Crystal facet-dependent electrocatalytic performance of metallic Cu in CO2 reduction reactions
Ho Zhng, Cihong He, Sumei Hn, Zeyng Du, Ling Wng, Qini Yun,Wenin Co, Bowei Zhng, Y-Hui Tin, Qipeng Lu,∗
a School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, China
b Department of Chemistry, City University of Hong Kong, Hong Kong, China
c Institute for Advanced Materials and Technology, University of Science and Technology Beijing, Beijing 100083, China
d Institute of Acoustics, Chinese Academy of Sciences, Beijing 10019, China
ABSTRACT Developing high-performance electrocatalysts for CO2 reduction reaction (CO2RR) is crucial since it is beneficial for environmental protection and the resulting value-add chemical products can act as an alternative to fossil feedstocks.Nonetheless, the direct reduction of CO2 into long-chain hydrocarbons and oxygenated hydrocarbons with high selectivity remains challenging.Copper (Cu) shows a distinctive advantage that it is the only pure metal catalyst for reducing CO2 into multi-carbon (C2+) products and the certain facets (e.g., (100), (111), (111)) of Cu nanocrystals exhibit relatively low energy barriers for the formation of specific products (e.g., CO, HCOOH, CH4, C2H4, C2H5OH, and other C2+ products).Therefore,extensive studies have been carried out to explore the relationship between the facets of Cu nanocrystals and corresponding catalytic products.In this review, we will discuss the crystal facet-dependent electrocatalytic CO2RR performance in metallic Cu catalysts, meanwhile, the detailed reaction mechanisms will be systematically summarized.In addition, we will provide a personal perspective for the future research directions in this emerging field.We believe this review is helpful to guide the design of high-selectivity Cu-based electrocatalysts for CO2RR.
Keywords:Copper CO2 electrochemical reduction Crystal facet-dependence Electrocatalysts
1 Introduction
The rapid industrial revolution and population growth have led to a large amount of global energy consumption [1,2].Energy heavily depends on the burning of fossil fuels, causing a significant increase of the content of CO2in atmosphere [3–5], which further results in the greenhouse effects such as melting glaciers, global warming, and gradually threatens human life [6–8].Therefore, we are facing a crisis for excessive emission of CO2.In this context,extensive studies have been carried out to control CO2emissions such as carbon capture and storage [9,10], carbon capture and utilization [11,12].Carbon capture and storage aims to capture and store CO2in the ocean or land [13], and carbon capture and utilization intends to use CO2as feedstock for generating a variety of economically valuable products [14], including producing the foodgrade CO2, mineral carbonation and reduction of CO2to chemicals by catalysis.Photocatalytic and electrocatalytic CO2reduction reactions (CO2RR) could convert CO2into valuable chemicals in an environmentally friendly way.Compared with photocatalytic CO2RR,electrocatalytic CO2RR is more efficient, which shows higher activity and selectivity for the desired products [15].Thus, electrocatalytic CO2RR becomes a hot spot in the field of energy utilization and environmental protection.
Extensive efforts have been devoted to electrocatalytic CO2RR.However, it has yet to meet industrial application targets owing to technological challenges [16].First, CO2is thermodynamically stable molecules with two C=O double bonds.A high overpotential is significantly required to achieve one-electron reduction of CO2to CO2−, (E=−1.90 Vvs.standard hydrogen electrode, SHE) [17],which is the key step in the reduction process.Thus, it is necessary to input a large amount of energy to meet the requirement of thermodynamic and kinetic processes.Second, during the electrocatalysis, there is a competitive relationship between CO2RR and severe side reactions (e.g., hydrogen evolution reaction (HER)).When HER overpowers CO2RR at the cathode side, the faradic efficiency(FE) of CO2RR significantly decreases [18].Furthermore, the products of CO2RR are complex, including CH4[19,20], CO [21], CH3OH[22], HCOOH [23–25], C2H4[26–29], C2H6[30,31], CH3CHO [32],CH3CH2OH [33], and C2+products [34,35].As shown in Table 1,these products require different numbers of transferred electrons[36,37].The complex process makes it difficult to achieve high selectivity for the desired products, especially for C2and C2+species.To solve the key issues above, it is urgent to develop superior catalysts to achieve desired valuable products through CO2RR.
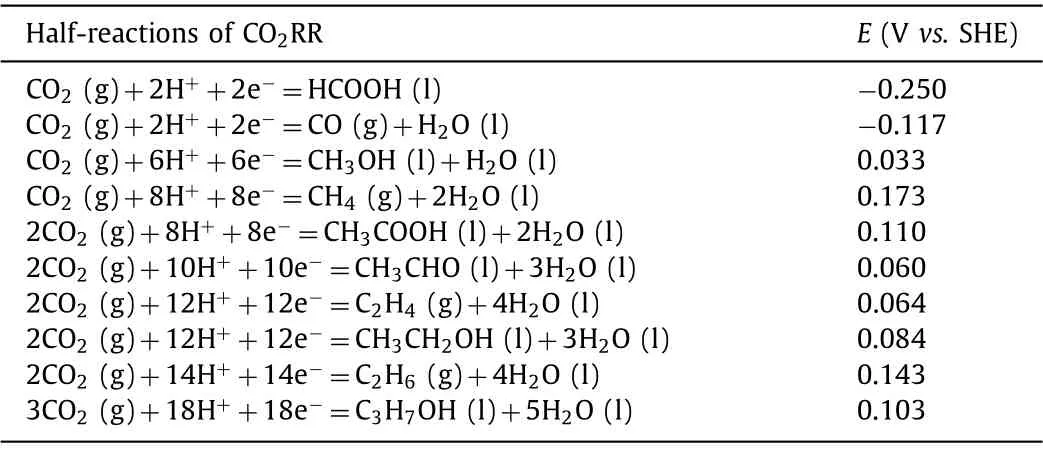
Table 1 Equilibrium potentials for CO2 electrolysis related reactions at pH 7 [36,37].
