Hyperbenzones A and B, two 1,2-seco and rearranged polycyclic polyprenylated acylphloroglucinols from Hypericum beanii
Weijia Lu, Yanqiu Zhang, Yawei Li, Shengtao Ye, Jun Luo, Lingyi Kong, Wenjun Xu
Jiangsu Key Laboratory of Bioactive Natural Product Research and State Key Laboratory of Natural Medicines, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 210009, China
ABSTRACT Two novel seco-polycyclic polyprenylated acylphloroglucinols (PPAPs), hyperbenzones A (1) and B (2),were isolated from the roots of Hypericum beanii, together with one known biosynthetic congener 3.Compound 1 incorporates a 6/5/5 ring system with an unprecedented spiro[bicyclo[3.3.0]octane-3,1ʹ-cyclohexane]-2,2ʹ–dione motif.The structures of 1 and 2 were determined by a combination of high resolution electrospray ionization mass spectroscopy (HRESIMS), nuclear magnetic resonance (NMR) spectroscopic analyses, gage-independent atomic orbital (GIAO) NMR chemical shift calculation with DP4+analyses, electronic circular dichroism (ECD) calculation, and X-ray diffraction analysis.A 1,2-seco retro-Claisen rearrangement from a bicyclo[3.3.1]nonane PPAP precursor and following chemodivergent radical cascade cyclizations are proposed as the key steps in the biosynthetic pathway to yield compounds 1 and 2.Biological investigations indicated that compounds 1 and 3 could decrease intracellular lipid accumulation in a palmitic acid-induced nonalcoholic steatohepatitis (NASH) cell model.
Keywords:seco-PPAPs Hypericum beanii Hyperbenzones A and B NASH 1,2-seco-Bicyclic derivatives
Polycyclic polyprenylated acylphloroglucinols (PPAPs), most of which feature highly oxygenated and densely substituted bicyclo[3.3.1]nonane-2,4,9-trione core, have become one of the hotspots in the natural products research field [1–3].The enolizableβ-diketones andβ,βʹ-triketones in the core are versatile units to undergo ring-opening and further rearrangement reactions, which have strikingly expanded the structural complexity and diversity of PPAPs [4,5].Up to date, 40 bicyclo[3.3.1]nonanederivedseco-PPAPs, including 1,2-seco, 2,3-seco, 3,4-seco, 1,9-seco,1,2;4,5-diseco, and their highly cyclized derivatives with intriguing architectures, have been reported [2,6-10].It is noting thatseco-PPAPs exhibit a wide range of biological activities, including antiinflammatory [8], anti-tumor [9,10], acetylcholinesterase (AChE) inhibitory activities [11], and multidrug resistance reversal activity to adriamycin resistant cancer cell lines [12].
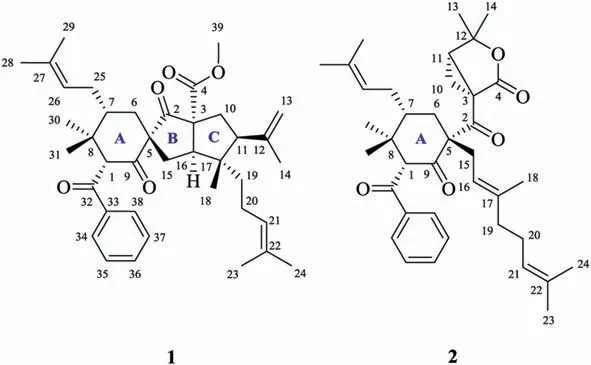
Fig.1.Structures of hyperbenzones A (1) and B (2).
In our continuous high-performance liquid chromatographydiode array detection (HPLC-DAD) and proton nuclear magnetic resonance (1H NMR) guided pursuit of benzoyl-PPAPs fromHypericum beanii(H.beanii) [13,14], hyperbenzones A (1) and B(2), together with one known biosynthetic congener otogirinin D(3) [15] were isolated (Fig.1 and Scheme 1).Notably, hyperbenzone A (1) is the first example of 1,2-seco-PPAP with an unprecedented spiro[bicyclo[3.3.0]octane-3,1ʹ-cyclohexane]-2,2ʹ–dione motif, while hyperbenzone B (2) possess an unusual 4,4-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one moiety.Compounds 1 and 2 could biogenetically arise from an assumed bicyclo[3.3.1]nonane PPAP precursorvia1,2-secoretro-Claisen rearrangement and chemodivergent radical cascade cyclizations.Determination of the planar structures of 1 and 2 based on high resolution electrospray ionization mass spectroscopy (HRESIMS) and two-dimensional nuclear magnetic resonance (2D NMR) experiments, while the establishment of their absolute configurations was confirmed by X-ray diffraction analysis, and gage-independent atomic orbital (GIAO)NMR chemical shift calculations with DP4+ analyses, and comparison of the experimental and calculated electronic circular dichroism (ECD) data.Detailed herein are the isolation, structural elucidation, and the plausible biosynthetic pathway of compounds 1–3 along with theirin vitroeffects against nonalcoholic steatohepatitis(NASH).
