment), and 75 healthy controls. All specimens were collected with the corresponding patient information, such as age, sex, and collection time. In addition, the tumor size, tumor location (evaluated according to the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, Version II[14]), pathological type, degree of differentiation (based on the World Health Organization Classification of Tumors of the Digestive System, 4thedition[15]), TNM stage and lymphatic invasion status (based on the American Joint Committee on Cancer Staging Manual, 7thedition[16]) of the corresponding patients were collected.
Plasma and serum samples were collected in BD vacutainer ethylenediamine tetraacetic acid tubes and BD vacutainer somatostatin tubes (BD Biosciences, NJ, United States), respectively. Preoperative samples of patients with gastric cancer were obtained at least 2 mo before surgery or after radiotherapy or chemotherapy. Postoperative samples from these patients were collected 7-10 d after surgery.Samples from patients with gastric stromal tumor or gastritis/peptic ulcer were collected before any treatment was administered. The healthy control samples were randomly collected from normal subjects. For plasma samples, a special centrifugation protocol (2348 × g for 30 min at 4 °C; 4696 × g for 5 min at 4 °C; 10733 × g for 5 min at 4 °C) was performed to prevent contamination with cellular nucleic acids. Plasma and IFCC samples were stored at -80 °C in 3 volumes of TRIzol®reagent (Qiagen, CA,United States) for further analysis. Serum samples were analyzed directly[17]. This study was approved by the Research Ethics Committee of Peking University People’s Hospital. Patient data and samples were treated according to the ethical and legal criteria adopted in the 2013 Declaration of Helsinki.Written informed consent for ethical approval and patient consent was obtained from all participants.
RNA extraction and quantitative real-time polymerase chain reaction
This method is the same as that described in our previously published article[17]. LncRNA was extracted from plasma using a Direct-zol™ RNA MiniPrep R2050i Kit (Zymo Research, CA, United States) according to the manufacturer’s protocol. The concentration and purity of total RNA were measured using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, MA, United States).Total RNA was then solubilized using RNase-free water, and reverse transcription was immediately performed using PrimeScript RT Master Mix (Takara Bio, Kyushu, Japan) according to the manufacturer’s instructions. After reverse transcription, quantitative polymerase chain reaction (qPCR) was performed in a LightCycler 480 Instrument II (Roche Diagnostics, Basel, Switzerland) using TransStart Green qPCR SuperMix (TransGen, Beijing, China) according to the supplier’s instructions. The primers used for qPCR were designed with Primer Premier V 5.0 (Premier Biosoft International, CA, United States) and were synthesized by Beijing Sunbiotech Co., Ltd. (Beijing, China). The sequences of all primers, including those specific for GAPDH, are listed in Table 1. PCR was carried out with initial denaturation at 95 °C for 3 min followed by 40 cycles of 1 min at 95 °C and 1 min at 60 °C with subsequent detection. Due to the stability of GAPDH in plasma, it was chosen as the internal control for data standardization. For the calculations, the equation ΔCq= CqselectedlncRNA- CqGAPDHlncRNAwas used,where ΔCqwas defined as the difference in the quantification cycle (Cq) values. Every specimen was analyzed in triplicate, and the experiment was repeated 3 times.
Cell culture
The human gastric cancer cell lines BGC-823 and SGC-7901 were a gift from Peking University People’s Hospital Gastrointestinal Surgery Laboratory and were maintained in RPMI 1640 medium (Invitrogen,CA, United States) supplemented with 10% fetal bovine serum (FBS) (Gibco, CA, United States). The flask was incubated in a humidified incubator at 37 °C under 5% CO2in air.
LncRNA silencing and overexpression
ZFAS1-siRNA against ZFAS1 was synthesized by GenePharma (Shanghai, China). BGC-823 and SGC7901 cells were grown in 6-well plates (2 × 105cells/well) and transfected for 36 h using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, CA, United States). The ZFAS1 siRNA sequences were as follows: ZFAS1 siRNA1, 5’-AGACGCGAAAGAACGAAUGTT-3’; ZFAS1 siRNA2, 5’-UUACAAGGCAGACUGAAUCTT-3’; and ZFAS1 siRNA3, 5’-UAUGCAGGUAGGCAGUUAGTT-3’.The GAPDH siRNA and negative control siRNA sequences were as follows: GAPDH siRNA, 5'-ACGUGACACGUUCGGAGAATT-3' and negative control siRNA, 5'-ACGUGCAC AGUACUAGGAATT-3'.The ZFAS1 overexpression short hairpin RNA (ZFAS1-shRNA1), sh-ZFAS1 directed against ZFAS1 short hairpin RNA (ZFAS1-shRNA2), and NC-shRNA short hairpin RNA constructs were synthesized by GenePharma (Shanghai, China). The sequences of ZFAS1-shRNA1, ZFAS1-shRNA2, and NC-shRNA are shown in Table 2. BGC-823 and SGC7901 cells were grown in 6-well plates (2 × 105cells/well) and transduced for 36 h using Polybrene (GenePharma, REVG0001) and Enhanced Infection Solution(GenePharma, REVG0002).