Until now, various electrocatalysts for CO2RR have been studied,and two kinds of compounds are focused: Solid materials and homogeneous metal complexes [38,39].The metallic electrocatalysts as one kind of solid materials have been applied in CO2RR due to their excellent catalytic performance, facile preparation and separation after the usage [40].According to the electrocatalytic products, single metal catalysts can be divided into four categories: (I)Metals with high selectivity to produce H2(e.g., Ni, Fe, Pt and Ti),(II) metals with high selectivity to produce HCOOH (e.g., Bi, Sn, Pb and In), (III) metals with high selectivity to produce CO (e.g., Au,Ag, Zn and Pd) and (IV) metals with high selectivity to produce C2and C2+products (e.g., Cu) [37,41,42].Among the above pure metal catalysts, Cu is the only one that is capable of reducing CO2into long-chain hydrocarbons and oxygenated hydrocarbons [37].Under certain conditions, up to 16 kinds of products can be detected on the surface of Cu catalysts during CO2RR [23].Importantly, tailoring crystal facet to optimize the catalytic activity and selectivity of Cu catalysts is a key approach to achieve high yields for multicarbon products.Meanwhile, different arrangements of atoms on the crystal facets could produce significantly diverse ratios of C1,C2and C2+products [43].However, up to now, there are rare reviews focusing on explaining the crystal facet-dependent selectivity and activity in Cu-based catalysts, understanding the elementary steps of reactions, revealing the corresponding CO2RR process and establishing relationship between structure and performance[44].
In this review, we will comprehensively introduce the recent developments of metallic Cu catalysts with different crystal facets in CO2RR, and systematically summarize the detailed reaction mechanisms for different valuable products.In addition, we will give some perspectives on the challenges and promising directions in this research field.
2.Reaction mechanisms of metallic Cu with different facets in CO2RR
Metallic Cu possesses thermodynamically stable face center cubic (fcc) crystal phase.Similar to other metals withfccphase,e.g., Au, Ag, Pd, Pt, the synthetic strategies for exposed facet control in Cu have been well-developed [45–48].Through thermodynamic and kinetic controls, Cu nanocrystals with controllable exposed facets and various morphologies could be prepared, such as nanoparticles (NPs) [49,50], nanowires (NWs) [51–55], polyhedrons exposing desired crystal facets and so on.
Among the numerous synthetic strategies, wet-chemical method is considered as a commonly used approach [56–62].In this method, capping agents are usually adopted for shape control, because they can adsorb on specific facets to change the surface free energy and tune the growth rate.As a result, the most favorable thermodynamic facets will be exposed [33,63-69].Besides, etching agents are often employed for the construction of the exposed facets.It is reported that crystal etching can act as the reverse process of crystal growth to endow nanocrystal with high-energy facets [58].In addition, the gas-phase-reduction method and electrochemical method have also been reported to synthesize Cu nanocrystals with various shapes (e.g., cube, octahedron and dodecahedron) [70–72].Compared with wet-chemical method, they can avoid the use of surfactants, thus facilitating the subsequent charge transfer in the electrocatalytic performance test[73].
This review does not discuss the detailed descriptions about the synthesis of metallic Cu nanostructures with different exposed facets by using these methods, since they have been reviewed previously [45–48].Herein, we summarize the established synthetic procedures and corresponding obtained metallic Cu nanostructures with different morphologies and list them in Table 2.In the following sections, we focus on the mechanisms of crystal facetdependent electrocatalytic performance of metallic Cu catalysts in CO2RR processes.

Table 2 Summary of established synthetic procedures for preparing Cu nanocrystals with different facets.