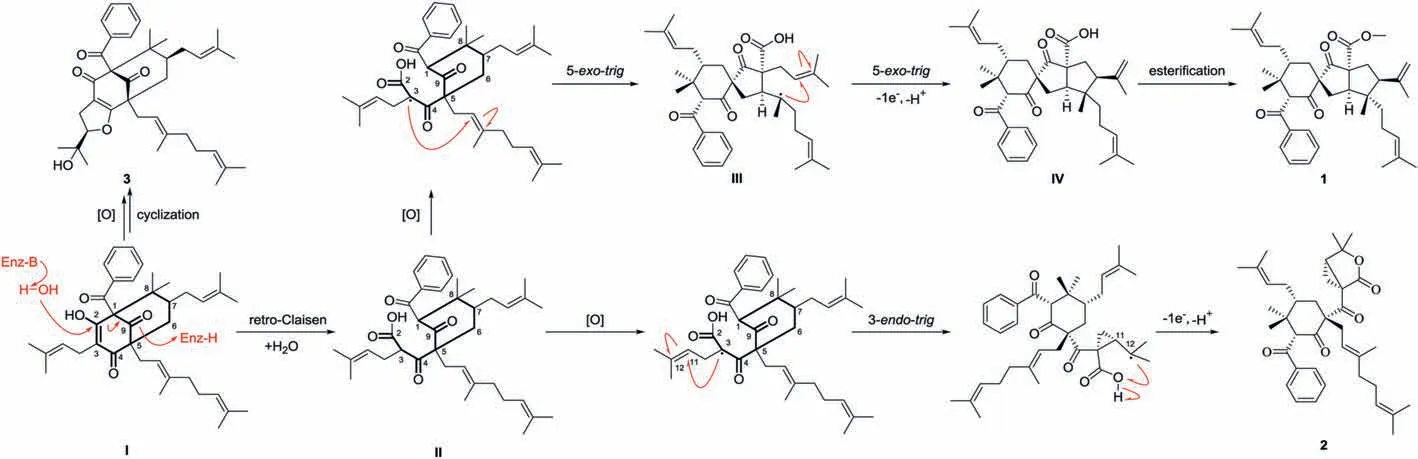
Scheme 1.Putative biosynthetic pathway for compounds 1–3.
Hyperbenzone A (1) was isolated as a white powder and eventually as colorless needle crystals from MeOH–H2O (90:10) system.The molecular formula was assigned from the HRESIMS protonated molecular ion atm/z601.3877 [M+H]+(calcd.601.3888) as C39H52O5, which indicated 14 indices of hydrogen deficiency.The1H NMR spectrum (Table 1) showed obvious signals of five aromatic protons (δH7.79, 2H, br d,J=7.2 Hz; 7.55, 1H, br t,J=7.4 Hz;and 7.46, 2H, br dd,J=7.4, 7.2 Hz), four olefinic protons (δH5.13, t,J=6.9 Hz; 5.09, t,J=7.2 Hz; 4.90, br s; and 4.68, br s), and nine methyls (δH3.53, s; 1.72, s, overlapped; 1.72, s, overlapped; 1.71,s; 1.64, s; 1.61, s; 1.17, s; 1.11, s; and 0.63, s).The13C NMR (Table 1) and distortionless enhancement by polarization transfer (DEPT)spectra, in conjunction with a heteronuclear single quantum coherence (HSQC) experiment, indicated characteristic resonances of two non-conjugated carbonyl groups (δC210.5 and 206.3), a benzoyl group (δC196.0, 139.0, 133.1, 128.8×2, and 127.8×2), an ester carbonyl group (δC170.2), two trisubstituted olefine (δC133.8, 131.9,124.5, and 122.8), and one terminal double bond (δC142.9 and 113.9).These functionalities accounted 11 out of 14 indices of hydrogen deficiency, and the remaining three ones were attributable to the existence of a tricyclic ring system in 1.