Table 1 List of all primers used to screen gastric cancer-associated long noncoding RNAs
MTT assay
After transduction with NC-shRNA, ZFAS1-shRNA1, and ZFAS1-shRNA2, BGC823 and SGC7901 cells were trypsinized, and six replicates of 12 × 103cells per well were seeded in 96-well plates, with wells without cells used as blank wells. Proliferation was measured by an MTT (Sigma Aldrich, St. Louis, MO,United States) transformation assay. Briefly, 20 mL of MTT solution (5 mg/mL) was added to each well,and cells were grown for 4 h at 37 °C. After adding 100 mL of the dissolved solution (10% SDS in 0.01 M HCl solution), cells were further cultured at 37 °C for 3 h. The specific optical density of all wells was then measured at 490 nm.
Transwell migration assay
BGC823 and SGC7901 cells were suspended in serum-free medium after transduction with NC-shRNA,ZFAS1-shRNA1, and ZFAS1-shRNA2 and inoculated into transwell chambers with inserts of 8 μm pore size. Medium containing 10% FBS was placed in the bottom chamber. After 36 h of incubation, cells that migrated through the membrane to the lower surface were fixed with paraformaldehyde, stained with crystal violet, counted, and photographed. The results are shown as the average of three independent experiments.
Colony formation assay
BGC823 and SGC7901 cells transduced with NC-shRNA, ZFAS1-shRNA1, and ZFAS1-shRNA2 were seeded into 6-well plates (1000 cells per well) and cultured in a humidified incubator at 37 °C in 5% CO2for 10 d. The medium was changed every 3 d. At the end of the incubation period, the cultured cells were fixed with 4% paraformaldehyde and stained with crystal violet to assess the number of colonies.The results are shown as the average of three independent experiments.
Prediction and expression of ZFAS1 binding proteins
Binding proteins of lncRNA ZFAS1 were predicted based on the starBase V2.0 database (http://starbase.sysu.edu.cn). The primers specific for the mRNA sequences of the genes encoding the binding proteins were designed according to the nucleic acid sequence information for the corresponding proteins in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) with a primer design tool (http://www.ncbi.nlm.nih.gov/tools/primer-Blast). The sequences of all primers, including those specific for GAPDH, are listed in Table 3.

Table 2 The sequences of ZNFX1-AS1-shRNA1, ZNFX1-AS1-shRNA2 and NC-shRNA
NC-shRNA, ZFAS1-shRNA1, and ZFAS1-shRNA2 were transduced into BGC823 and SGC7901 cells,and mRNA was then reverse transcribed. The mRNA expression levels of the genes encoding the candidate lncRNA ZFAS 1 binding proteins were determined by quantitative real-time-PCR (qRT-PCR)using the primers listed in Table 3. The experimental method was the same as that described previously.
Protein extraction and enzyme-linked immunosorbent assay
Stably transfected cells, as well as tumor/lymph node tissue from BALB/c nude mice, were lysed with NP40 cell lysis buffer (Invitrogen, CA, United States) supplemented with SigmaFASTTMprotease inhibitor tablets (Sigma Aldrich, St. Louis, MO, United States). LIN28, CAPRIN1, CEA, and CK20 were detected by enzyme-linked immunosorbent assay (ELISA) using a Mouse Protein lin-28 homolog A(Lin28a) ELISA Kit (ELISAGenie, Dublin, Ireland), a Human Caprin 1 (CAPRIN1) ELISA Kit (Abbexa,Cambridge, United Kingdom), a CEA ELISA Kit (Bioss, United Kingdom) and CK20 ELISA kits (FKBIO,Chengdu, China), respectively.
In vivo tumor formation assay
Four-week-old female athymic BALB/c nude mice were maintained under specific pathogen-free conditions and operated on according to the protocol approved by the Beijing Medical Laboratory Animal Management Committee. NC-shRNA-, ZFAS1-shRNA1-, and ZFAS1-shRNA2-transduced BGC823 cells were harvested. For tumor formation assays, 107cells were injected subcutaneously into one side of each mouse. Tumor growth was examined every week, and the tumor volume was calculated using the equation V = 0.5 × D × d2 (V, volume; D, longitudinal diameter; d, latitudinal diameter). The expression of lncRNA ZFAS1 in BALB/c mouse blood was detected by qRT-PCR every week, and the primer sequences are listed in Table 1. The expression of LIN28 and CAPRIN1 proteins in BALB/c nude mouse tumors and the expression of CEA and CK20 in BALB/c nude mouse lymph nodes were detected by ELISA after four weeks. This study was conducted in strict accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The program was approved by the Animal Experiment Ethics Committee of Peking University People’s Hospital.
Statistical analysis
The formula 2-ΔΔCtwere used to calculate the levels of relative lncRNA expression in plasma[18]. Based on this formula, all relative lncRNA expression levels in plasma were evaluated. Continuous variables were described by the mean ± SD (normal distribution), or the median and interquartile range M (P25,P75) (non-normal distribution). Thet-test or Mann-WhitneyUtest was used according to the data distribution to identify statistically significant differences. For categorical variables, the percentages of patients in each category were calculated using theχ2test or Fisher’s exact test. In addition, receiver operating characteristic (ROC) curve analysis was performed to assess the diagnostic value of circulating lncRNA and traditional tumor markers in distinguishing between gastric carcinoma patients and healthy subjects. Statistical analysis was performed using SPSS software version 19.0 (IBM Corp.,Armonk, NY, United States).Pvalues less than 0.05 were considered significant.