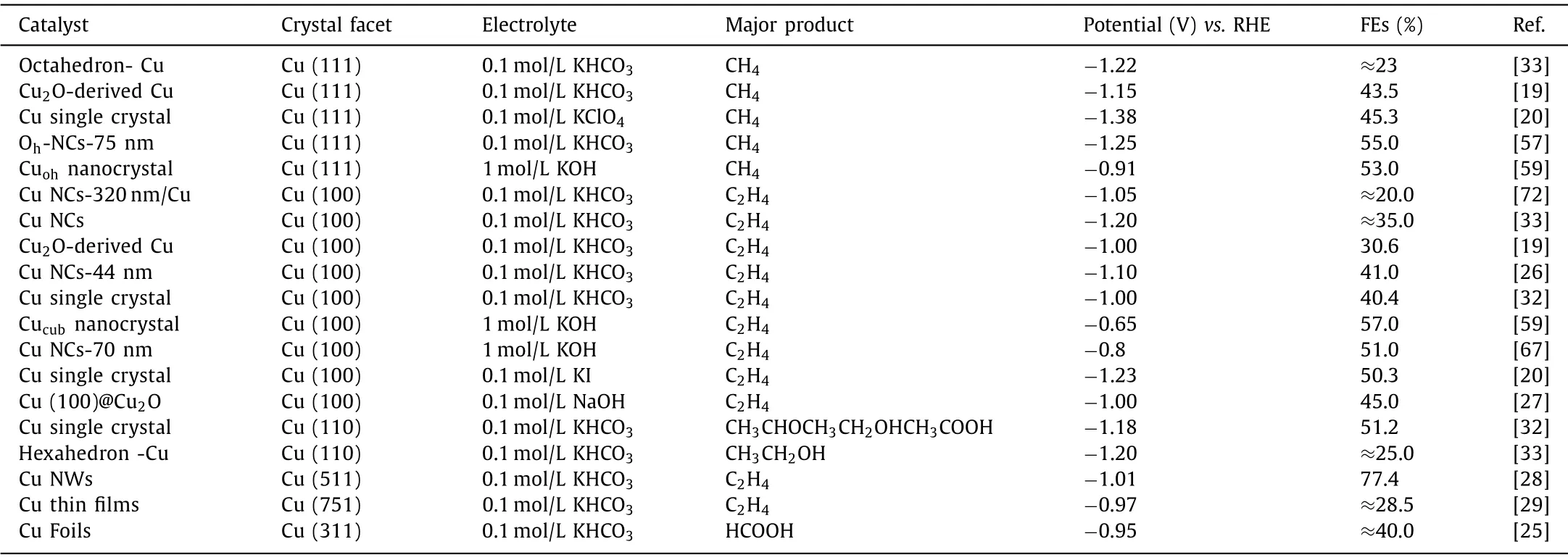
Table 3 Summarization of preferable product on the different Cu crystal facets in CO2RR.
2.1.The initial adsorption modes of CO2 on Cu catalysts
In the pioneering research conducted by Hori and co-workers,Cu was demonstrated to be the unique electrocatalyst that can reduce CO2into valuable products [32,41,74].However, the mechanism of CO2RR is still an open question, and there is no consensus even up to now.As is well known, CO2is initially adsorbed on the catalyst surface and then reduced to CO2−by receiving one electron (Fig.1, step a), which requires a high overpotential [17].Peterson and co-workers first performed density functional theory (DFT) calculations to propose insights into the detailed reaction pathways of CO2RR on Cu surface [75].The results demonstrated that two different adsorption modes of CO2−exist: (I) If oxygen atoms are adsorbed on the surface of the catalyst (Fig.1,step b),∗OCHO intermediate will be obtained after protonation of CO2−, thus generating HCOO−/HCOOH; (II) if the carbon atoms are adsorbed on the surface of catalysts (Fig.1, step c), the carboxyl∗COOH intermediate will be produced followed by the formation of∗CO as a very important intermediate in CO2RR [76].It is proposed that Cu has negative adsorption energy for∗CO through DFT calculations, which avoids the desorption of CO and ensures its subsequent reduction to generate desirable products [77].As discussed in the followed sections, bifurcating reaction pathways will occur on different Cu facets after intermediate∗CO was formed,which is described as “facet-dependence”.Therefore, many studies have attached importance to controlling shapes and structures ofCu nanocrystals with specific exposed facets, including (111), (100)and (110), to obtain desired products in CO2RR [33,67,78,79].
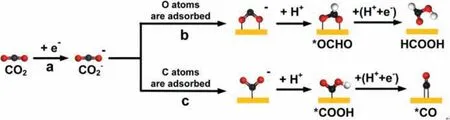
Fig.1.Possible initial reaction steps of CO2RR.The red, black, and white balls represent the O atom, C atom and H atom, respectively.
2.2.Reaction mechanisms on Cu (111) facet
Previous research demonstrated that CH4is the main product on Cu (111) facet through a series of proton-electron transfers at near neutral pH (Table 3).Unavoidably, other byproducts could be obtained simultaneously as well [37,74,80-84].
It is controversial that whether the∗CO intermediate is initially reduced to a formyl∗CHO by forming a C−H bond or a∗COH intermediateviaforming an O−H bond.One viewpoint holds that CH4is derived from∗CHO.Zhao and co-workers used the climbing image nudged elastic band method to calculate the saddle points and minimum energy paths (MEPs) of∗CO protonation into∗CHO and∗COH intermediates on Cu (111) (Fig.2a).The results suggested that the formation barriers of∗CHO and∗COH from∗CO on Cu (111) are 0.85 and 1.39 eV, respectively [85].Thus, the formation of∗CHO is more thermodynamically favorable.Moreover,Peterson and co-workers found that∗CHO can be obtained much easier than∗COH on Cu (111) based on DFT calculation, and regard the protonation of∗CO to∗CHO as the rate-determining step(RDS) (Fig.2b) [75], which is consistent with the conclusions of Nørskov and co-workers [75,86-88].In addition, they summarized that formaldehyde∗CH2O and methoxy∗CH3O are intermediates for CH4production, and proposed the overall pathway proceeding as∗CO →∗CHO →∗CH2O →∗CH3O →∗CH4+∗O →CH4+∗OH →CH4+H2O, which is indicated in Fig.2c1.