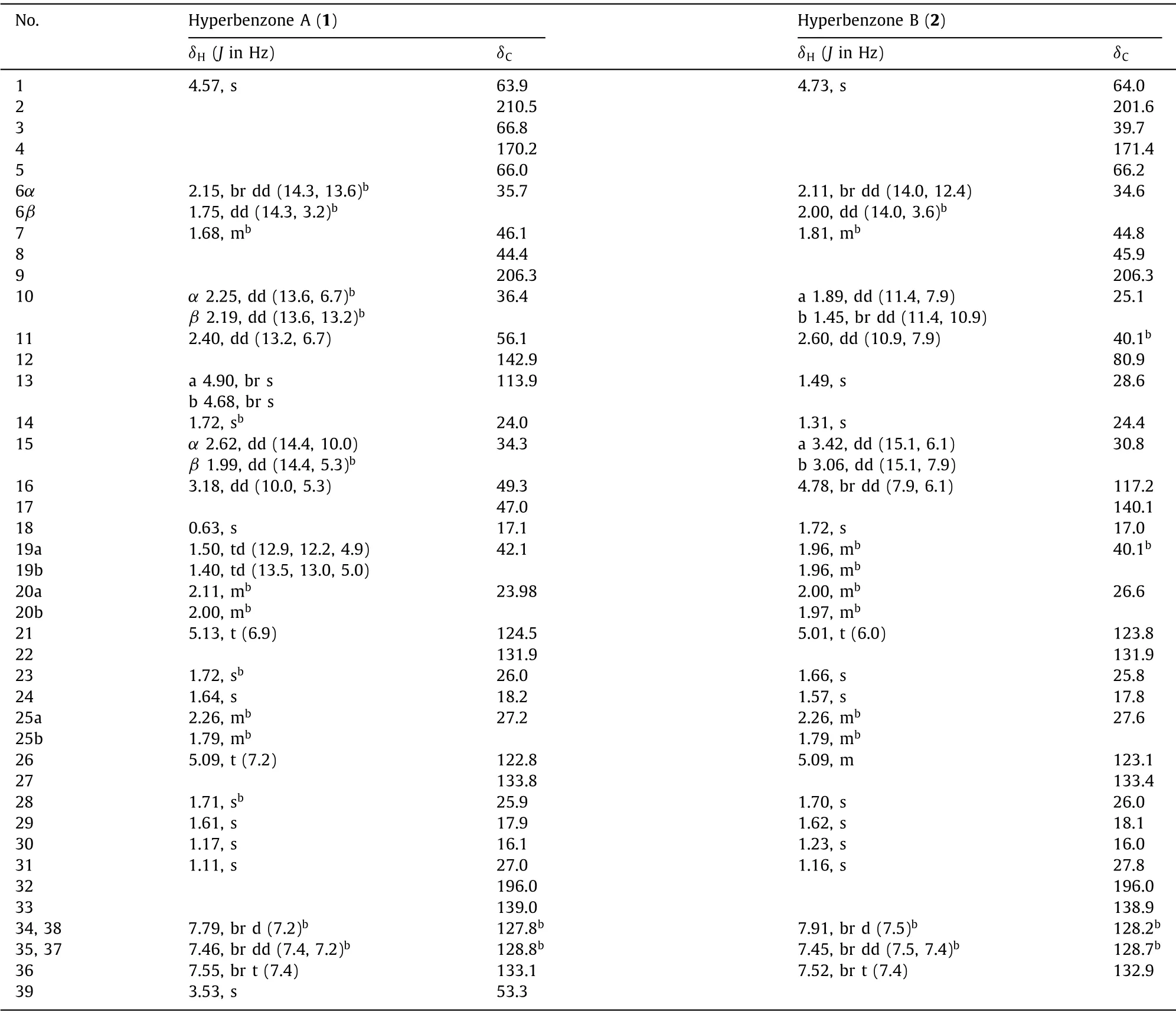
Table 1 1H (600 MHz) and 13C (150 MHz) NMR data of 1a and 2a.