Table 3 List of primers used to detect mRNA expression of long noncoding RNA binding proteins
RESULTS
Relative expression of a selected subgroup of lncRNAs (plasma) from 15 gastric cancer patients and 15 healthy control subjects
Fifteen matched samples from preoperative and postoperative gastric cancer patients and samples from 15 healthy control subjects were used as the sample cohort. The general characteristics and clinicopathological features of all subjects are provided in Table 4. The relative expression of the 15 lncRNAs,namely, uc001 Lsz/HOTAIR/CCAT1/H19/GACAT2/ABHD11-AS1/GACAT3 /SUMO1P3/CHET1/TUG1/SNHG12/GAS5/PVT1/LINC00152, and ZFAS1, was evaluated in the plasma of all subjects. As mentioned in Figure 1, the levels of lncRNA ZFAS1 in preoperative patient plasma were significantly higher than those in postoperative patient and healthy control subject plasma (P< 0.01).
Relative levels of the lncRNA ZFAS1 in the plasma of healthy control subjects, gastritis/peptic ulcer patients, GIST patients, and preoperative and postoperative gastric cancer patients
The general characteristics and clinicopathological features of 60 healthy control subjects, 60 gastritis/peptic ulcer patients, 60 GIST patients, and 60 patients with matched preoperative and postoperative data are summarized in Table 5. The relative levels of ZFAS1 in the plasma of all subjects were assessed by qRT-PCR. As shown in Figure 2A, the levels of lncRNA ZFAS1 in preoperative patient plasma were significantly higher than those in plasma from the individuals in the other four groups (P<0.01).
Relative levels of lncRNA ZFAS1 (plasma) in preoperative gastric carcinoma patients (different TNM stages)
We used the ΔΔCt formula to calculate the relative levels of lncRNA ZFAS1 in the samples (plasma) of patients with different TNM stages, including 20 patients with stage I and II disease and 40 patients with stage III and IV disease. As mentioned in Figure 2B, the median relative levels of lncRNA ZFAS1 in healthy controls, patients with stage I and II disease, and patients with stage III and IV disease were 0.81, 1.61, and 2.52, respectively. The relative levels in early- and advanced-stage patients were significantly higher than that in healthy controls (P< 0.01).
Relative levels of lncRNA ZFAS1 in 60 matched plasma samples from pre-operative and postoperative gastric carcinoma patients
Sixty matched pre-operative and post-operative samples (plasma) were included in our research. We found that the median relative levels of ZFAS1 before and after surgery were 2.22 and 1.01, respectively.The level decreased in 55 of the 60 gastric cancer patients (92%) approximately 10 d after surgery (P<0.01; Figure 2C).
Correlation between the relative level of lncRNA ZFAS1 in IFCC and plasma samples
Sixty matched preoperative patient plasma and IFCC samples were collected. We used the ΔΔCt method to evaluate the relative levels of ZFAS1 in plasma and IFCC. As shown in Figure 2D, the relative levels of ZFAS1 in plasma and IFCC were strongly positively correlated (R2= 0.76,P< 0.01).

