Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
Chengxiang Shi, Hui Li, Xiaofeng Shi, Liang Zhao, Hongdeng Qiu
a CAS Key Laboratory of Chemistry of Northwestern Plant Resources and Key Laboratory for Natural Medicine of Gansu Province, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China
b University of Chinese Academy of Sciences, Beijing 100049, China
c Department of Gansu Provincial Cancer Hospital, Institute of Materia Medica, Lanzhou 730000, China
d College of Chemistry, Zhengzhou University, Zhengzhou 450001, China
e College of Chemistry and Chemical Engineering, Gannan Normal University, Ganzhou 341000, China
ABSTRACT Chiral pillar[n]arenes have shown great research value and application prospect in construction of chiral materials and chiral applications, due to their inherent planar chiral configurations, chiral recognition ability, easy modification and highly symmetric hydrophobic cavity.This review systematically summarized the conformation inversion factors of planar chiral pillar[5]arenes (pR/pS), such as solvents, temperature, substituent size, alkyl chains, chiral and achiral guest molecules.We firstly introduced the applications of chiral pillar[n]arenes for constructing chiral materials and pointed out that planar conformation inversion showed a great potential role in constructing chiral materials.Then, we mainly concluded the chiral applications of chiral and planar chiral pillar[n]arenes like chiral enantiomer analysis by circular dichroism, electrochemistry or chiral fluorescence sensing.From this review, we found that the inherent planar chiral conformation of chiral pillar[n]arenes have played a very important role in chiral field in the future.
Keywords:Chiral pillar[n]arene Planar chiral configuration Chiral supramolecular materials Chiral separation and analysis Conformation inversion
1.Introduction
Pillar[n]arenes (n=5, 6…) have been widely used in material preparation, life sciences and separation analysis as soon as it was first synthesized by Ogoshi, due to their excellent characteristics such as symmetrical hydrophobic cavity, electron-rich and easy edge modification [1–11].Pillar[5]arene is benzene pentamers with 1,4-methylene bridged hydroquinone derivatives, which cavity inner diameter is around 4.7(Fig.1), and the bond angle of−CH2−is 111.3° which is closely to the sp3hybrid orbital bond angle of carbon atoms of 109.5° and the pentagonal internal angle of 108°, so it has a relatively stable pentagonal configuration.Pillar[6]arene was then prepared with a larger cavity and found to have one more repeating unit than pillar[5]arene (Fig.1A) [12], the inner diameter of pillar[6]arene cavity approximately 6.7(Fig.1B)[13], then more pillar[n]arenes with larger cavity were successfully prepared.Among these pillar[n]arenes (n=5, 6) have always been widely synthesized and applied due to their high thermodynamic stability and high yield.
Chiral pillar[n]arenes can be easily obtained by introducing different chiral groups at the edge or conformation inversion.Small molecular groups on the benzene ring can be elastically reversed with its units undergoing oxygen-through-the-annulus rotation,which produces conformational isomers and leads to various conformations of pillar[n]arenes (Fig.2).They were five enantiomers of pillar[6]arene, including of (pS,pS,pS,pS,pS,pS)/(pR,pR,pR,pR,pR,pR), (pR,pS,pS,pS,pS,pS)/(pS,pR,pR,pR,pR,pR), (pR,pR,pS,pS,pS,pS)/(pS,pS,pR,pR,pR,pR), (pR,pS,pR,pS,pS,pS)/(pS,pR,pS,pR,pR,pR), (pR,pS,pS,pR,pS,pS)/(pS,pR,pR,pS,pR,pR).Although the conformation of pillar[n]arenes can be reversed, the conformations with the lowest energy state become its dominant conformation due to the different potential barriers of conformational isomers.For all alkoxy pillar[n]arenes, due to steric effect, the energy of (pS,pS,pS,pS,pS,pS)/(pR,pR,pR,pR,pR,pR) in allRor allSconformations is the lowest [14–16].
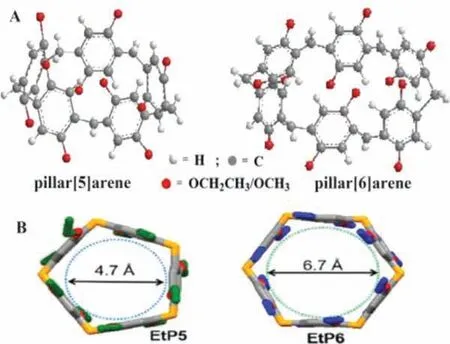
Fig.1.(A) Structure of pillar[n]arenes (n=5, 6).(B) Top view of pillar[5]arene and pillar[6]arene, respectively.Reproduced with permission [13].Copyright 2018,American Chemical Society.

Fig.2.(A) Rotation of paraphenylene methylene units of pillar[n]arenes represented by permethylated pillar[5]arene.Reproduced with permission [15].Copyright 2019,the Chemical Society of Japan.(B) Conformers of pillar[6]arenes.Reproduced with permission [16].Copyright 2013, the Royal Society of Chemistry.
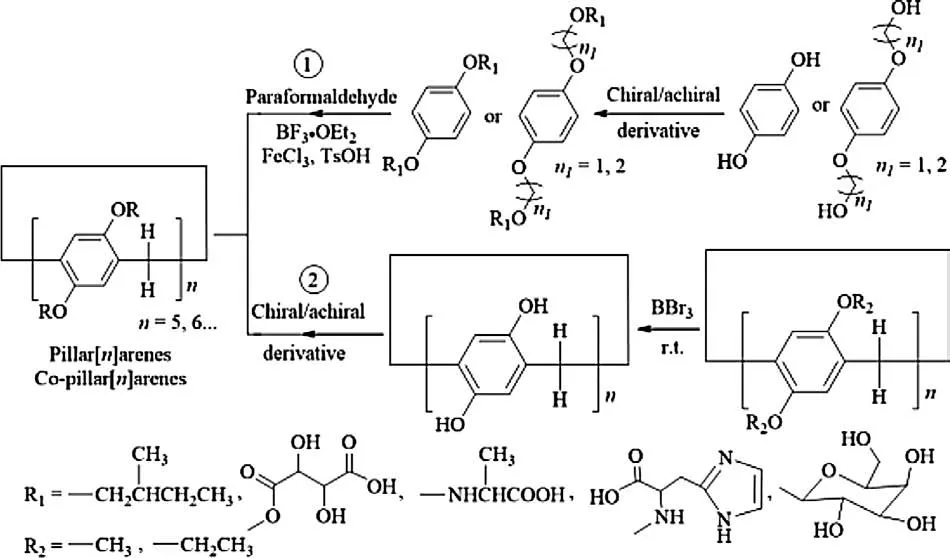
Fig.3.Synthesis method of chiral pillar[n]arenes.
