Mg掺杂对二维自旋系统Cu3B2O6磁化率的研究
何 顺(1.苏州科技大学 物理科学与技术学院,江苏 苏州 215009;2.江苏省微纳热流技术与能源应用重点实验室,江苏 苏州 215009)
1 Introduction
Recently low dimensional quantum spin systems receive much attention because spin-singlet ground states with the spin gaps in these systems are closely related to the mechanism of the high-Tcsuperconductivity.For one-dimensional spin system,many compounds have already been discovered.For example,it has been reported for the Haldane gap in PbNi2V2O8[1],spin-Peierls transition in CuGeO3[2]and spin ladder structure in SrCu2O3[3].But for two-dimensional spin system,only a few systems with singlet ground state were found,such as the plaquette system CaV4O9[4-5]and the orthogonal dimer system SrCu2(BO3)2[6].The classical magnetically ordered state is normally considered more stable than the quantum disordered state.So finding compounds of two-dimensional spin system with a spin gap becomes a valuble task for physicists.
Cu3B2O6has a quasi two-dimensional structure and belongs to the copper oxide compounds.Many research works have already been conducted on this system.Petakovskii et al first measured the temperature dependence of the magnetic susceptibility of the single crystal and suggested that the ground state is possibly a singlet ground state[7].Kudo et al measured the magnetic susceptibilities in a magnetic field of 1 T and the specific heat in magnetic fields of 0~9 T for single crystals,then claimed that there exists a three dimensional antiferromagnetic phase transition around the transition temperature[8].Their group also measured the magnetization curve in mag netic fields up to 30 T and the thermal conductivity in magnetic fields up to 14 T.Then it was concluded that the thermal conductivity strongly correlates with the spin state[9].Fukaya et al observed an oscillation of muon spinsat low temperatures,which indicates that the ground state is a long-range-ordered one[10].They thought that the magnetic transition is not an ordinary second-order phase transition because a critical slowing down of the fluctuations of electronic spins is not observed near the transition temperature.By measuring the magnetic susceptibility,heat capacity,neutron scattering,muon spin relaxation and electron paramagnetic resonance in Cu3B2O6,Petakovskii et al concluded that the spin subsystem of the compound undergoes a transition to a state representing a superposition of the singlet(for clusters)and magnetically ordered(for single spins)states[11].
The effect of the Zn-substitution of the two-dimensional spin system Cu3B2O6has also been carefully studied.Sakurai et al prepared the powder samples of(Cu1-xZnx)3B2O6and measured the magnetic susceptibilities under the applied magnetic field of 0.5 T[12].Nuclear magnetic resonance(NMR)measurements have also been performed,which suggested that the magnetic system has a modulated spin-density-wave ground state,rather than a simple antiferromagnetically ordered one.Kudo et al investigated the thermal conductivity in the(Cu1-xZnx)3B2O6single crystals and found that the anomalous peak observed in Cu3B2O6is largely reduced after Zn substitution[13].They concluded that the peak in Cu3B2O6is considered as being due to the enhancement of magnon.
In this paper,we design an experiment to observe the Mg-substitution effect in the two-dimensional antiferromagnetic system Cu3B2O6.The magnetic susceptibilities with different doping levels will be carefully investigated.One of the chief aims is to find the difference between the magnetic susceptibilities of Mg-substitution and Zn-substitution.This will help us to understand the ground state of the Cu3B2O6system.
2 Experimental details
Cu3B2O6has a two-dimensional crystal structure with triclinic symmetry.It belongs to space group P1ˉ[7].As shown in Fig.1,all the magnetic Cu2+ion in the 2D layer are surrounded by O2-ions,which form octahedrons,squares and pyramids.The mean Cu-O distance in the bc plane is much smaller than the minimum Cu-O distance between layers.Thus we may consider the system as a quasi-two-dimensional spin system.
The single crystal growth was carried out at University of Toronto,using the floating zone method.Powder CuO with purity 99.995%,B2O3with purity 99.98% and MgO with purity 99.95%are mixed together to prepare the compound(Cu1-xMgx)3B2O6.Excess B2O3of 3% in molecular ratio were added to compensate the evaporation of B2O3.The mixed powder was heated in the air at 900℃for 24 hours and cooled down to room temperature naturally.In order to have a well-reacted sample,the powder were ground again and heated at 900℃for 24 hours twice.The final(Cu1-xMgx)3B2O6compounds have dark green color.