Table 4 General characteristics and clinicopathological factors of gastric cancer patients and healthy control subjects
Diagnostic accuracy of plasma ZFAS1 and traditional serum biomarkers
ROC analysis revealed that the area under the curve (AUC) value of plasma ZFAS1 for discriminating gastric cancer patients from healthy control subjects was 0.85 (Figure 2E). The highest AUC value of any traditional serum biomarker was 0.63, while the AUC value of the other traditional biomarkers was lower than that of CEA. The highest accuracy of plasma ZFAS1 was obtained at a cutoff level of 1.00,where its sensitivity and specificity for identifying gastric carcinoma patients were 0.82 and 0.72,respectively.
ZFAS1 amplification is correlated with poor prognosis in gastric cancer
To explore the correlations between the relative ZFAS1 level and gastric cancer clinicopathological characteristics, we divided the patients into groups by sex, age, tumor site/size/lymphatic invasion/pathological differentiation status and TNM classification (Table 6). Data analysis indicated that the relative ZFAS1 expression level was strongly associated with tumor size (P= 0.01), TNM classification (P= 0.02), and lymphatic invasion (P= 0.03). We then divided patients into two groups by the median relative ZFAS1 level. Kaplan-Meier survival analysis was used to assess the potential correlation between the relative ZFAS1 expression level and patient prognosis. As shown in Figures 2F and G,patients with a high ZFAS1 level had shorter overall survival (OS) and PFS times than those with a low ZFAS1 level. This result suggests that ZFAS1 is a potential prognostic biomarker in gastric cancer patients.
ZFAS1 knockdown inhibited the viability, migration, and proliferation of gastric cancer cells
The expression level of ZFAS1 was positively correlated with lymph node metastasis, suggesting that ZFAS1 may be involved in tumor metastasis. Therefore, we studied the effect of ZFAS1 knockdown ongastric cancer cells. To verify the efficiency of ZFAS1 knockdown and prevent off-target effects, we first transfected three targeted siRNAs, ZFAS1-siRNA1, ZFAS1-siRNA2, and ZFAS1-siRNA3, into BGC823 and SGC7901 cells. Fluorescence microscopy was used to test the transfection efficiency. As shown in Figure 3, all three siRNAs significantly reduced the expression level of ZFAS1 in transfected BGC823 and SGC7901 cells. Next, we constructed ZFAS1-shRNA2 (with a sequence based on that of ZFAS1-siRNA2) and transduced it into BGC823 and SGC7901 cells. The MTT and colony formation assay results showed that compared to control cells transduced with NC-shRNA, BGC823 and SGC7901 cells with ZFAS1 knockdown showed significant decreases in viability and proliferation (Figures 4 and 5).The transwell migration assay showed that ZFAS1 knockdown in BGC823 and SGC7901 cells significantly inhibited cell migration (Figure 6). In summary, our data suggest that knockdown of ZFAS1 reduces the viability, proliferation, and migration of gastric cancer cells.
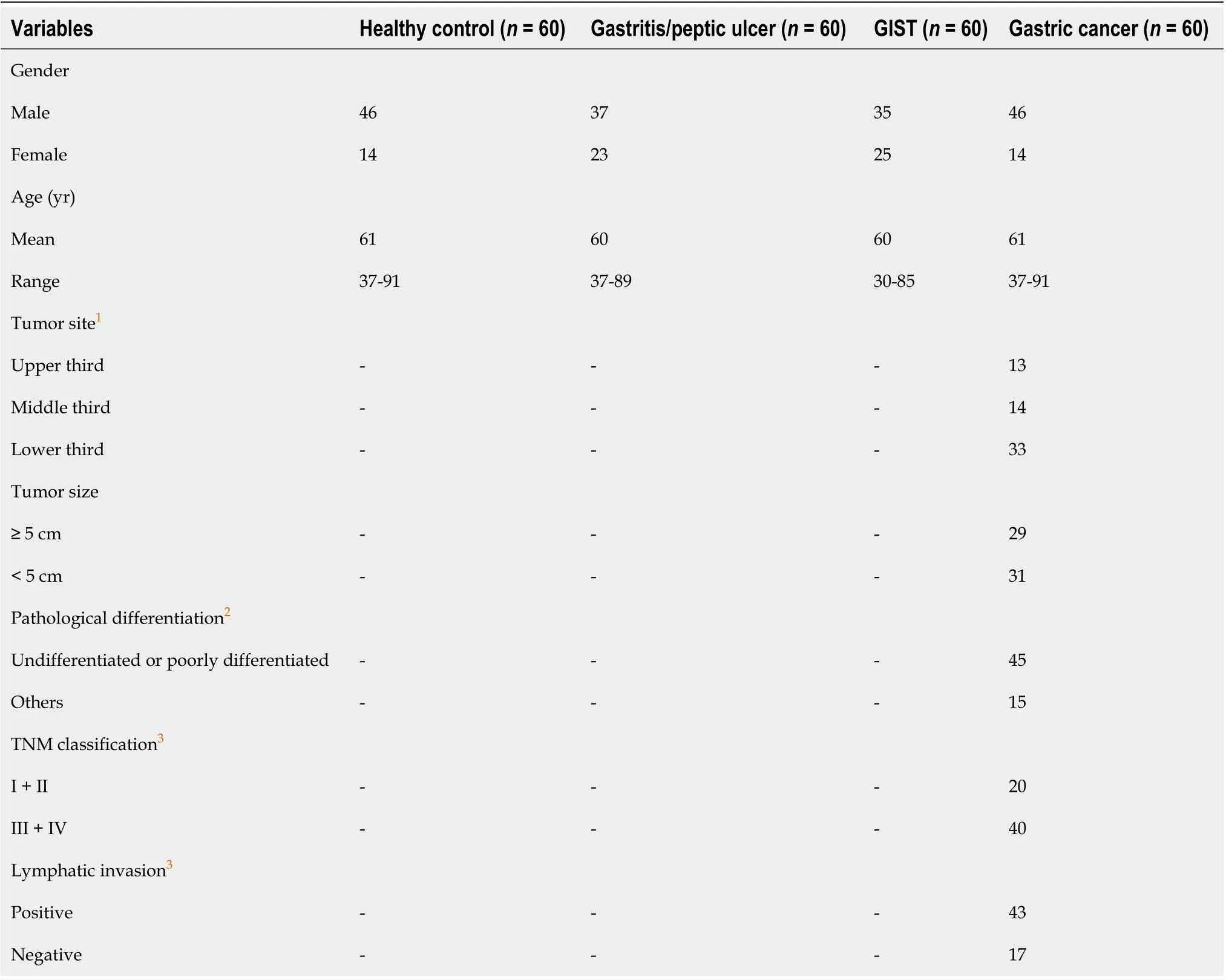
Table 5 General characteristics and clinicopathological factors of gastric cancer patients and healthy control subjects
ZFAS1 overexpression enhanced the viability, migration, and proliferation of gastric cancer cells
To further investigate the function of ZFAS1 in gastric cancer, we transduced ZFAS1-shRNA1 into BGC823 and SGC7901 cells to overexpress ZFAS1 and evaluated the transduction efficiency by fluorescence microscopy. As shown in Figure 7, the expression level of ZFAS1 was significantly increased in transduced BGC823 and SGC7901 cells. The MTT and colony formation assay results showed that compared to control cells transfected with NC-shRNA, BGC823 and SGC7901 cells overexpressing ZFAS1 showed significant increases in viability and proliferation (Figures 4 and 5). The transwell migration assay showed that ZFAS1 overexpression in BGC823 and SGC7901 cells significantly promoted cell migration (Figure 6). In summary, our data indicate that overexpression of ZFAS1 enhances the viability, proliferation, and migration of gastric cancer cells.