There are usually two main synthesis methods for both chiral and achiral pillar[n]arenes including derivatization followed by ring polymerization and ring polymerization followed by derivatization of repeating units, mainly catalyzed by BF3·OEt2, FeCl3or TsOH under mild conditions with paraformaldehyde as polymerization agent.The first method mainly uses homogeneous or mixed hydroquinone derivatives as repeating units to obtain homogeneous or copolymerization pillar[n]arenes [17–25], and then construction of dimeric, trimeric or tetrameric (Fig.3) [26–28].In contrast, the second synthesis method involves dealkylation of methoxy or ethoxy pillar[5]arene (MP5/EP5)first by BBr3to obtain homogeneous hydroxyl pillar[n]arene (P5), and then alkyl chain, carboxyl, amine,sulfonic acid or chiral groups were introduced to prepare a series of functionalized pillar[5]arenes.Therefore, chiral pillar[n]arenes(n=5, 6…) can be accurately synthesized by selectively optimizing and designing the chiral molecules or chiral precursors at its edge use the same strategy.
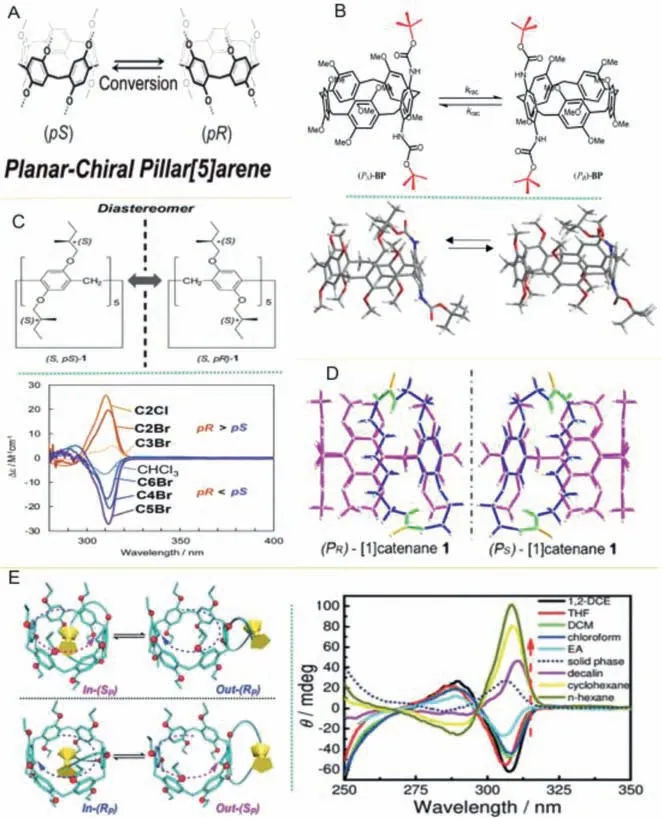
Fig 4.(A) Chemical structures of pS/pR-pillar[5]arene.Reproduced with permission[25].Copyright 2011, American Chemical Society.(B) Chemical structures and the stick model of planar chiral of butoxycarbonyl (Boc)-protected pillar[4]-arene[1]-diaminobenzene (PS)-BP and (PR)-(BP).Reproduced with permission [24].Copyright 2019, Multidisciplinary Digital Publishing Institute.(C) Diastereomer of pillar[5]arene carrying 10 stereogenic carbons and CD spectra.Reproduced with permission [58].Copyright 2020, Royal Society of Chemistry.(D) Two isomers of MSM1.Reproduced with permission [20].Copyright 2016, Elsevier.(E) In-RP and out-SP conformations of enantiomeric EMUJ1 and CD spectra.Reproduced with permission[59].Copyright 2020, Wiley.
Chiral pillar[n]arenes (n=5, 6…) can be used to construct novel chiral supramolecular polymers or planar chiral control systems, due to their specialized electron-rich cavity, easy to modify and polyfunctional groups.Chiral or achiral guest molecules of appropriate structural can be encapsulated into its cavities, as well as used for chiral fluorescence and chiral electrochemical sensing.Pillar[n]arene is a kind of good chiral sensor molecule, and its two enantiomers have the property of inversion, which makes it show special chiral response.Therefore,pillar[n]arene molecules have great application potential in chiral separation and analysis.Some reports have been published and mostly focus on the synthesis, sensing, separation or analysis of pillar[n]arenes [29–52].This review we focused on chiral information like conformation inversion of planar chiral conformation pillar[5]arenes (pR/pS),pillar[n]arene-based chiral materials and their applications of in chiral separation analysis.Through reading this review, we hope to provide readers with a powerful reference for the preparation,performance and chiral applications of chiral pillar[n]arenes.
2.Conformation inversion of chiral pillar[n]arenes
Chiral conformation inversion of pillar[5]arene may be caused by epimerization or racemization caused by rotation of units at the molecular level [53], and the reversal of the planar chiral configurations of the guest molecule induced pillar[n]arenes (n=5, 6…)maybe a new strategy to construct the planar chiral control system, chiral supramolecular self-assembly and polymers [54,55].Researchers have found that the inversion of the planar conformation(pR/pS) of pillar[n]arenes contain a variety of inducing factors, such as chiral or achiral guest molecules (anion and cation), length of alkyl chain, solvents, temperature or substituted radical hindrance.
2.1.Conformation inversion induced by chiral guest molecules
The inversion of the planar chiral conformations (pR/pS) of the pillar[5]arene is mainly due to different steric effect between chiral guest molecules and its cavity edge, and the most basic reason is the different binding model between pillar[5]arene cavity andαside chain group of chiral guest molecules.Chenet al.found that the chiralα-side chain group of L-Arg-ethyl ester hydrochloride faced the cavity of water-soluble pillar[5]arene WP5, which can induce itspRconformation to reverse.Other 18 types of L-amino acid ethyl ester hydrochlorides can also induce thepSconformation to reverse [53].
2.2.Conformation inversion induced by achiral guest molecules
The rotation speed of units and its direction on pillar[n]arenes can be affected by the interactions between pillar[n]arenes and achiral guest molecules, which then induce the reversal ofpR/pSof pillar[n]arenes.Ogoshiet al.prepared chiral pillar[5]arene with 1,4-bis[2(S)-methylbutoxy]-benzene as repeating units, they found that when octyltrimethylammonium hexafluorophosphate (OTMA)was added, the negative signal intensity of circular dichroism (CD)was significantly enhanced and conformation reversal was also induced [25].Then they found that introducing substituents containingπ-πconjugated units and appropriate guest molecules to pillar[5]arenes could effectively control the reversal and change the planar chiral structure, which provides a new strategy for inducing planar chiral conformations [21].Chenget al.used the guest molecule 1,4-dicyanobutane as a competitor molecule to induce the conformation conversion of mechanically self-locked molecules(MSM1 and MSM2) under the conditions of increasing solvent polarity [20].Leeet al.found that guest molecule of Hg2+can lead the in-pS-Lconformation of bicyclic pillar[5]arene to reverse to the out-pR-Lin the presence of ClO4–or NO3–[22].In addition, changing the length and addition amount of achiral guest molecules pyridine, imidazole and alkyl chain containing terminal hydroxyl or methyl can lead to the conformation reversal of L/D-Ala-tertbutyl ester hydrochloride derived pillar[5]arenes [54].Jiet al.found that amino acid derivatives can induce pillar[5]arene with alkyl chains of different lengths to generate extremely strong CD signal values[56].Yaoet al.found that pressure could also drive the planar chirality switching of molecular universal joints based on cyclophanopillar[5]arenes [57].The structures, preparation, influencing factors, advantages and applications of chiral and planar chiral pillar[5]arenes were summarized in detail in Table 1.