Two-dimensional NMR spectra analysis allowed the assembly of units a and b and their connectivity in 1 (Fig.2).The correlations from thegem–dimethyl groups Me-30 and Me-31 to C-1, C-7 and C-8, from H2-6 to C-5, C-7 and C-9, and from H-1 to C-7, C-8 and C-9 in the heteronuclear multiple bond correlation (HMBC) spectrum, as well as1H–1H correlation spectroscopy (COSY) cross-peak of H2-6/H-7 established a cyclohexanone ring A in unit a.The locations of the benzoyl and an isoprenyl group at C-1 and C-7, respectively, were assigned by the HMBC correlations from H-1 to C-32, and from H2-25 to C-6, C-7, C-8 and C-26.Thus, unit a with a cyclohexanone ring A was defined as shown in Fig.2.
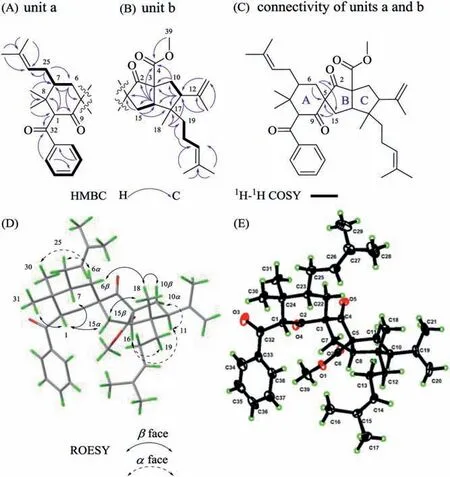
Fig.2.Two-dimensional NMR spectra analysis and crystallographic analysis of 1.(A–D) Key HMBC, 1H–1H COSY, and ROESY correlations of 1.(E) The X-ray Oak Ridge thermal ellipsoid plot (ORTEP) drawing of 1.
The HMBC correlations from H2-15 to C-2, C-5, C-16 and C-17, from H-16 to C-2, C-3, C-5, C-10, C-11, C-15 and C-17, and from H-11 to C-3, C-10, C-16 and C-17 as well as the spin systems of H2-10/H-11, and H2–15/H-16 indicated the presence of a hexahydropentalen-1(2H)-one moiety (rings B and C) in unit b.The correlations from H2-10 and H-16 to C-4, from Me-39 to C-4, from Me-14 and H2-13 to C-11, from Me-18 and H2-19 to C-11, C-16, and C-17, and from H2-20 to C-19 and C-21 in the HMBC spectrum indicated the locations of a methyl ester, an isopropenyl, a methyl,and a homoprenyl group in unit b.Finally, the diagnostic-HMBC correlations from H2-6 to C-2, C-5, and C-15, and from H2-15 to C-5 and C-9 disclosed the connectivity of units a and bviaa C-5 spirocenter.Thus, an unprecedented spiro[bicyclo[3.3.0]octane-3,1ʹ-cyclohexane]-2,2ʹ–dione scaffold in 1 was identified as depicted(Fig.2).
The relative configuration of hyperbenzone A (1) was determined using a rotating-frame Overhauser effect spectroscopy(ROESY) experiment, in which correlations of H-1/H-7, H-1/Me-31, H-6α/Me-30, and H-6α/H-25b supported that H-1 and H-7 in unit a areβ-oriented (Fig.2D).The ROESY cross-peaks of H-1/H-7, H-10α/H-11, Me-18/H-10β, and Me-18/H-15βindicated theαorientations of H-11 and H-16, and theβ-orientation of Me-18(unit b).In addition, the key ROESY correlations of H-1/H-15α, and Me-18/H-6βindicated that the C-15 to C-17 hemi-sphere in unit b is located at the upside of the C-5 spirocenter (Fig.2D).However,due to the absence of obvious ROESY correlations between Me-39 with other protons, the relative configuration of C-3 was still indistinct.To tackle this problem, we carried out computational modeling of NMR chemical shifts of two possible C-3 epimers, named(3R∗)-1a and (3S∗)-1b.The absolute difference between calculated and experimental13C NMR chemical shifts of (3R∗)-1a were within 5 ppm, while the calculated13C NMR chemical shifts of (3S∗)-1b showed large discrepancies at C-1, C-5, C-10, C-12, C-16, C-17, C-33,and C-37 (Fig.3A).Further, the DP4+ NMR chemical shift calculation method confirmed the assignment of anR∗configuration for C-3 (Fig.3B and Fig.S1-1A in Supporting information).Thus, the C-3 methyl ester group in 1 was assigned asα-orientation (3R∗).Then, we tried many recipes to obtain a suitable single crystal for X-Ray diffraction analysis, but only got a thin needle crystal for Mo Kαradiation analysis.And the relative configuration of 1 was thus confirmed by the subsequent crystallographic analysis (Fig.2E) [CCDC 2091426], which is also consistent with that of (3R∗)-1a.On the basis of this well-defined relative configuration of 1, the absolute configuration of 1 was confirmed as 1S,3R,5R,7R,11S,16R,17Sby ECD theoretical calculations (Fig.3C).