Table 6 Correlation between the relative expression of long noncoding RNA HULC or ZNFX1-AS1 and clinicopathologic factors
LIN28 and CAPRIN1 are the key downstream mediators of ZFAS1 in gastric cancer cells
To further investigate the mechanism by which lncRNA ZFAS1 affects the aggressiveness of gastric cancer, we used the starBase V2.0 database to analyze binding proteins of lncRNA ZFAS1[19]. The results showed that a total of 10 proteins had more than three binding sites for lncRNA ZFAS1. These proteins were UPF1, eIF4AIII, IGF2BP1, FMRP, LIN28, IGF2BP2, FUS, ZC3H7B, IGF2BP3, and CAPRIN1. We then analyzed the mRNA expression levels of these proteins in BGC823 and SGC7901 cells transduced with ZFAS1-shRNA1 and ZFAS1-shRNA2. Our goal was to determine which proteins exhibited changes in expression consistent with the change in ZFAS1 expression. The qRT-PCR results showed that ZFAS1 overexpression significantly increased the expression of LIN28 and CAPRIN1 (P<0.05) and that ZFAS1 knockdown significantly decreased the expression of LIN28 and CAPRIN1 (P<0.05) (Figure 8). ELISA showed the same results (Figure 9).
Association of ZFAS1 with the tumorigenesis of gastric cancer cells in vivo

Figure 1 Relative expression levels of a selected subset of plasma long noncoding RNAs in 15 matched preoperative and postoperative gastric cancer patients and 15 healthy control subjects. Scatter plot of long noncoding RNA relative expression levels in the plasma of preoperative patients (preoperative; n = 15), postoperative patients (postoperative; n = 15), and healthy control subjects (normal; n = 15), as assessed by quantitative polymerase chain reaction. The upper and lower bars indicate the ± SD values, and the middle bar indicates the median value. P values (aP < 0.05; bP < 0.01) were determined using the t-test.
To further investigate whether ZFAS1 overexpression or knockdown affects tumor growthin vivo,BGC823 cells stably transduced with NC-shRNA, ZFAS1-shRNA1 and ZFAS1-shRNA2 were inoculated into 4-wk female athymic BALB/c nude mice. The expression of lncRNA ZFAS1 in nude mice was detected weekly by qRT-PCR. The results showed that the expression of lncRNA ZFAS1 in the three groups of nude mice increased over time. The expression level in the ZFAS1-shRNA1 group was higher than that in the control group, and the expression level in the ZFAS1-shRNA2 group was lower than that in the control group (Figure 10A). Images of tumors from nude mice are shown in Figures 10B and C. Tumor size and weight were measured weekly; compared with tumors in the control group, tumors in the ZFAS1-shRNA1 group were significantly larger and heavier, and tumors in the ZFAS1-shRNA2 group were significantly smaller and lighter (Figures 10D and E). Next, in tumor tissue, ELISA confirmed that the expression of LIN28 and CAPRIN1 was significantly increased in tumors in the ZFAS1-shRNA1 group compared with those in the control group (P< 0.05), while the expression of LIN28 and CAPRIN1 was significantly decreased in tumors in the ZFAS1-shRNA2 group (P< 0.05)(Figure 10F). In addition, ELISA showed significant increases in CEA and CK20 expression in lymph nodes in the ZFAS1-shRNA1 group compared with the control group (P< 0.05) and significant decreases in CEA and CK20 expression in lymph nodes in the ZFAS1-shRNA2 group (P< 0.05)(Figure 10G), confirming the relationship between lncRNA ZFAS1 expression and lymph node metastasis in gastric cancer cells. In conclusion, our results indicate that ZFAS1 overexpression promotes the growth of gastric cancer cellsin vivoand that ZFAS1 knockdown inhibits the growth of gastric cancer cellsin vivo.