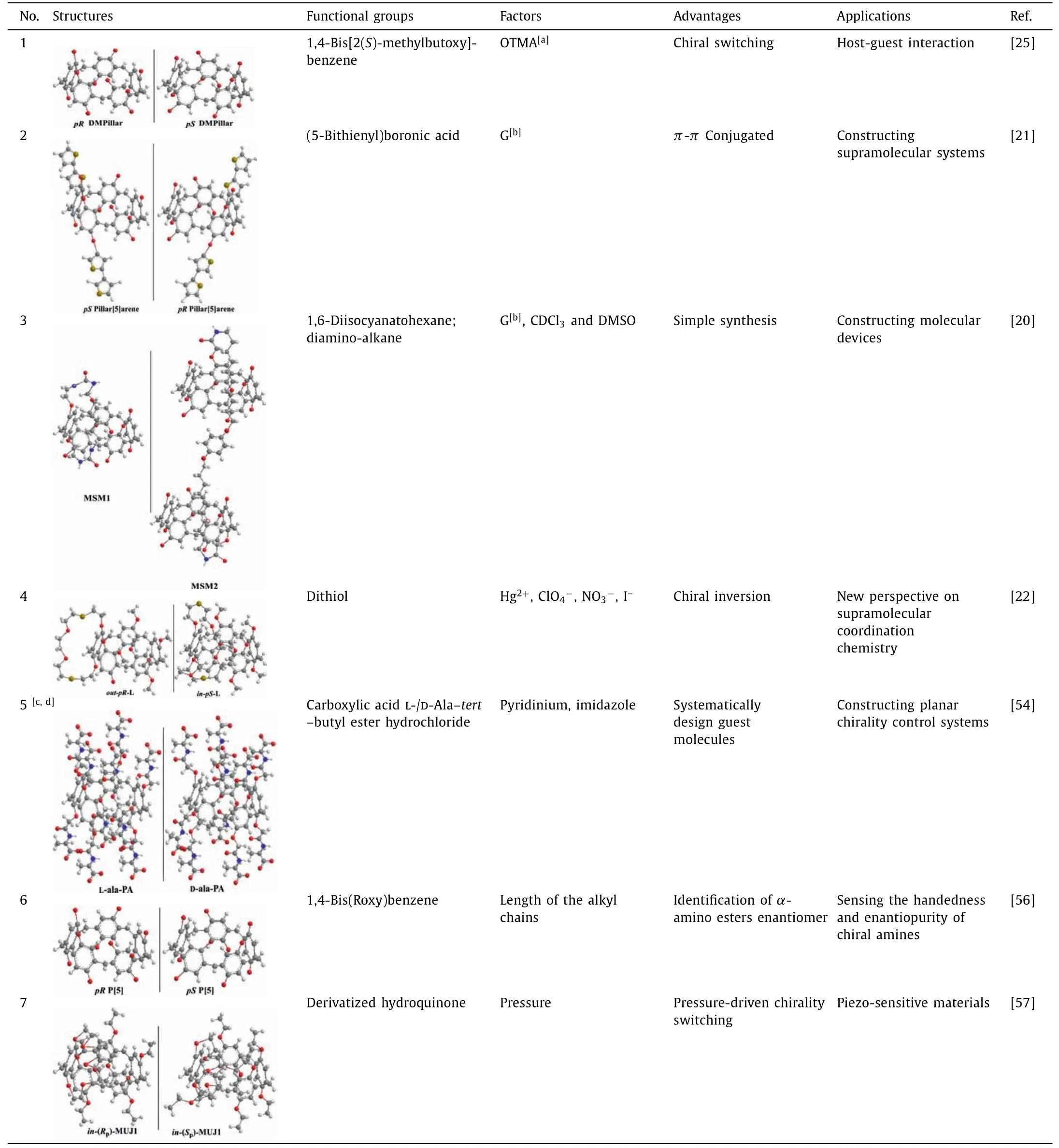
Table 1 Conformation inversion of pillar[5]arenes inducted by achiral guest molecules.
2.3.Conformation inversion induced by solvents
Solvent molecules can be encapsulated into pillar[n]arenes cavity due to their hydrogen bonding, repulsion, C–H···πinteraction and space interaction to form stable encapsulated structure, which may lead to different rotation or swing speed of pillar[n]arenes under different dielectric constants, resulting in configurations inversion.Ogoshiet al.found that solvents with high dielectric constant could induce the conformation inversion of 2-(S)-methylbutoxy modified pillar[5]arene (Fig.4A), mainly because the substituents of pillar[5]arene ring swung and rotated rapidly in such solvents, which led to the decrease of CD signal intensity [25].The stablepS/pRconformation of butoxycarbonyl (Boc)-protected pillar[4]arene[1]-diaminobenzene (BP) BPf1 and BP-f2 (Fig.4B), mainly depended on the size of solvent molecules(n-hexane or dichloromethane) and encapsulation in the cavity [24].Nagataet al.found that 2-(S)-methyl butoxy substituted chiral pillar[5]arene mainly existed inpRconformation in short linear dihaloalkane solvents.While in long linear solvents,it mainly existed inpSconformation (Fig.4C) [58].Chenget al.revealed planar chiral conformation of the mechanically self-locking molecule can be inversed when changing the polarity of mixed solvents (Fig.4D) [20].In addition, the planar chiral conformations of electrochemically responsive molecular universal joints(EMUJ) out-(SP) EMUJ1-f1 and in-(RP) EMUJ1-f2 of the electrochemically responsive molecular universal joint EMUJ based on pillar[n]arene can also be controlled by the polarity of solvents.In the polar solvents (Fig.4E), EMUJ1 exists in out-(SP) conformation.While in nonpolar solvents, it exists in-(RP) conformation [59].Therefore, the inversion of the planar chiral conformations of pillar[n]arenes (n=5, 6…) can be effectively controlled by changing the types, polarity or amounts of solvents, further applied to be used in the preparation of chiral materials, chiral separation and analysis.
2.4.Conformation inversion induced by temperature
The rotation and oscillation rate of the substituents o n the edge of the pillar[n]arene cavity may be caused by the temperature, and resulted in excessive diastereomers or conformations inversion for pillar[5]arene.Chiral pillar[5]arenes with 1,4-bis(2-(S)-methyl–butyl)benzene as the repeating units can be induced conformation reversal when the temperature was in the range of 0–60°C with the ee value as high as 24.2% (Fig.5A)[25].(RS)-(±)−1-phenylethane-1-acetamide decasubstituted chiral pillar[5]arene derivatives can induce enantiomers of different proportions, and theireevalues vary by 14%−40% in the temperature range of 50–72°C [60].ThepSconformation reversal of pillar[4]arene[1]-diaminobenzene BP protected by butoxy carbonyl Boc can be accelerated by increasing the temperature inn-hexane[24].The absolute conformation of the universal joint MUJ based on ethoxy substituted pillar[5] arene can also be switched reversibly by controlling the temperature (Figs.5B and C) [61].Similarly, L/D-Ala-derivatived pillar[5]arenes can only exist inpRconformation at low temperature, and the interaction between LAla-PA and guest molecule Py-11-OH is stronger at 35°C than at 45°C.It was mainly due to the strong hydrophobic and electrostatic interaction of L-Ala-PA at low temperature, which made it easier to reverse the CD signal.(Fig.5D) [54].In addition, Fanet al.synthesized temperature-driven chiral switching molecular universal joints materials using ethoxy-substituted pillar[5]arene,and realized a single form of in-(Sp) or out-(Rp) with temperature changes (Figs.5E and F) [62].Therefore, we can use this strategy to obtain single enantiomer of pillar[5]arene by changing the temperature and apply them to construct new chiral materials.