Hyperbenzone B (2) was purified as a yellow oil.The molecular formula of 2 was determined as C38H50O5on the basis of its HRESIMS and13C NMR data (Table 1) and indicated 14 indices of hydrogen deficiency.Comparison of its 1D (Table 1) and 2D NMR spectroscopy data with those of 1 indicated that 2 had the same benzoyl group and cyclohexanone ring A.A comprehensive analysis of DEPT and HSQC spectra suggested that C-3 (δC39.7) is a quaternary carbon, C-11 (δC40.1, overlapped) is a tertiary carbon, and C-19 is a secondary carbon (δC40.1, overlapped).The prominent HMBC correlations from H-10a to C-3, from H-10b to C-11, and from H-11 to C-3 and C-10, as well as1H–1H COSY cross-peaks of H2-10/H-11 established a cyclopropane ring in compound 2.And the HMBC correlations from to H2-10 to C-4 and C-12, from H-11 to C-4 and C-12, and fromgem–dimethyl groups Me-13 and Me-14 to C-11 and C-12 indicated the presence of a 4,4-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one moiety in compound 2 (Fig.4A), which is similar to that in a known compound hypercohin K [16].In addition, a geranyl group was deduced by the HMBC correlations from allylic Me-18 to C-16, C-17 and C-19, from H-21 to C-19, C-20 and C-22.Furthermore, the correlations from H2-15 to C-2, C-5, C-6 and C-9, and from H2-6, H2-10, and H-11 to C-2 in the HMBC spectrum indicated the locations of the geranyl group and 4,4-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one moiety (Fig.4A).Thus, the planar structure of 2 was determined.
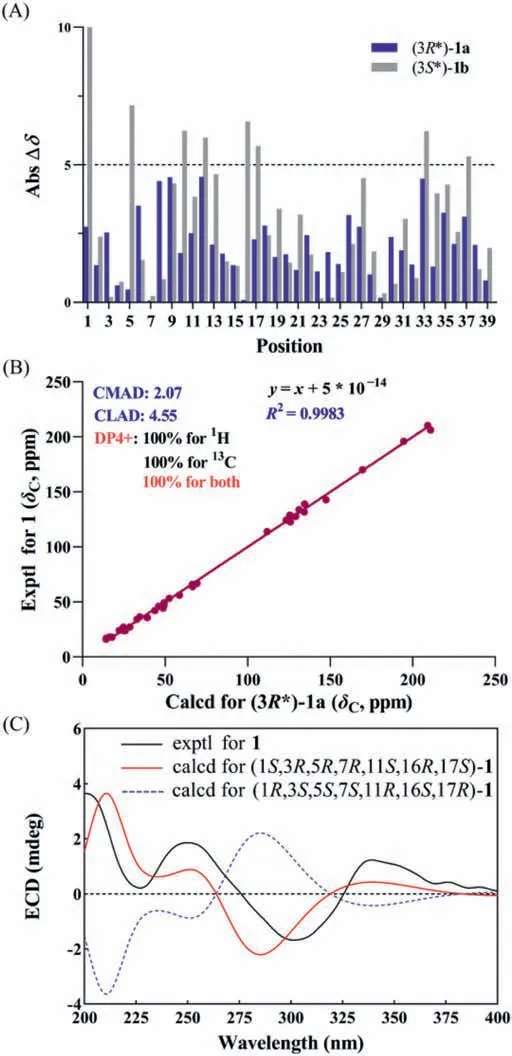
Fig.3.A comprehensive methodology for determination of relative and absolute configurations of compound 1: (A) Absolute difference between calculated and experimental 13C NMR chemical shifts of (3R∗)-1a and (3S∗)-1b.(B) NMR calculations with DP4+ analyses for (3R∗)-1a.(C) Experimental and calculated ECD spectra of 1.