Figure 2 ZNFX1-AS1 is a potential diagnostic and prognostic biomarker. A: Scatter plot of long noncoding RNA (lncRNA) relative expression levels in the plasma of healthy control subjects (normal; n = 60), gastritis/peptic ulcer patients (Gs/PU patients; n = 60), gastric stromal tumor patients (GIST; n = 60),preoperative patients (pre-opt; n = 60), and postoperative patients (post-opt; n = 60), as assessed by real-time polymerase chain reaction (PCR); B: Scatter plot of lncRNA relative expression levels in the plasma of healthy control subjects (normal; n = 60), early-stage patients (TNM I + II; n = 20), and advanced-stage patients(TNM III + IV; n = 40), as assessed by real-time PCR; C: The endpoints indicate the relative expression levels of lncRNA ZNFX1-AS1 (ZFAS1) in preoperative (preopt) and postoperative (post-opt) patient plasma, while the lines connecting the pairs of endpoints indicate the trends in the relative expression levels in the matched preoperative and postoperative patient plasma; D: The linear correlations between the relative expression of lncRNAs in plasma and infrequent clonal complexes were analyzed. P values (aP < 0.05; bP < 0.01; cP < 0.001) were determined using the t-test; E: Receiver operating characteristic curves showing the area under the curve values of plasma ZFAS1 and traditional serum biomarkers; F: Progression-free survival times indicating the potential prognostic value of ZFAS1 in gastric cancer patients; G: Overall survival times indicating the potential prognostic value of ZFAS1 in gastric cancer patients. Gs/PU: Gastritis/peptic ulcer patients; GIST:Gastric stromal tumor; AUC: Area under the curve; CEA: Carcinoembryonic antigen; AFP: Alpha fetoprotein; CA-199: Carbohydrate antigen 199; OS: Overall survival;PFS: Progression-free survival.
DlSCUSSlON
Gastric cancer is a common malignancy that is particularly difficult to diagnose early[20]. However,traditional biomarkers have low specificity and low sensitivity. As lncRNA research has intensified,many lncRNAs have been determined to be involved in the development, progression, and prognosis of gastric cancer[21], but their diagnostic value as gastric cancer markers needs further study. By reviewing the literature, we selected 15 candidate lncRNAs that have high expression in gastric tumor tissues. Next, the relative levels of these 15 candidate lncRNAs were evaluated in the plasma of 15 normal subjects and 15 matched samples from preoperative and postoperative patients with gastric cancer. The results showed that the plasma level of only ZFAS1 was significantly higher in preoperative gastric cancer patients than in normal subjects and postoperative gastric cancer patients (Figure 1).Therefore, we selected ZFAS1 as a follow-up research object. Moreover, we confirmed that lncRNAs can exist stably in peripheral blood, consistent with the findings of Liet al[22]. Therefore, lncRNA ZFAS1 may be a potential plasma biomarker for gastric cancer.
We recollected plasma from 60 healthy control subjects, 60 gastritis/peptic ulcer patients, 60 GIST patients, and 60 matched preoperative and postoperative patients to further validate the aforementioned results. Our data indicated that the expression of ZFAS1 in the peripheral blood of patients with gastric cancer was significantly higher than that of healthy controls and patients with benign gastrointestinal disease (Figure 2A), consistent with previous reports. Our data also showed that ZFAS1 is significantly associated with advanced TNM stage and lymphatic invasion (Figure 2B). There was a sharp decrease in the relative level of ZFAS1 in peripheral blood after surgery (Figure 2C). We also evaluated traditional serological markers such as CEA, CA-199, and alpha fetoprotein in the 60 preoperative patients. Compared with traditional biomarkers, plasma lncRNA ZFAS1 had higher sensitivity and specificity (Figure 2E). In addition, the relative level of ZFAS1 in plasma was significantly correlated with that in IFCCs (Figure 2D). This indicates that lncRNA ZFAS1 is released into the peripheral blood by gastric tumors. This suggests that ZFAS1 may promote the progression of gastric cancer. Both the PFS and OS analysis results showed (Figures 2F and G) that a high level of lncRNAs in peripheral blood was not favorable for patients. Thus, lncRNAs may be used not only for early diagnosis but also for postoperative evaluation of patients.

Figure 3 Fluorescence microscopy was used to verify the transfection efficiency of ZNFX1-AS1-siRNA1, ZNFX1-AS1-siRNA2 and ZNFX1-AS1-siRNA3. A: The same position of BGC823 cells and SGC7901 cells under a normal microscope and fluorescence microscope, respectively; B: The relative expression of long noncoding RNA ZNFX1-AS1 (ZFAS1) in five groups of cells (None, NC-siRNA, ZFAS1-siRNA1, ZFAS1-siRNA2, ZFAS1-siRNA3) was evaluated by quantitative reverse transcription polymerase chain reaction. ZFAS1: ZNFX1-AS1. aP < 0.05; bP < 0.01.

Figure 4 MTT assays were performed to determine the viability of ZGCS1-shRNA1- and ZNFX1-AS1-shRNA2-transduced BGC823 and SGC7901 cells. OD: Optical density.

Figure 5 Colony formation assays were performed to determine the proliferation of BGC823 and SGC7901 cells transduced with ZNFX1-AS1-shRNA1 and ZNFX1-AS1-shRNA2. A: BGC823 cells and SGC7901 cells were subjected to colony formation assay after transduction, and the number of spots was analyzed; B: The number of spots for the three groups [NC-shRNA, ZNFX1-AS1 (ZFAS1)-shRNA1, ZFAS1-shRNA2] in the colony formation assays were compared. ZFAS1: ZNFX1-AS1. aP < 0.05; bP < 0.01.