2.5.Conformation inversion induced by substituents
The introduction of bulky groups at the edge of pillar[n]arenes to increase the steric hindrance, which can effectively inhibit the rotation of pillar[n] arene units and induce the planar conformation reversal, and this can be judged by the integral area of the splitting peak.Ogoshiet al.found that the rotation of the pillar[5]arene unit was inhibited by the introduction of percyclohexylmethyl substituents at the edge of the pillar[5]arene [63].Al-Azemiet al.demonstrated large substituents of planar chiral pillar[5]arene derivatives substituted by chiral reagents (S)-(+)-Mosher-inhibited the rotation of their units and converted them into compounds with a single planar chiral conformation [55].In addition, Panet al.introduced bulky substituents on pillar[5]arene,and found that the conformations of pillar[5]arene could be reversed in solid state [64].
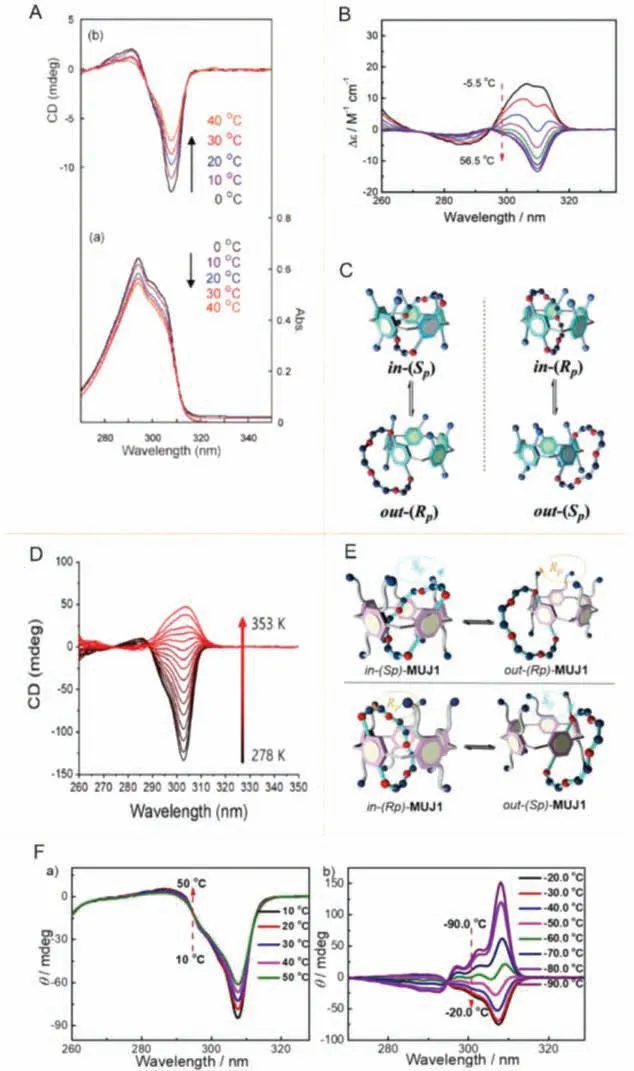
Fig.5.(A) CD spectra of pillar[5]arene carrying 2(S)-methylbutoxy moieties.Reproduced with permission [25].Copyright 2010, American Chemical Society.(B, C)Representations for the in-out equilibrium of the MUJ1 enantiomeric pair and CD spectra of MUJ1-f1 in CHCl3.Reproduced with permission [61].Copyright 2017,Wiley.(D) CD spectra of aqueous solution of L-Ala-PA and Py-11-OH.Reproduced with permission [54].Copyright 2019, American Chemical Society.(E, F) Schematic representations of the in/out equilibrium of a pair of MUJ enantiomers and CD spectra of in-(Sp)/out-(Rp) MUJ1.Reproduced with permission [62].Copyright 2019,Wiley.
2.6.Significance of chiral inversion
Chiral pillar[5]arenes can be used to construct a large number of chiral materials like chiral memory materials and chiral mechanical self-locking molecules, because of the unique chiral conformation reversal properties or chiral modification, and widely used in chiral biosensing, electrochemical sensing and chiral separation analysis and other fields.Faet al.found that when added the regulator 1,4-dibromobutane, the planar chirality of pillar[5]arene can be induced by chiral inducer and accurately regulated by regulator.Moreover, during the chiral induction period, the addition sequence of chiral inducers and regulators will have a certain impact on the chiral induction behavior of pillar[5]arene [65].This may provide a new strategy to the applications of pillar[5]arene in the construction of chiral control system materials and expand the applications range of chiral pillar[5]arene.
3.Pillar[n]arene-based chiral materials
Novel chiral macrocyclic materials derived from single enantiomer of planar chiral or chiral pillar[5]arene, which mainly can be constructed by the modification of pillar[5]arene on its cavity edge or polymerization of hydroquinone derivatives.In this way,it may provide a new strategy for the construction and application on pillar[n]arene-based chiral materials.In this chapter, we focused on several types of pillar[n]arene-based chiral materials and their preparation methods.