However, some research proposed a different conclusion that CH4is originated from∗COH since the∗COH is more favorable to be producedviathe proton-electron transfer [89,90].As shown in the H-shuttling model on the Cu (111) facet (Fig.2d), the distance between the hydrated proton and the oxygen atom of∗CO became shorter from the initial, transition to final states in the formation of∗COH.By using DFT to calculate the free energies for several reaction paths of CO2RR on Cu(111) facet, Nie and co-workers found that the activation barriers for∗CHO and∗COH formation derived from∗CO is 0.39 and 0.21 eV at −1.15 V (vs.RHE), respectively [82,83].As a result,∗COH was the key intermediate for the formation of CH4on Cu (111).As shown in Fig.2c2, the other pathway was proposed:∗CO →∗COH →∗C →∗CH →∗CH2→∗CH3→CH4.Meanwhile,they also pointed out that the pathway in Fig.2c1 is thermodynamically unfavorable.According to the relative free energy diagram for reaction as shown in Fig.2e,∗CH3O tends to produce CH3OH rather than CH4because the barrier for hydrogenation of oxygen atom is smaller than that of carbon atom.Additionally, Additionally, Buonsanti and co-workers synthesized octahedral Cu nanocrystals (Oh-NCs) with different sizes (i.e., 75 nm, 150 nm and 310 nm, Fig.2f), and further investigated their electrochemical properties [57].The results indicated that CH4is the favored product for all samples, and the Oh-NCs with 75 nm achieved the best performance with 77% FEs towards CO2RR and 55% FEs for CH4at−1.25 V (Fig.2g).Because the Cu atoms at corner and edge with low coordination number (CN) are active sites for CO2RR.Therefore, the smaller Cu Oh-NCs with highly exposed active sites exhibited the best catalytic performance.Moreover, CH3OH was demonstrated to be the byproduct on Cu (111) in CO2RR [75].Therefore,it can be confirmed that Cu (111) tends to generate CH4through∗COH as the intermediate.
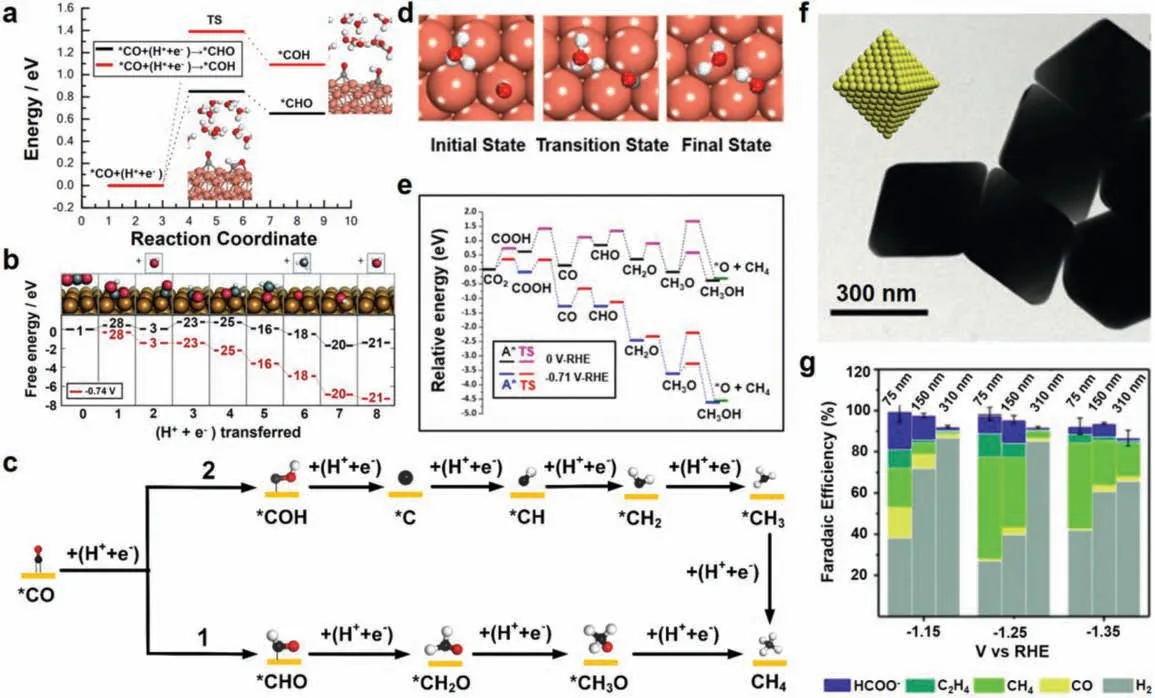
Fig.2.(a) The MEP analysis of ∗CO reduction into ∗CHO and ∗COH intermediates on Cu (111).Reproduced with permission [85].Copyright 2019, American Chemical Society.(b) Free energy diagrams for the lowest energy pathways to CH4.Reproduced with permission [75].Copyright 2010, Royal Society of Chemistry.(c) Possible pathways for CH4 on Cu (111).The red, black, and white balls represent the O atom, C atom and H atom, respectively.(d) Optimized structures of different states involved in ∗CO reduction to ∗COH in the H-shuttling model on the Cu (111) facet.Reproduced with permission [90].Copyright 2016, American Chemical Society.(e) Relative free energy diagrams of pathway through a ∗CHO intermediate.Reproduced with permission [82].Copyright 2014, Elsevier.(f) TEM image of the octahedral Cu nanocrystals.(g) FEs of the three sizes of Cu Oh-NCs.Reproduced with permission [57].Copyright 2019, Royal Society of Chemistry.