Figure 6 Transwell migration assays were performed to determine the migration ability of ZNFX1-AS1-shRNA1- and ZNFX1-AS1-shRNA2-transduced BGC823 and SGC7901 cells. A: BGC823 cells and SGC7901 cells were subjected to transwell migration assay after transduction, and the number of cell was calculated; B: The number of cells for the three groups [NC-shRNA, ZNFX1-AS1 (ZFAS1)-shRNA1, ZFAS1-shRNA2] in the transwell migration assays were compared. aP < 0.05; bP < 0.01. ZFAS1: ZNFX1-AS1.
In addition, ZFAS1 is reported to be involved in metabolism, prognosis, and cell proliferation in liver cancer[23,24]. It has been reported that the expression of ZFAS1 in gastric cancer tissues is significantly higher than that in adjacent tissues and that ZFAS1 expression is related to the prognosis of gastric cancer patients[25,26]. siRNA transfection techniques were used to determine the appropriate lncRNA ZFAS1 interference sequence. We used three types of siRNAs (ZFAS1 siRNA1, ZFAS1 siRNA2, and ZFAS1 siRNA3) to interfere with the expression of ZFAS1 in cell lines to prevent off-target effects. All these siRNAs significantly reduced the expression of lncRNA ZFAS1 in BGC823 cells and SGC7901 cells(Figure 3). We finally selected the ZFAS1 siRNA2 sequence to construct ZFAS1-shRNA2 and successfully generated BGC823 cells and SGC7901 cells with low expression of lncRNA ZFAS1. BGC823 cells and SGC7901 cells with stable knockdown of lncRNA ZFAS1 were also successfully cultured. We further employed loss-of-function and gain-of-function studies to assess the role of ZFAS1 in gastric cancer cell proliferation and migration by MTT, transwell, and colony formation assays (Figures 4, 5 and 6). We observed that ZFAS1 knockdown inhibited gastric cancer cell proliferation and migration,whereas ZFAS1 overexpression had the opposite effects.
We then studied the mechanism by which ZFAS1 affects the proliferation and invasion of gastric cancer cells. The starBase V2.0 database was used to analyze the lncRNA ZFAS1 binding proteins[19].Only 10 proteins had more than three binding sites for lncRNA ZFAS1. We found that the changes in the mRNA expression of LIN28 and CAPRIN1 were consistent with the change in ZFAS1 expression in gastric cancer cells (Figure 8). The LIN28 protein is thought to play an important role in gastrointestinal tumors[27,28]. In colorectal cancer, LIN28 has been shown to enhance tumor cell invasionviathe Wnt pathway, whereas overexpression of LIN28 also recruits the microRNA let-7 to enhance tumor cell metastasis[28]. In gastric cancer, LIN28 affects the human epidermal growth factor receptor 2 level through posttranscriptional regulation, which in turn affects the invasive ability of gastric cancer cells.In addition, LIN28 is an independent risk factor for the prognosis of gastric cancer. This result suggests that LIN28 may be a key protein downstream of lncRNA ZFAS1 and that lncRNA ZFAS1 may enhance the proliferation and invasion of gastric cancer cells by regulating LIN28. CAPRIN1 is thought to be involved in cell invasion in a variety of tumors[29-31]. In osteosarcoma, CAPRIN1 has been shown to cause cisplatin resistance and abnormal apoptosis of tumor cellsviathe Akt pathway and the ERK1/2 pathway[31], and it can also be used as an independent risk predictor for breast cancer. The changes in the mRNA expression of LIN28 and CAPRIN1 were consistent with knockdown and overexpression of ZFAS1, whereas LIN28 and CAPRIN1 were significantly associated with tumor invasion, which explains the mechanism connecting ZFAS1 with lymphatic invasion in tumor patients. ZFAS1 has been shown to silence the expression of Kruppel-like factor 2 and naked cuticle homolog 2 by binding to polycomb repressive complex 2 and lysine-specific demethylase 1, leading to the development of gastric cancer[25]. In addition, it promotes the development of liver cancer tumor cells[23]. CAPRIN1 may play a key role in the regulation of tumor cell proliferation and invasionvialncRNA ZFAS1.

Figure 7 Fluorescence microscopy was used to test the transduction efficiency of ZNFX1-AS1-shRNA1 and ZNFX1-AS1-shRNA2. A: The same position of BGC823 cells and SGC7901 cells under a normal microscope and fluorescence microscope, respectively; B: The relative expression of long noncoding RNA ZNFX1-AS1 (ZFAS1) in four groups of cells (None, NC-shRNA, ZFAS1-shRNA1, ZFAS1-shRNA2) was evaluated by quantitative reverse transcription polymerase chain reaction. ZFAS1: ZNFX1-AS1. aP < 0.05; bP < 0.01.

Figure 8 The relative mRNA levels of genes encoding ZNFX1-AS1 binding proteins in transduced BGC823 and SGC7901 cells were determined by quantitative reverse transcription polymerase chain reaction. A: UFP1; B: eIF4AIII; C: IGF2BP1; D: FMRP; E: LIN28; F: IGF2BP2; G:FUS; H: ZC3H7B; I: IGF2BP3; J: CAPRIN1. AFAS1: ZNFX1-AS1. aP < 0.05; bP < 0.01.

Figure 9 The relative protein levels of LlN28 and CAPRlN1 in transduced BGC823 and SGC7901 cells were determined by enzyme-linked immunosorbent assay. A: The levels of CAPRIN1 in transduced BGC823 cells; B: The levels of CAPRIN1 in transduced SGC7901 cells; C: The levels of LIN28 in transduced BGC823 cells; D: The levels of LIN28 in transduced SGC7901 cells. aP < 0.05; bP < 0.01.