3.1.Chiral mechanical self-locking molecules/rotaxane
Chiral mechanical self-locking molecules or rotaxane can be efficiently synthesized by selective modification or derivatization on symmetric co-pillar[n]arene.Moreover, the inversion of planar chiral configuration has great influence on the construction of chiral rotaxane enantiomers, and has great application potential in chiral separation and analysis.Jiaet al.synthesized diesterfunctionalized pillar[5]arene by one-pot method, which can be used as intermediate of complex system polymers and mechanically interlocking molecules [66].Ogoshiet al.used per-ethylated pillar[5]arene as wheels, and pyridinium derivatives containing alkynyl and trans-azobenzene moieties as the axis, to construct planar chiral [2/3]-rotaxanes [67].The planar chiral conformations inversion of pseud[1]rotaxane, including in-pS-1 and in-pR-1, out-pS-1 and out-pR-1 (Fig.6A), can be controlled, which is of great significance for the preparation of chiral switches or sensors [68].Liet al.prepared A1/A2-dicarboxy-DMP[5]A by alkylation of 4-dimethoxy pillar[4]arene[1]hydroquinone with ethyl bromoacetate, and then reacted with 1,8-diaminooctane DA-8 to prepare pseudo[1]rotaxane and gemini-catenanes (Figs.6B and C)[69].Moreover, Trinhet al.prepared amphiphilic pillar[5]arenecontaining [2]rotaxane (Fig.6D), which could form a stable Langmuir film [70].Chenget al.used 1,6-diisocyanate hexane and diamino-functionalized pillar[5]arene to construct mechanical selflocking molecular pseudo[1]naphthene [20].Holleret al.used dodecanedioyl dichloride to form diamide[2]rotaxaneby associating with all ethoxy pillar[5]arene (Fig.6E).This research opened a new method for the preparation of rotaxanes [71].Lianget al.complexed a carboxyl bifunctionalized pillar[5]arene host with alkyl diamine guest to form pseudo[1]naphthene PN4 with high affinity in and out configuration with good acid or alkali response performance (Fig.6F), which was of great significance for the construction of acid and alkali responsive materials [72].Chiral rotaxane prepared by Oxacalix[4]arene bridging pillar[5]arene dimer realized chiral enantiomerpR/pSresolution (Fig.6G) [73].The pseudo[4]rotaxane material prepared by the reaction of amphiphilic tetraphenylethylene derivative TPEA,γ-cyclodextrin (γ-CD) and water-soluble pillar[5]arene has achieved chiral metrological control [74].Recently, Liet al.prepared chiral[3]rotaxane based on pillar[5]arene macrocycles with the thiourea moieties and the ethoxy group [75].
3.2.Chiral bio-interface materials
Chiral biological interface materials formed by chiral pillar[5]arene immobilized on biological interfaces may exhibit better performance, due to their self-assembly, inclusion and easy modification.L/D-His-self-assembled on the D-tartaric acid modified pillar[5]arene interface to construct a chiral interface D-TP5 (Fig.7A),which was greatly effective for L-His and D-His and the recognition ability wasKL/KD=4.6.In addition to recognizing amino acid enantiomers, D/L-AP5 interface, prepared by attaching D/L-Alapillar[5]arene to gold surface, were applied to highly selective adsorption ofctDNA and proteins (Fig.7B) [76,77].Similarly, Yanetal.prepared chiral biological interface materialsR-PEA@WP5 andS-PEA@WP5 through reacting phenythylamine with pillar[5]arene(Fig.7C), which shows high enantioselectivity to proteins, andRPEA@WP5 has the fastest adsorption rate and large adsorption capacity [78].This may provide a new strategy for constructing new chiral platform to study chiral phenomena in biological systems.
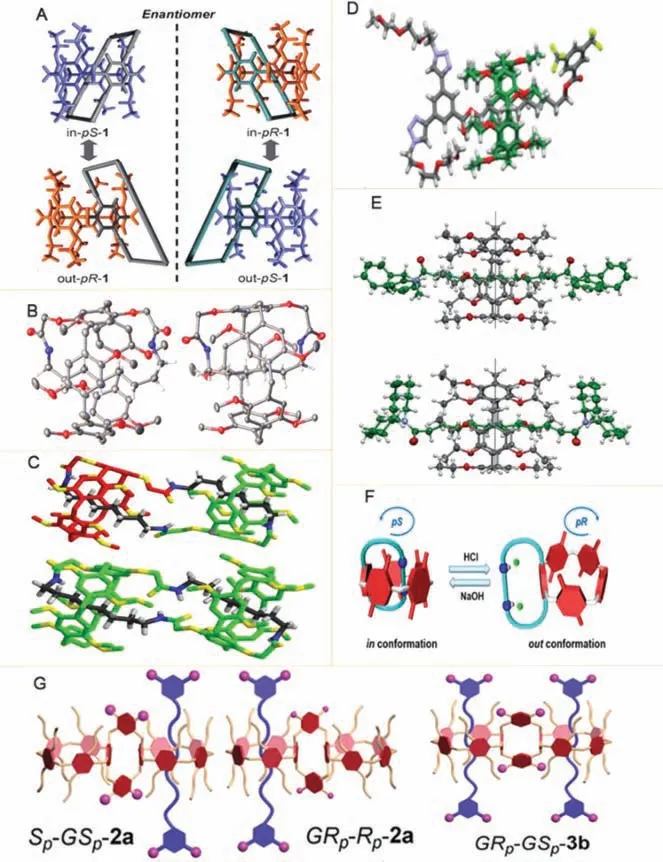
Fig.6.(A) Four conformers of a pseudo[1]catenane 1.Reproduced with permission [68].Copyright 2013, Wiley.(B, C) Crystal structures of pseudo[1]catenanes and geometries of selflocked gemini-catenanes.Reproduced with permission [69].Copyright 2015, Macmillan Publishers Limited.(D) X-ray crystal structure of amphiphilic[2]rotaxanes.Reproduced with permission [70].Copyright 2015, Wiley.(E)ORTEP plots of the structure of diamide[2]rotaxanes.Reproduced with permission[71].Copyright 2019, Wiley.(F) Chirality switching of PN4.Reproduced with permission [72].Copyright 2020, Journal of the American Chemical Society.(G) Cartoon representations of 2a and 3b.Reproduced with permission [73].Copyright 2020,Royal Society of Chemistry.
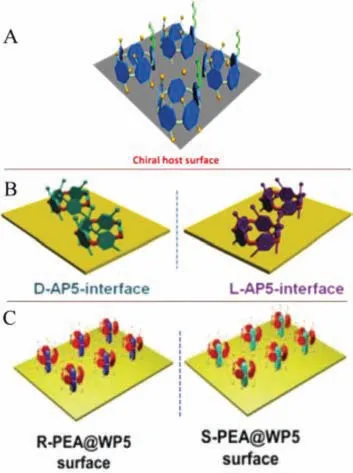
Fig.7.(A) Modification of D-TP5 on a Si-surface.Reproduced with permission[76].Copyright 2018, American Chemical Society.(B) D/L-AP5-interfaces.Reproduced with permission [77].Copyright 2019, Royal Society of Chemistry.(C) The picture of chiral R-PEA@WP5/S-PEA@WP5 surface.Reproduced with permission [78].Copyright 2020, Wiley.
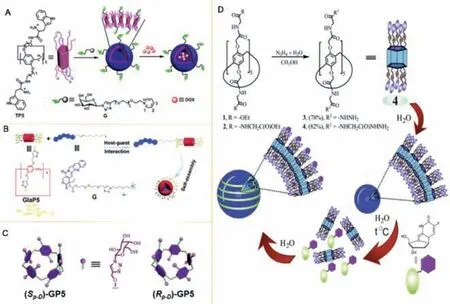
Fig.8.(A) Representation of the self-assembly and drug loading process of TP5G.Reproduced with permission [80].Copyright 2016, American Chemical Society.(B)Schematic of the construction of supramolecular prodrug nanoparticles.Reproduced with permission [81].Copyright 2017, Royal Society of Chemistry.(C) Structure illustration of (Rp-D/Sp-D)-GP5.Reproduced with permission [82].Copyright 2019, Frontiers in Chemistry.(D) Pillar[5]arene 4 self-assembly and self-organization of the 4/FUDR system into monodisperse spherical particles.Reproduced with permission[83].Copyright 2018, Elsevier.