In addition, the product distribution on Cu (111) in CO2RR is determined by electrocatalytic reaction conditions [84,91-95].By taking the kinetic barrier and solvent effect into account, first quantum mechanics (QM) study showed that the C1and C2pathways on Cu (111) are competitive and the final products vary with the pH value [96].As shown in Fig.3, at low pH, C2pathways are kinetically unfavorable while C1pathways are promotedvia∗COH intermediate to form CH4.At neutral pH, the C1and C2pathways share the common intermediate,i.e.,∗COH, and the multi-carbon products formed by CO−COH coupling [80].At high pH, the coverage of∗H decreases, which leads to a possibly larger portion of∗CO on the surface.C1pathway is suppressed, and C−C coupling through∗CO dimerization increases.Huang and co-workers demonstrated that the high coverage of∗CO favors the∗CO dimerization on Cu (111) [19].The∗CO coverage from 5/18 to 10/18 monolayer (ML) resulted in a decreased energy barrier around 0.5 eV, which is caused by the weakening of the Cu−∗CO bond at higher∗CO coverage.Besides, the FEs of C2H4could achieve 30.6%when the applied potential was −1.0 V (vs.RHE).Additionally, in CO2RR, graphitic carbon species (Cu=∗CH2) have been detected by X-ray photoelectron spectroscopy and Auger electron spectroscopy measurement.Besides,∗CH2was regarded as the precursor of C2H4[97].Therefore, it is not surprising to find the generation of a bit C2H4as byproduct on Cu (111) during the formation of CH4.
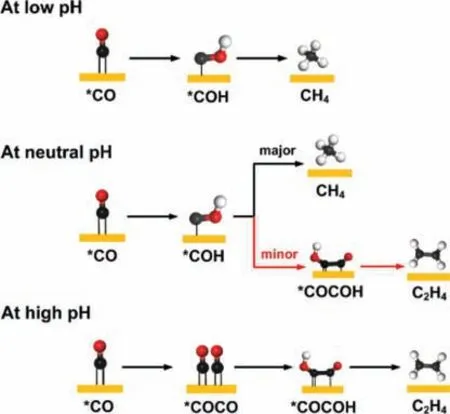
Fig.3.The pH-dependent mechanism of CO2RR on Cu (111).The red, black, and white balls represent the O atom, C atom and H atom, respectively.
2.3.Reaction mechanisms on Cu (100) facet
The CO2RR processes and corresponding mechanisms on Cu(100) are more complicated compared with those on Cu (111).The detailed experimental analyses combined with the DFT calculations have been carried out in the published literatures.Herein, we list the overall roadmap of CO2RR on Cu (100) based on the revealed mechanisms in Figs.4a, b and d.
Currently, Cu (100) is regarded as the suitable facet for the formation of C2H4[86,98].Three pathways to C−C coupling formation on Cu (100) have been summarized: (I)∗CO+∗CHO →∗COCHO,(II)∗CO+∗COH →∗COCOH, (III)∗CO+∗CO →∗COCO.These pathways are considered to be related with the applied potential (U).Head-Gordon and co-workers have developed a micro-kinetic model to predict the Gibbs free energy of elementary steps involved in CO2RR [99].They deemed that C−C bond formation proceedsvia∗CO dimerization at low overpotential, whileviathe combination of∗CO and∗CHO at high overpotentials.By employing the ab initio molecular metadynamics simulations, Goddard and co-workers proposed a reaction mechanism of C2H4for all potentials [100].WhenU <−0.8 V (vs.RHE),∗CHO formation is through protonation of∗CO, furthermore, the obtained∗CHO tends to react with∗CO to generate∗COCHO as an initial intermediate.As Fig.4a shows, a more concrete pathway is proposed:∗COCHO →∗CHOCHO →∗CHOCH2O →∗CHOCH2OH →∗CH2CHO →C2H4[76].WhenU >−0.6 V (vs.RHE), the RDS,i.e., the formation of∗COCO dimer through the coupling of∗CO intermediate,leads to a pH-independent C2H4production [43,81,101,102].By calculating the free-energy barrier for the transition state saddle point (ΔG‡), the explicit explanation is offered:∗COCO dimer is first hydrated to∗COCOH followed by the generation of∗COHCOH instead of∗CCO.TheΔG‡for the formation of∗COHCOH is 0.67 eV, lower than that of∗CCO, which makes it thermodynamically favorable [100].As shown in Fig.4b, the pathway was raised:∗CO+∗CO →∗COCO →∗COCOH →∗COHCOH →∗C=COH →∗CH=COH →∗CH=C→∗CH=CH →∗CH2=CH →C2H4.This mechanism can give a proper explanation for the experimental observation that Cu (100) possess a high activity for C−C coupling at low overpotentials.Through successive oxidative-reductive cycles, Nilsson and co-workers transformed the starting polycrystalline Cu into the Cu cubic nanostructure, which means that the (100) facets are exposed (Fig.4c) [71].The obtained CO2RR performance indicated that Cu (100) favors the C−C coupling with the highest selectivity for C2H4formation at the lowest onset potential (−0.6 Vvs.RHE) compared with other Cu surfaces.However, by using the computational hydrogen electrode (CHE) model [103], Calle-Vallejo and co-workers proposed another mechanism to explain the reaction processes on Cu (100) at low overpotential [104,105].They agreed with the initial reaction steps (∗CO+∗CO →∗COCO →∗COCOH) but proposed different views on the follow-up steps.They illustrated that∗COCOH tended to form∗CCO and projected the pathway:∗CO+∗CO →∗COCO →∗COCOH →∗CCO →∗CHCO →∗CHCHO →∗CH2CHO →C2H4+H2O (Fig.4d).It is also reported that∗CO dimerization process is geometry-sensitive and shows a preference for the Cu (100) surface, which possesses square symmetry of atoms arrangement [106].When −0.8V < U <−0.6 V (vs.RHE),the increase of∗H binding energy leads to a higher coverage content of∗H by displacing∗CO on the surface sites.This conclusion is in accordance with the experimental observation (Fig.4e) [99].Consequently, HER is promoted with restraining the C2formation(Fig.4f).