To make a feed rod,powder in the latex balloon with the steel frame of 8 mm diameter and the 130 mm length was isostatically cold-pressed at 7.8 MPa for several minutes.The shaped powder rod was fired at 950℃in air for 12 hours.The crystal growth process is performed in an infrared heating furnace with a double ellipsoidal mirror.In our experiments solvent is not necessary,we choose single crystal Cu3B2O6as the seed for different doping levels.The upper and lower shafts were rotated in the opposite directions with a speed of 10 r·min-1and the liquid in the molten zone can be heated homogeneously.The growth rate was set as 20 mm·h-1,and thereal process was performed in the oxygen gas of 0.8 MPa to prevent the B2O3from evaporating.At the beginning and the end of the crystal growth,the heating and cooling rate are set as 0.20 V·min-1and 0.05 V·min-1,respectively.Finally four crystals with different doping levels were grown.The magnetic susceptibilities of the single crystals were measured from 2 K to 100 K,using a Superconducting Quantum Interference Device(Quantum Design,Model MPMS).
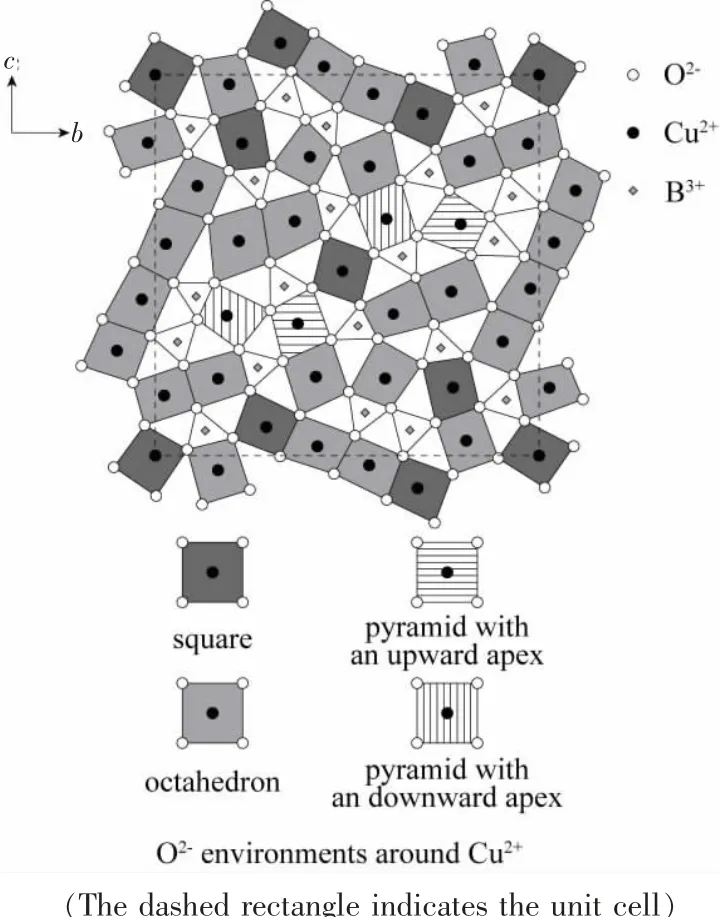
Fig.1 Crystal structure in the bc plane of the Cu3B2O6
3 Results and discussion
Fig.2 shows the temperature dependence of the magnetic susceptibility χ of single crystal Cu3B2O6in a magnetic field of 0.5 T paralleling to the bc plane.The magnetic field was applied after zero field cooling(ZFC).For the single crystal Cu3B2O6,the magnetic susceptibility exhibits a magnetic transition temperature at 10 K.It also shows a broad peak around 40 K and a small sink around 4 K.These features are in good agreement with the previous reports[7-12].The sharp drop of the susceptibility below the transition temperature suggests that the ground state of Cu3B2O6is an antiferromagnetically one,with the Neel temperature TN=10 K.The rise of the susceptibility below 4 K is considered to be due to the lattice defects and impurities.The susceptibility between 2 K and 10 K was estimated from the data fitting by the following equation

where the first term represents the component of the susceptibility with a finite energy gapΔ,the Cuire-Weiss term due to the impurities,and the constant term independent of the temperature.The energy gap ofΔwas estimated to be 33.3 K,which agrees with the previous reports[7,12].The value of A,Ci,θiand χ0were found to be 4.46×10-2emu·mol-1,4.14×10-1emu·K·mol-1,-108.85 K and-2.46×10-3emu·mol-1,respectively.