3.3.Chiral framework active domain materials
Chiral metal organic framework materials with homochiral active domains can be prepared by pillar[n]arene.Struttet al.incorporated pillar[5]arene into Zn4O clusters and synthesizedrac-P5A-MOF-1, which had an active domain.These active domains could selectively absorb a large number of electron-deficient guest pyridinium cations andp-dinitrobenzene.At the same time, they also usedrac-P5A-MOF-1 as chiral stationary phase of HPLC and successfully separated enantiomers of methyl pillar[5]arene [79].This study may provide a significant reference for the construction of chiral frameworks with active domain based on chiral pillar[n]arenes.
3.4.Chiral nanomaterials
Chiral nanoparticles or asymmetric biomolecular molecules may be constructed with chiral pillar[5]arene due to its molecular size, functional group, self-assemblyor inversion of chiral configuration.Shurpik et al.prepared (pS/pR)-deca-substitution modified chiral nanoparticles containing secondary amide fragments pillar[5]arene.The content ratio of enantiomerspR/pScan be changed by increasing or decreasing the temperature, which proved that chiral inversion plays an important role in constructing chiral nanomaterials [60].In addition, Yanget al.used galactose derivatives combined with Trp-modified chiral pillar[5]arene to synthesize chiral nanocarrier supramolecular vesicles TP5G (Fig.8A).TP5G loaded with DOX was relatively stable under physiological conditions and could be used for controlled release [80].Under normal physiological conditions, Liuet al.prepared the targeted drug delivery system GalP5⊃G based on alkyne-substituted pillar[5]arene (Fig.8B), which had good stability.However, in the cell environment with high GSH concentration, camptothecin prodrug G could be released effectively [81].Similarly, Sunet al.synthesized (SP-D)-GP5 and (RP-D)-GP5 which could capture DNS-CPT with planar chiral pillar[5]arene, then using them to prepare planar chiralSPandRPnanomaterials with good stability (Fig.8C).This work also proves that the inversion of chiral planar conformation can affect the construction of chiral nanomaterials [82].Nanoparticles, based on the self-assembly of glycine decasubstituted pillar[5]arene hydrazide compounds, combined with antitumor drugs floxuridine (FUDR) to form a complex (Fig.8D).It could significantly reduce the toxicity of FUDR when applied to drug delivery [83].
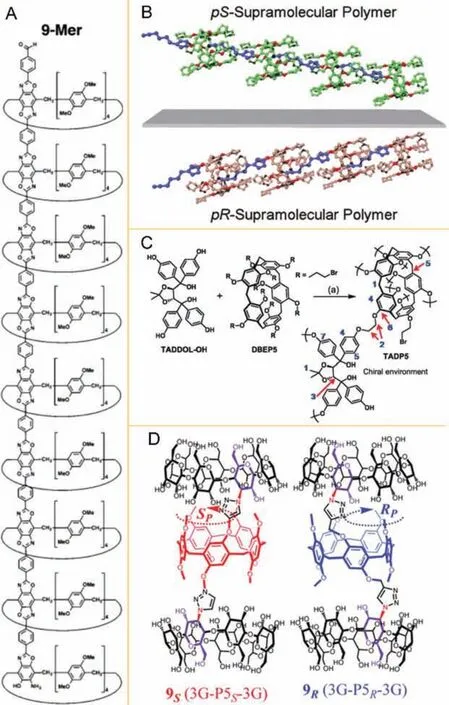
Fig.9.(A) Chemical structure of the rigid pillar[5]arene-containing oligomers 9-Mer.Reproduced with permission [84].Copyright 2014, Wiley.(B) Cartoon representation of pS and pR planar-chiral supramolecular polymers.Reproduced with permission [85].Copyright 2016, Royal Society of Chemistry.(C) Synthetic procedures of TADP5.Reproduced with permission [87].Copyright 2017, Royal Society of Chemistry.(D) Chemical structure of chiral 9.Reproduced with permission [88].Copyright 2020, Royal Society of Chemistry.
3.5.Chiral polymer materials
Chiral polymer materials can be successfully constructed due to its advantages of chiral pillar[5]arene, such as structural modifiability, hydrogen bond, covalent bond, bridging and self-assembly layer by layer of pillar[n]arenes.Struttet al.reacted mono (2,5-diamino-1,4-benzoquinone) pillar[5]arene with terephthalaldehyde to produce AB-type monomers.After being used to synthesize oligomers, the initial monomer and extended viologen form a 1:1 complex dimer tubular structures (Fig.9A) [84].Ogoshiet al.propargylated the monohydroxy pillar[5]arene derivatives to obtain pillar[5]arene derivatives, and then reacted with 5-azidopentanenitrile through alkyne-azide cycloaddition CuAAC reaction to prepare planar chiral supramolecular polymer hostguest conjugates (Fig.9B), which provides a new way to construct chiral supramolecular polymer based on chiral pillar[n]arene,were affected by planar chiral configuration [85].Furthermore,chiral nanotubes with specific lengths and diameters were obtained by chiral functionalized pillar[5]arene, which contained chiral building block of 2,5-dihydroxybenzaldehyde as repeating units.In the study, pre-regulation of chiral molecules was designed, which improved the planar chirality of chiral pillar[5]arene and provided a reference for the construction of chiral nanomaterials based on chiral pillar[n]arenes [86].Zhuet al.combined the (2-bromoethyl)pillar[5]arene DBEP5 with(R,R)-tetraaryl-1,3-dioxolane-4,5-dimethanol derivative (TADDOL-OH) to construct a new chiral 3D polymer network TADP5 that had good heterogeneous asymmetric catalytic ability and recycling ability (Fig.9C).It is very important to synthesize chiral catalysts [87].In addition, a supramolecular polymer three-cavity hostβ-cyclodextrinpillar[5]arene can be constructed by combining the planar chiral pillar[5]arene withβ-cyclodextrin (Fig.9D), and its stereoselectivity depends on the absolute configuration of the central pillar[5]arene and the conjugate position onβ-cyclodextrin, in which the inversion of planar chiral configuration plays an extremely important role [88].
4.Applications of chiral pillar[n]arenes
Chiral pillar[n]arenes can easily interact with chiral or achiral guest molecules due to their chiral functional groups, electronrich cavities and intrinsic planar chiral conformation.They are also widely used in chiral separation and analytical chemistry, especially in fluorescence detection of chiral guest molecules, electrochemical detection of chiral enantiomers and circular dichroism detection of chiral compounds.In addition, pillar[n]arenes also play an important role in biological separation or life processes.