Figure 10 Effect of ZNFX1-AS1 on gastric cancer cell tumorigenesis in vivo. A: The expression of long noncoding RNA ZNFX1-AS1 in BALB/c nude mice was detected weekly by quantitative reverse transcription polymerase chain reaction; B and C: Images of tumors from BALB/c nude mice; D and E: Tumor size and weight were measured weekly; F: Expression of LIN28 and CAPRIN1 in tumors of BALB/c nude mice; G: Expression of carcinoembryonic antigen and CK20 in lymph nodes of BALB/c nude mice. CEA: Carcinoembryonic antigen; ZFAS1: ZNFX1-AS1. aP < 0.05; bP < 0.01; cP < 0.001.
In this study, we validated the above hypothesis by establishing tumor-bearing mice. The expression level of lncRNA ZFAS1 in the plasma of mice injected with tumor cells with high expression of lncRNA ZFAS1 was significantly higher than that in mice in the other two groups (Figure 10A). Thein vivoexperiments also indicated that ZFAS1 overexpression promoted gastric cancer cell proliferation and migration, whereas ZFAS1 knockdown had the opposite effect (Figures 10B-E). The data also demonstrated that LIN28 and CAPRIN1 are still regulated by lncRNA ZFAS1 in mice (Figure 10F).CK20 and CEA are classical biomarkers of gastric cancer cells in tissues. The CEA and CK20 levels in the lymph nodes of nude mice injected with tumor cells with high expression of lncRNA ZFAS1 were significantly higher than those in mice in the other two groups (Figure 10G). This further demonstrated that high expression of lncRNA ZFAS1 may enhance the invasion of gastric cancer cells.
CONCLUSlON
In conclusion, we demonstrated in this study that the lncRNA ZFAS1 level is increased in the plasma of gastric cancer patients. LncRNA ZFAS1 promotes the invasion and proliferation of gastric cancer cells by modulating LIN28 and CAPRIN1 expression. These findings indicate that ZFAS1 plays an oncogenic role in gastric cancer and can be used as a potential diagnostic biomarker and a new therapeutic target for gastric cancer.
ARTlCLE HlGHLlGHTS
Research background
Long noncoding RNA (lncRNA) ZNFX1-AS1 (ZFAS1) is a newly discovered lncRNA, but its diagnostic value in gastric cancer is unclear.
Research motivation
We investigated the biological effects of ZFAS1 on the survival, proliferation, and migration of gastric cancer cells.
Research objectives
This study aimed to investigate the potential role of ZFAS1 in gastric cancer and to evaluate the clinical significance of ZFAS1 as a biomarker for gastric cancer screening.
Research methods
RNA extraction, quantitative realtime polymerase chain reaction, lncRNA silencing and overexpression,MTT assay, transwell migration assay, Colony formation assay, protein extraction, immunosorbent assay, andin vivotumor formation assay were performed in this study.
Research results
ZFAS1 amplification was correlated with poor prognosis in gastric cancer. ZFAS1 knockdown inhibited the viability, migration, and proliferation of gastric cancer cells. ZFAS1 overexpression enhanced the viability, migration, and proliferation of gastric cancer cells. LIN28 and CAPRIN1 were the key downstream mediators of ZFAS1 in gastric cancer cells. ZFAS1 was associated with the tumorigenesis of gastric cancer cellsin vivo.
Research conclusions
LncRNA ZFAS1 is a potential biomarker for gastric cancer.
Research perspectives
ZFAS1 plays an oncogenic role in gastric cancer and can be used as a potential diagnostic biomarker and a new therapeutic target for gastric cancer.
ACKNOWLEDGEMENTS
The authors would like to thank Peking University People’s Hospital Gastrointestinal Surgery Laboratory for providing the cell lines.
FOOTNOTES
Author contributions:Zhuo ZL analyzed the experimental data and completed the draft of the manuscript; Xian HP completed all of the experiments; Sun YJ, Long Y, and Liu C collected all of the clinical data; Liang B and Zhao XT are responsible for designing the work and for final approval of the version to be published.
Supported byBeijing Natural Science Foundation, No. 7172225.
lnstitutional review board statement:This study was approved by the Research Ethics Committee of Peking University People’s Hospital. Patient data and samples were treated according to the ethical and legal criteria adopted in the 2013 Declaration of Helsinki. Written informed consent for ethical approval and patient consent was obtained from all participants.
lnstitutional animal care and use committee statement:This study was approved by the Peking University People’s Hospital Animal Use Protocol & Ethic Review.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ARRlVE guidelines statement:The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Zhong-Ling Zhuo 0000-0001-9284-4785; Xiao-Tao Zhao 0000-0001-6104-8989.
S-Editor:Wang JJ
L-Editor:Webster JR
P-Editor:Chen YX
 World Journal of Gastroenterology2022年34期
World Journal of Gastroenterology2022年34期
- World Journal of Gastroenterology的其它文章
- Therapeutic strategies for post-transplant recurrence of hepatocellular carcinoma
- Pregnancy and fetal outcomes of chronic hepatitis C mothers with viremia in China
- Spontaneous expulsion of a duodenal lipoma after endoscopic biopsy: A case report
- Trends in hospitalization for alcoholic hepatitis from 2011 to 2017: A USA nationwide study
- Analysis of invasiveness and tumor-associated macrophages infiltration in solid pseudopapillary tumors of pancreas
- lmpact of adalimumab on disease burden in moderate-to-severe ulcerative colitis patients: The one-year, real-world UCanADA study