The ROESY correlations observed from H-1/H-7, H-1/Me-31, H-6α/Me-30, H2-15/H-1, H2-15/H-6β, and H2-15/H-7 implied that H-1, H-7 and the methylene H2-15 were on the same face withβorientations, while the benzoyl group, the prenyl group at C-7 and C-2 carbonyl group were on the same face withα-orientations(Fig.4B).Further, the 16Econfiguration of C-5 geranyl moiety was confirmed by the ROESY correlations occurring between Me-18 and H-15a and between H2-19 and H-16 (Fig.4B).The structural inflexibility of the 3-oxabicyclo[3.1.0]hexan moiety in 2 restricted that the relative configurations of the bridgehead C-3 and C-11 should be 3S∗,11R∗or 3R∗,11S∗.This assignment was also confirmed by the cross-peaks of H-11/Me-13, H-11/H-10a, and H-10b/Me-14 in its ROESY spectrum.To further confirm the relative configuration of C-3 and C-11, a quantum chemical prediction of1H and13C chemical shifts of two isomers (1S∗,3S∗,5R∗,7R∗,11R∗)-2a and(1S∗,3R∗,5R∗,7R∗,11S∗)-2b were conducted.The absolute difference between calculated and experimental13C NMR chemical shifts of(1S∗,3R∗,5R∗,7R∗,11S∗)-2b were within 5 ppm, while the calculated13C NMR chemical shifts of (1S∗,3S∗,5R∗,7R∗,11R∗)-2a showed large discrepancies at C-3, C-11, and C-33 (Table S3-4 in Supporting information).Further, applying the DP4+ algorithm to shielding tensors produced 100% confidence that 1S∗,3R∗,5R∗,7R∗,11S∗best fits the experimental data (Fig.4C and Fig.S1-1B in Supporting information).The absolute configuration of 2 was determined by comparative analysis of computed and experimental ECD spectra,which assigned the 1R,3S,5S,7S,11R(Fig.4D) absolute configuration for 2.
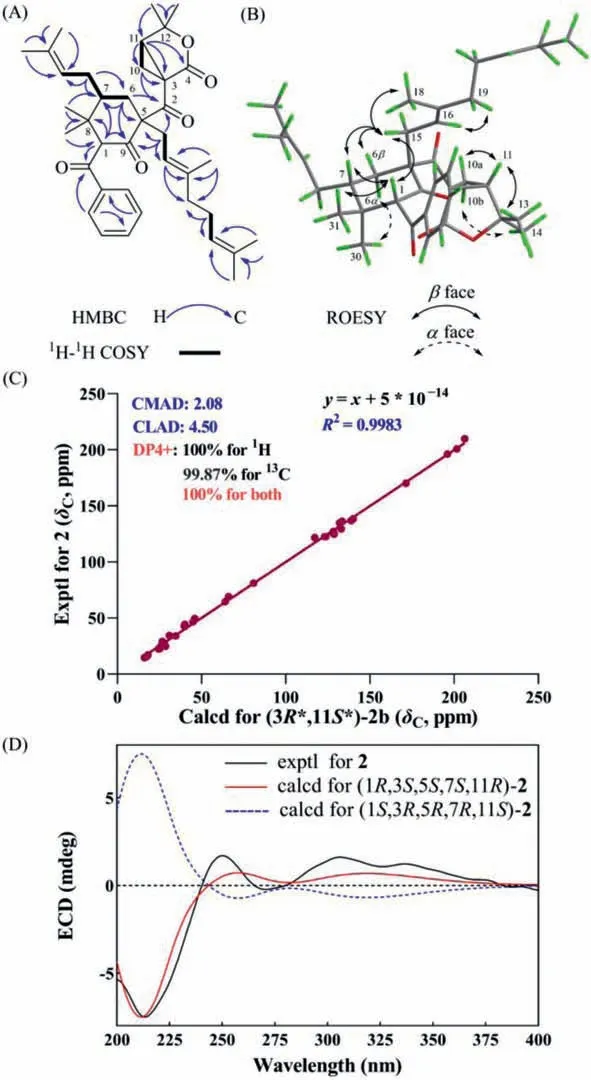
Fig.4.2D NMR spectra analysis and assignment of the relative and absolute configurations of 2.(A) Key HMBC and 1H–1H COSY correlations of 2.(B) Key ROESY correlations of 2.(C) NMR calculations with DP4+ analyses for (3R∗,11S∗)-2b.(D)Experimental and calculated ECD spectra of 2.