4.1.Chiral fluorescence
Chiral pillar[n]arenes c an easily form stable complexes with guest molecules through electrostatic, hydrophobic bonding, hydrogen bonds,π-π, C–H···O and C–H···π.Linet al.found that LMet-could induce the cationic water-soluble pillar[5]arene AWP5 to show a strong fluorescence signal [89].Similarly, L-Arg-could significantly enhance the fluorescence intensity of naphthoyl hydrazone group pillar[5]arene chemical sensor PNS, while other amino acids hardly changed its fluorescence intensity [90].Zhanget al.prepared theN-(2-aminoethyl)−2-(hexylthio)acetamide functionalized pillar[5]arene fluorescence sensor SNP5, which has achieved high sensitivity and selectivity for L-Trp-detection [91].A pillar[5]arene fluorescence sensor SP5 with two-site cooperative ester functionalization was constructed by Yanget al.They found that only L-Met-can obviously reduce the fluorescence signal intensity of SP5 [92].Chenet al.synthesized planar chiral organoborane fluorescent molecules P5NN and P5BN, and applied chiral pillar[n]arenes in chiral luminescence sensing, chiral supramolecular assembly and chiral fluorescent electronic devices [93].Other small molecular fluorescent structures like pigment molecules with good adsorption and fluorescence properties attached to pillar[n]arenes,could significantly affect the fluorescence intensity [94,95].Tanet al.prepared gold nanoparticle CP5@Au-NPs and used them to detect L-carnitine in human serum and milk samples, with the lowest detection limit of 0.067 μmol/L [96].Weiet al.synthesized a fluorescence sensor BTAP5 and used for high sensitivity detecting LTrp [97].Specific information, such as functional groups, chiral analytes, driving force, mechanisms and LOD of chiral pillar[5]arene,has been summarized in Table 2.
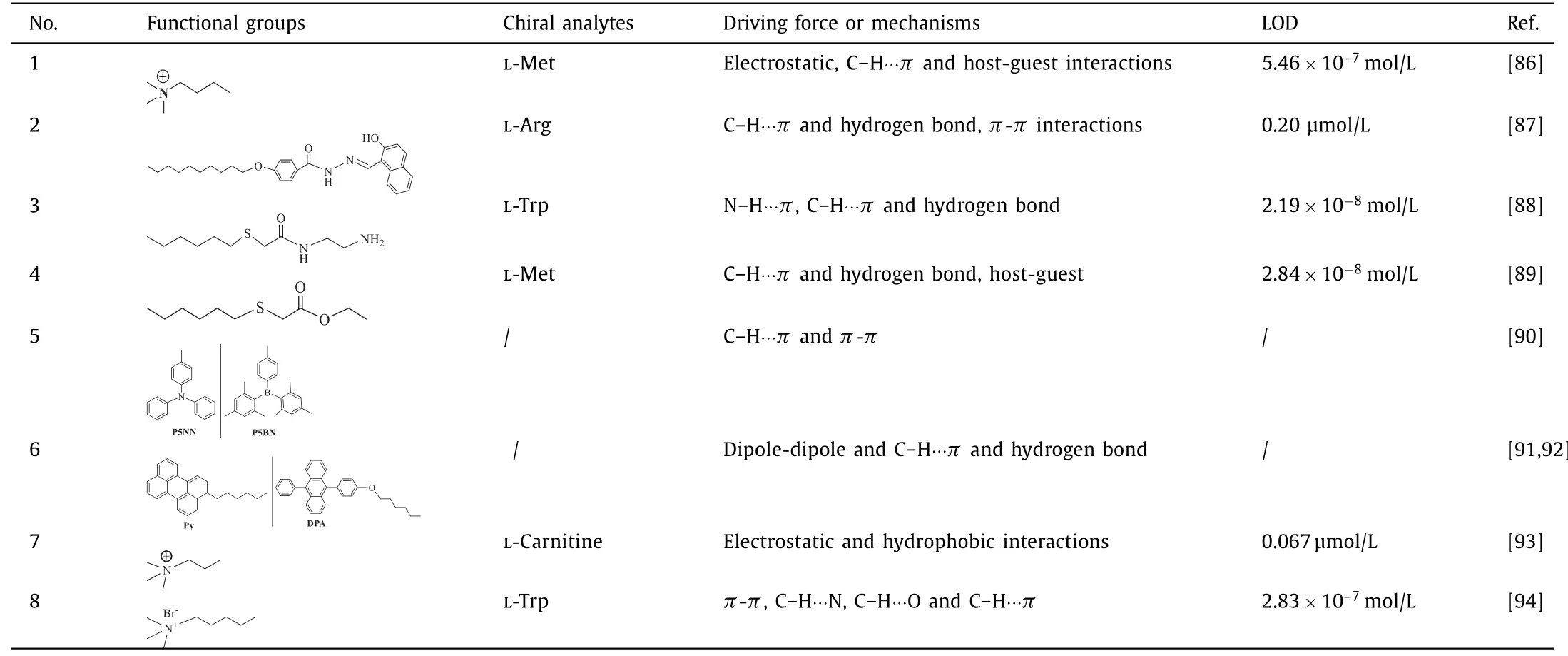
Table 2 Fluorescence detection of chiral guest molecules.
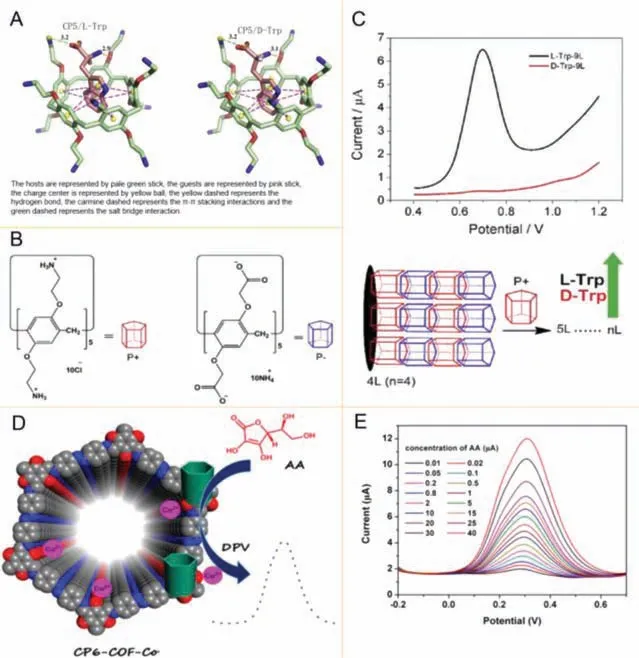
Fig.10.(A-C) The binding models of CP5/L-Trp, CP5/D-Trp, the structures of P- and P+ and LbL assembly of anionic-/cationic-pillar[5]arene multilayer films and the recognition of Trp-isomers.Reproduced with permission [98].Copyright 2018, Elsevier.(D, E) Electrochemical sensing application of the novel nanomaterial CP6-COFCo and quantitative determination current value versus various concentrations of AA at CP6-COF-Co/GCE.Reproduced with permission [100].Copyright 2020, American chemical society.