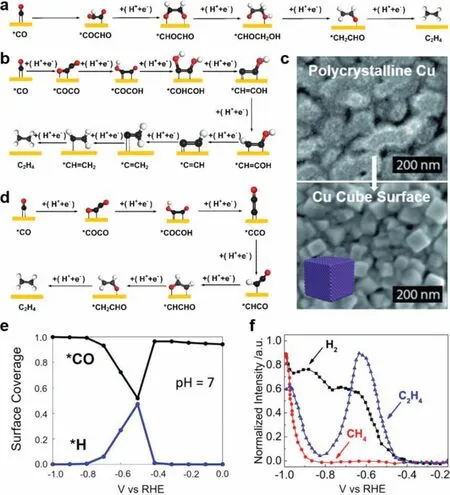
Fig.4.(a) Possible pathways of CO2RR on Cu (100) at high potential.(b) Possible pathways of CO2RR on Cu (100) at low potential.(c) SEM images of the change process from polycrystalline Cu to the Cu Cube surface.Reproduced with permission [71].Copyright 2015, Wiley-VCH.(d) The other possible pathway of CO2RR on Cu (100) at low potential.(e) Diagram of the surface coverage of ∗CO and ∗H at different potential.Reproduced with permission [99].Copyright 2016, American Chemical Society.(f) Diagram of reduction of CO to C2H4, H2, and CH4, determined with OLEMS.Reproduced with permission [100].Copyright 2017, National Academy of Sciences.In (a), (b) and (d), the red, black, and white balls represent the O atom, C atom and H atom, respectively.
It is reported that there are other C2products formed on Cu(100) as well [27,107,108], because∗CH2CHO intermediate will bifurcate into other pathways to produce∗CH3CH2OH, CH3CHO and CH3COOH although Cu (110) is the most favorable facet for the formation of them [32,74].The related mechanisms will be discussed in Section 2.4.
2.4.Reaction mechanisms on Cu (110) facet
In the early 2000s, Hori and co-workers have demonstrated that Cu (110) favors the production of oxygenated hydrocarbon in CO2RR compared with Cu (100) and Cu (111), such as CH3CH2OH,CH3CHO and CH3COOH [32,74].But it does not mean that oxygenates are major products.Recently, it is speculated that sites of Cu (110) facet primarily produce CH3CHO, followed by the further reduction to CH3CH2OH [78].Huang and co-workers demonstrated that CH3CH2OH formed on Cu (110) with better selectivity and activity than those on Cu (100) and Cu (111) [19].However, the mechanism investigations conducted on the Cu (110) surface are rare, which is primarily related to the instability of Cu (110) facet due to the surface reconstructions during the process of electrocatalysis [109].
There is certain association among the formation of CH3COOH,CH3CHO and CH3CH2OH and the current efficiency of CH3CHO,CH3CH2OH is positively correlated with that of CH3COOH (Fig.5a)[32].It is reported that∗CH2CHO is the commonly shared intermediate of them.As Fig.5b shows,∗CH2CHO is first hydrogenated to vinyl alcohol∗CH2CHOH [76].After the rearrangement of∗CH2CHOH, the CH3CHO can be obtained since∗CH2CHOH is the tautomer of CH3CHO.Meanwhile, CH3CH2OH can be obtained through consecutive protonation of∗CH2CHOH.Chen and co-workers prepared the hexarhombic docadehedron-like (H−Cu),cube-like, octahedron-like Cu nanoscale single crystals [33].The morphology of H−Cu is shown in Fig.5c, H−Cu possesses the most edges among the three samples based on the geometry.Furthermore, it is well known that the atomic arrangement along the edge side can be regarded as the (110) facet.They used H−Cu as the electrocatalyst for CO2RR and performed theoretical calculation to compute the formation energy of key steps.The results indicated that Cu (110) favored a route to CH3CHO, because the formation energy of CH3CHO was lower than that of adsorbed∗O and C2H4(Fig.5d).Moreover, they thought that CH3CHO would further be reduced to CH3CH2OH, and as can be seen in Fig.5e, the H−Cu could reduce CO2to CH3CH2OH with FEs ≈25% at −1.2 V and with FEs>10% from −1.15 V to −1.25 V.