Inspired by the structure of a co-isolated known PPAP (3)with a C-5 geranyl-decorated bicyclo[3.3.1]nonane core, a plausible biogenetic pathway (Scheme 1) was proposed, in which compounds 1–3 were deemed to be derived from an assumed bicyclo[3.3.1]nonane PPAP precursor (Ⅰ).Starting from the precursor Ⅰ, anO-cyclization step led to compound 3.Then, through a pivotal retro-Claisen rearrangement of the enolizableβ,β′-triketone motif, the key intermediate Ⅱwith a typical 1,2-secobicyclo[3.3.1]nonane-derived core was constructed [17–19].Further,the chemodivergent radical cascade cyclizations between aβ-keto acid group and pendent prenyl or geranyl groups in the structure of Ⅱyielded compounds 1 and 2.The 5-exo-trigradical cyclization of a functionalizedβ-keto acid group and geranyl group led to the intermediate Ⅲ, which would undergo another 5-exo-trigradical cyclization to the intermediate Ⅳand then esterification to give compound 1 [20].Referring to the similar radical cyclization, Ⅱcould also undergo a 3-endo-trigradical cyclization of theβ-keto acid moiety andΔ11,12double bond in the pendent prenyl moiety to afford compound 2 [20].

Fig.5.Lipophilic dye BODIPY 493/503 images of compounds 1 (A) and 3 (B) anti-NASH screening in HepG2 cells.DAPI: 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride; BODIPY: Boron-dipyrromethene, 4,4-difluoro-4-bora-3a,4a-diaza-sindacene.Scale bar: 20 μm.
Growing evidences have supported the beneficial role of natural products in treating NASH [21–23].In the previous reports, many sub-types of PPAPs exhibited significant anti-NASH activity [24,25].Compound 1 showed no cytotoxicity against HepG2 cell lines up to 200 μmol/L, while compounds 2 and 3 showed no cytotoxicity against HepG2 cell lines up to 50 μmol/L (Fig.S1-4 in Supporting information).The effects of 1–3 on cellular lipid levels were explored in a palmitic acid-exposed [26,27] HepG2 cell model using the lipophilic dye BODIPY 493/503 [28].The images of lipophilic dye BODIPY 493/503 in Fig.5 showed that 1 and 3 could inhibit lipid accumulation in HepG2 cells.Furthermore, quantitative analysis of intracellular lipid droplets showed that 1 and 3 could decrease the lipid droplets in a dose-dependent manner (Fig.S1-5 in Supporting information).These results indicated that compounds 1 and 3 might be appealing to medicinal chemists for further investigation as a potential protective agent against NASH development.
In summary, 1,2-secoand rearranged bicyclo[3.3.1]nonane PPAP possessing an unprecedented spiro[bicyclo[3.3.0]octane-3,1ʹ-cyclohexane]-2,2ʹ–dione core and an unusual 4,4-dimethyl-3-oxabicyclo[3.1.0]hexan-2-one moiety, hyperbenzones A (1) and B(2), respectively, along with a known biosynthetic congener otogirinin D (3) [15], were isolated fromH.beanii.Biogenetically,the 1,2-secoretro-Claisen rearrangement in combination with the following chemodivergent radical cyclizations from a bicyclo[3.3.1]nonane PPAP precursor afforded compounds 1 and 2.In addition, the bioassay showed that both compounds 1 and 3 could decrease intracellular lipid accumulation in a palmitic acid-induced NASH cell model.This work indicated that the cleavage and further cyclization of the enolizableβ,βʹ-triketone moiety in the bicyclo[3.3.1]nonane core of PPAP could continuously generate significant structural complexity and diversity of PPAPs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.31900287 and 81773886), the 111 Project from Ministry of Education of China and the State Administration of Foreign Export Affairs of China (No.B18056), and Special fund from the Central Committee for guiding local scientific and technological development of Shenzhen (No.2021Szvup161).
Appendix A.Supplementary data
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.011.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