4.2.Chiral electrochemistry
Pillar[n]arenes with electron-rich system when they interact with guest molecules, may cause electron transfer.The mechanism between pillar[5]arenes and chiral substances by electrochemical method may be that the spatial conformation of chiral isomers of guest molecules is opposite, and the interaction between isomers and pillar[n]arene host is different, which leads to obvious distinguishable electrochemical differences in peak currents for identifying chiral isomers (Figs.10A and B).Zhaoet al.assembled watersoluble cations and anion pillar[5]arene on carboxyl-graphene CGra to form glassy carbon electrode, and then used differential pulse voltammetry DPV to electrochemically identify L/D-Trp (Fig.10C).It indicated that pillar[5]arene-based chiral interface material can be used to identify tryptophan isomers at a low detection limit[98].Yuet al.prepared a chiral sensor based on pillar[n]arenes and used it for electrochemical detection of chiral drug propranolol(R/S-PPL) [99].In addition, Tanet al.prepared a CP6-COF-Co glass electrode with pillar[6]arene loaded with transition metal ions and cations (Fig.10D), and used it for electrochemical detection of AA in acetic acid buffer solution.The oxidation peak current values of ascorbic acid (AA) in CP6-COF-Co/GCE gradually increased when gruadlly increase concentration of AA (Fig.10E), the detection limit could reach 0.26 μmol/L (S/N=3) [100].
4.3.Chiral circular dichroism
Detection of chiral enantiomers by circular dichroism mainly relies on the host-guest interaction between pillar[n]arenes and chiral guest molecules to form a relatively stable host-guest complex,which changes the corresponding wavelength values of maximum CD signal intensity, thus realizing the recognition of chiral enantiomers.Jiet al.synthesized amino acid based pillar[5]arene, when chiral guest molecules entrapped in the cavity of pillar[5]arene,may induce to produce a strong CD signal [58].Furthermore, Zhuet al.used chiral amplification induced by pillar[5]arene host-guest complexs to detect D/L-Arg-by circular dichroism [101].Chenet al.also claimed that WP5 and its homologues can be used as detectors to study the recognition of different chiral regions of a single chiralα-amino acid derivative [53,102].In addition, Zhuet al.synthesizedpS/pRpillar[5]arene derivatives by triflate pillar[5]arene and 4-pyridylphenyl borate through Suzuki coupling reaction, and then reacted with chiral metal ring of Pt(II) receptors with 60° and 90° respectively to synthesize platinum triangle rows withpS/pRplanar chirality, and their enantiomers show mirror images of CD signals at the same wavelength.Moreover,the chiral platinum triangles have the property of circularly polarized light.This study provides a new method to construct planar chiral coordination-driven metal-organic complexes (MOCs) and know chiral supramolecular self-assembly [103].Table 3 summa-rizes the above-mentioned pillar[n]arene edge modification functional groups, analytes and CD signal values and intensity.
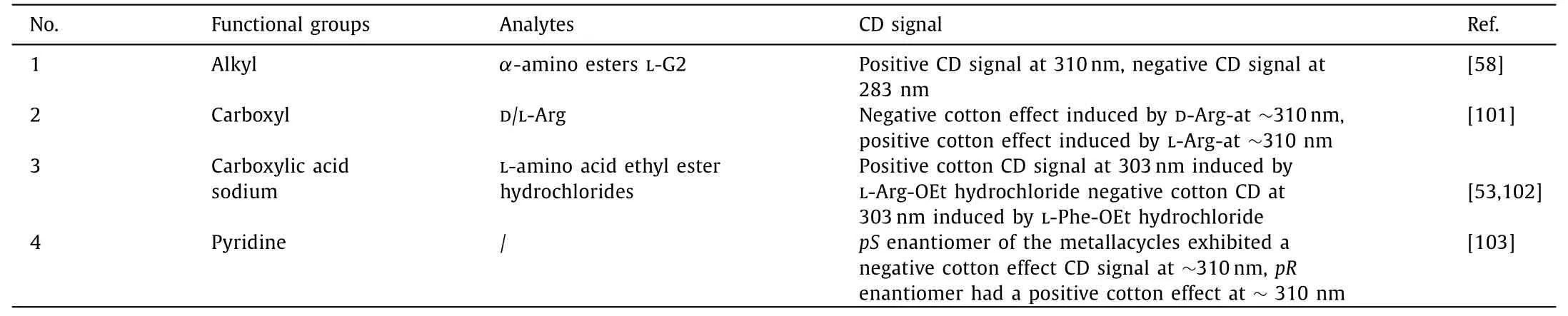
Table 3 The application of circular dichroism in the detection of chiral guest molecules.
4.4.Other chiral applications
Chiral pillar[n]arenes may have more chiral materials and applications, due to their unique structure, shape or intrinsic planar chirality.Therefore, in this section we will summarize other reports on chiral separation and analysis of chiral pillar[n]arenes.Liet al.synthesized peptide-linked pillar[5]arene biomimetic nanochannelspR/pS-pH by using phenylalanine and pillar[5]arene reaction.This study laid a solid foundation for developing high-efficiency artificial water-based biomimetic membranes for water purification.The chiral biomimetic nanochannels were influenced by planar chiral configuration which plays an important potential role in chiral separation analysis [104].By immobilizing nanochannelscontaining liposomes on the active layer of the reverse osmosis (RO) membranes, Limet al.prepared pillararene-based NBMpRPH membranes, which gave a water permeability higher than commercial RO membranes [105].Furthermore, Nierengartenet al.found that the chiral pillar[n]arene modified with mannose clusters can be used as an inhibitor of adhesion between urinary pathogenicEscherichia colistrains and red blood cells [106].Al-Azemiet al.prepared planar chiral pillar[5]arene derivatized with Mosher acid chloride [(S)-(+)-α–methoxy-α-trifluoromethyl phenylacetyl chloride], which can identify chiral alkylammonium salt guest molecules.This may be because the hydrophobic cavity of the pillar[n]arene forms an inclusion compound with the chiral guest molecule, which leads to the shift of the nuclear magnetic peak of the host or guest molecule to the corresponding high field or low field, so that the enantiomers detection of the chiral guest molecule can be realized [107].Isothermal titration calorimetry can also detect chiral enantiomers.Lvet al.reacted dicarboxylic acid pillar[5]arene with monohydroxy calix[4]pyrrole to prepare chiral mandelic acid ester macrocyclic compound PC.The affinity and selectivity of its enantiomer to chiral mandelic acid ester may mainly depend on the structure of chiral guest molecules, that is, based on structural complementarity [108].
5.Conclusions and perspectives
Chiral pillar[n]arenes have shown excellent results in constructing chiral supramolecular materials, detecting chiral small molecules or amino acid enantiomers.Other chiral pillar[n]arenes(n=7, 8…) with larger hydrophobic cavity or more chiral groups,may be more effective in the separation and analysis of chiral analytes with larger volume and more complex structure.The inversion of planar chiral conformation has great potential influence on chiral separation analysis, especially the development of chiral separation materials.Based on the special chiral inversion performance of chiral pillar[n]arenes, we believe that the research on the planar chiral conformation inversion of pillar[n]arenes can promote the remarkable development of chiral pillar[n]arenes in the fields of design, preparation and application of chiral photoelectric devices, chiral asymmetric catalysis and chiral supramolecular materials.
Declaration of competing interest
The authors declare that they have no known competing financial interestsor personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (Nos.21822407, 21974146), the Foundation for Science and Tech Research Project of Gansu Province (No.20JR10RA052).
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
- Crystal facet-dependent electrocatalytic performance of metallic Cu in CO2 reduction reactions