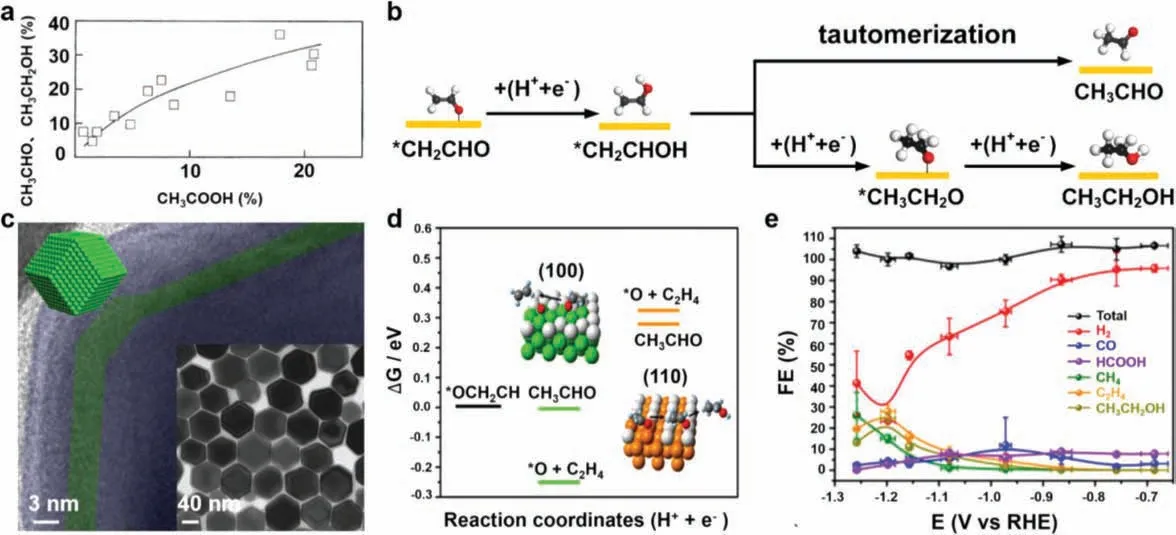
Fig.5.(a) Correlation of the sum of the current efficiency of CH3CHO and C2H5OH with that of CH3COOH.Reproduced with permission [32].Copyright 2003, Elsevier.(b)Possible mechanistic pathways of CO2RR on Cu (110).The red, black, and white balls represent the O atom, C atom and H atom, respectively.(c) HR-TEM image of rhombic dodecahedron-like Cu nanocrystal.The insight is the TEM image.(d) Calculated formation energy of ∗OCH2CH, ∗OCH2CH2, CH3CHO, ∗O, and C2H4 on Cu (100) and Cu (110).(e) Potential-dependent FEs curves of gas-phase products and liquid-phase products.Reproduced with permission [33].Copyright 2019, American Chemical Society.
As shown in Table 3, CH3COOH is another product of the CO2RR on Cu (110), while the reaction mechanisms have not received much attention.Koper and co-workers suggested that, at high potentials, the local alkaline environment near the electrode can promote CH3CHO to yield CH3CH2OH and CH3COO−viaa Cannizzarotype reaction: 2CH3CHO+OH−→CH3CH2OH+CH3COO−[110].However, the observed ratio of these two products is not in accordance with the stoichiometric ratio.CH3COO−is the predominant product.Furthermore, the potential required for the formation of CH3COO−from CH3CHO (−1.5 to −2.0 Vvs.RHE) is higher than that of CO2RR [76].Thus, there must be additional pathways to CH3COO−apart from the Cannizzaro mechanism.Unfortunately, to date, there have been few studies about the pathways of CH3COOH on Cu (110) facet in CO2RR.Therefore, it is necessary to clarify the related mechanisms in future work.
2.5.Reaction mechanisms on high-index facets
High-index facets are a kind of open structures, which are denoted by a set of Miller indices (hkl) with at least an index being greater than one [29,111].Usually, in terms of catalytic performance, metals with high-index facets are considered to be one of the best candidates for catalytic applications owing to their high density of low-coordinated atoms on step, edge, and kink sites.However, the high-index facets with high chemical reactivity always own poor stability, which are not widely accessible compared with low-index facets [112].The reported experimental results have been listed in Table 3.
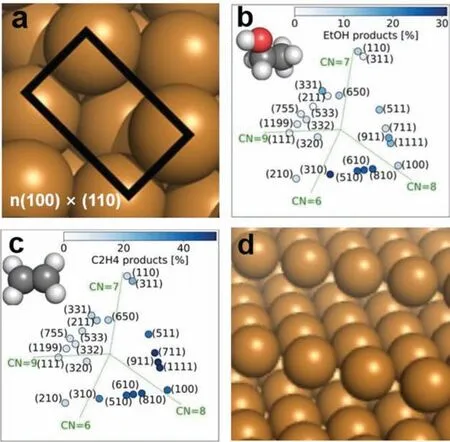
Fig.6.(a) Identification of active sites of the n(100)×(110) step producing CH3CH2OH.(b) Selectivity of CH3CH2OH among different Cu crystal facets.(c) Selectivity of C2H4 among different Cu crystal facets.Reproduced with permission [78].Copyright 2019, American Chemical Society.(d) 3D atomic models of (211) facet from perspective view.Reproduced with permission [114].Copyright 2011, Elsevier.