The effects of the partial substitution of Mg for Cu are also shown in Fig.3.Since Mg2+is nonmagnetic ion with spin quantum number S=0,replaceing Mg2+by Cu2+ion(S=1/2)will gradually destroy the antiferromagnetic order.The magnetic susceptibilities develop systematically with x’s changing.For x=0.02,the Neel temperature becomes hard to find and the material is almost paramagnetic.In the case of x=0.04,the transition temperature totally disappears and the material becomes purely paramagnetic.In contrast to the two-dimensional square lattice,which needs 0.35~0.40 substitution of nonmagnetic ion to destroy the antiferromagnetic order,Cu3B2O6system only needs 0.02~0.04 to reach the same result.
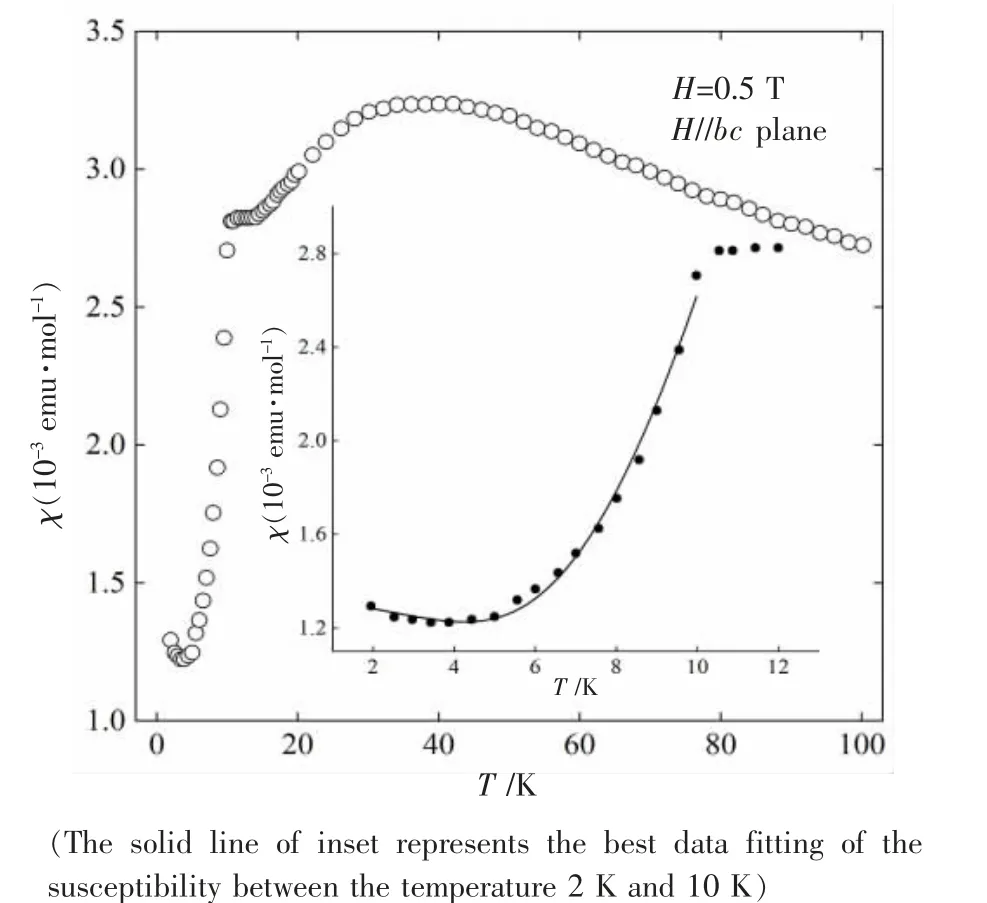
Fig.2 Temperature dependence of the magnetic susceptibility of single crystal Cu3B2O6 in a magnetic field of 0.5 T parallel to the bc plane
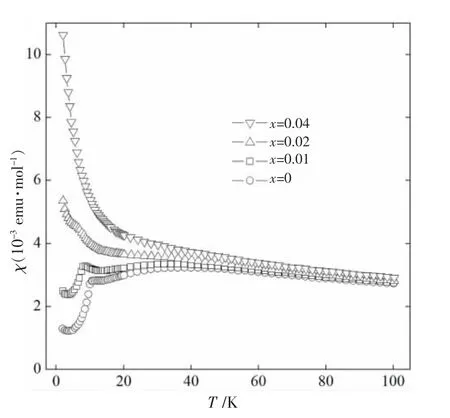
Fig.3 Temperature dependences of the magnetic susceptibilities in a magnetic field of 0.5 T parallel to the bc plane of(Cu1-xMgx)3B2O6
The Zn-substitution effect on the physical properties of the Cu3B2O6system has already been reported[12-13].A comparison can be made between the Mg2+doping and the Zn2+substitution.Because Zn2+is also nonmagnetic ion with spin quantum number S=0,substituting Zn2+for Cu2+has the similar effect with Mg2+substitution.The small difference is:for Zn2+doping,a broad maximum of susceptibility still exists in the case of x=0.02[12],while in our results case x=0.02 becomes almost paramagnetic.This means that we can dope less Mg than Zn to destroy the antiferromagnetic order in the two-dimensional Cu3B2O6system.
Fig.4 exhibits the dependence of the Neel temperature TNof(Cu1-xMgx)3B2O6on the doping level x.The function of fitting line is TN(x)=-249x+11.0.We can also calculate the rate of TNsuppression
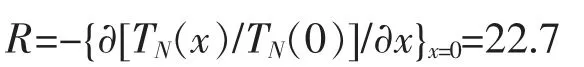
This result is almost the same as that in Zn2+substitution.The fitting line for Zn2+is TN(x)=-250x+10.9,and TNsuppression rate is R=22.9[12].Compared with the errors of fitting,the difference between two fitting lines is relatively small.We can conclude that Mg2+and Zn2+substitution have almost the same effect on the suppression of the Neel temperature TNin the antiferromagnetic Cu3B2O6system.
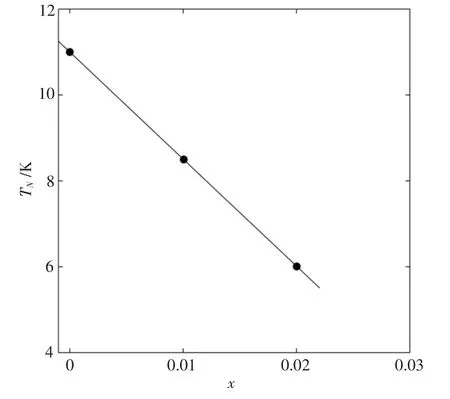
Fig.4 The doping level x of the Neel temperature of(Cu1-xMgx)3B2O6 and the fitting line for the points
4 Summary
The ground state of the two-dimensional spin system Cu3B2O6is still not clear.The transition temperature of the magnetic susceptibility observed in the bc plane suggests that the system may have the antiferromagnetic order.We have grown four single crystals of(Cu1-xMgx)3B2O6with different doping level(x=0,0.01,0.02 and 0.04)and measured the magnetic susceptibilities.For single crystal Cu3B2O6,our result agrees well with the previous reports.When the doping level x increases,the doping results show that the antiferromagnetic order in the material is gradually destroyed.These phenomena are similar to those for the Zn doping reported in the previous paper.In the future,we will do the neutron scattering of single crystals(Cu1-xMgx)3B2O6to examine the spin excitation in the system.Also we will try to dope Li in the Cu3B2O6system to reveal the ground state.
This work was supported by Jiangsu Key Disciplines of the Fourteenth Five-Year Plan(Grant No.2021135).