For the first time, Hori and co-workers explored the Cu electrodes with high-index facet-based electrocatalysts in CO2RR[32,74].For example, the Cu (110) step was introduced into Cu(100) terrace and acquired electrode surface comprisesnatomic rows of (100) terrace and one atomic height of (110) step.According to the Lang, Joyner and Somorjai notation, these surfaces can be named as Cu (S)-[n(100)×(110)] [113].Rossmeisl and coworkers analyzed the Cu facets with respect to the CN, allowing for a unique identification of active sites (Fig.6a) [78].They demonstrated that the (100)×(110) step could act as the special site for CH3CH2OH formation.With the decrease ofnvalue, the step density increases, leading to the increased FEs of CH3CH2OH.As shown in Fig.6b, Cu (310)-[3(100)×(110)] shows a higher selectivity for CH3CH2OH compared with Cu (510)-[5(100)×(110)],Cu (610)-[6(100)×(110)] and Cu (810)-[8(100)×(110)].However, as for the Cu (210) with the high step density, the FEs of CH3CH2OH decrease to less than 10%, which is inconsistent with the above tendency.The authors claimed that the optimal surface structure also needs a certain density of Cu (100) basal plane.Meanwhile, by incorporation of Cu (100) terrace with the Cu (111) step, another high-index facet could be created,i.e., Cu (S)-[n(100)×(111)].The results demonstrated that the surfaces containing well-ordered Cu(100) domains combined with a Cu (111) step show high selectivity for C2H4.Recently, Huang and co-workers successfully prepared the Cu NWs with Cu (511)-[3(100)×(111)] facet throughin-situelectrochemical surface activation of pre-grown Cu NWs with (100)facets [28].The calculation results demonstrate that the formed step sites on Cu (511) lead to a 0.17 eV higher affinity for a single∗CO adsorption compared with that of Cu (100).Moreover, Cu(511) shows a higher selectivity towards C2H4since the free energy of two adjacent∗CO adsorptions on Cu (511) is 0.44 eV lower than that on Cu (100).This result is consistent with the study of Rossmeisl’s group (Fig.6c).
The (100) step was also introduced into (111) terrace to form Cu (S)-[n(111)×(100)].The product distribution of Cu (S)-[n(111)×(100)] resembles that of the Cu (111) single crystal.High gaseous products are detected and the yield of C2+products (e.g.,aldehydes, alcohols) is low.In this series, Cu (211)-[3(111)×(100)]has been studied extensively [75,114-118].It contains a terrace of atoms in the (111) geometry with 3-fold coordination and a (100)step containing 4-fold coordination.The atomic models of (211)facet are shown in Fig.6d.Remarkably, the step sites exhibit higher activity in activating bonds compared with the flat facets.Nørskov and co-workers found that Cu (211) favors the formation of CH4[117].The DFT calculation results demonstrated that∗CHO is more stable than∗CO on Cu (211), resulting in the key step that protonation of∗CO to∗CHO being thermodynamically favorable.Besides, other series of Cu electrodes with high-index facets have been prepared as well, such as Cu (S)-[n(111)×(111)] and Cu (S)-[n(110)×(100)] [25,32].
3.Summary and outlook
Overall, in this review, we point out the importance of CO2RR to solve the current energy crisis and environmental problems,and it is exhilarating that metallic Cu acts as a unique metal to electrochemically reduce CO2into various value-add products, including CO, HCOOH, CH4, CH3OH, C2H4, C2H6, C2H5OH, CH3CHO,CH3COOH and other C2+products.Subsequently, we summarize the synthesis methods of Cu nanocrystals with different facets exposed, such as wet-chemical and electrochemical methods.Moreover, by taking the various theories into account, we discuss the reaction mechanisms of CO2RR among different Cu crystal facets.It is clear that CO2RR is commonly initiated by one-electron injection to the CO2reactant to form anion radical CO2−, which is adsorbed on the catalyst surface.The conversion of CO2−to∗CO or∗HCOO depends on the way in which CO2−is adsorbed on the catalysts surface.The major challenge of CO2RR is to understand the bifurcating pathways from∗CO to other products since∗CO will be further reduced to various products on different Cu facets, which is described as “facet-dependence”.Facet-dependence means that a facet exhibits a relatively lower reaction barrier for the formation of a specific product.Unfortunately, the mechanisms are still not elucidated clearly.Herein, we would like to put forward the following points to be explored in future:
(1) It is urgent to develop the operando characterization techniques to detect the intermediates and identify the active sites during the CO2RR process (e.g., the products protonated from∗CO).Meanwhile, the theoretical calculations are important for going deep into the study of the reaction mechanisms of CO2RR, such as the C−C coupling modes.These experimental results and corresponding mechanism studies are vital to set the guidelines for the rational design of the high-performance CO2RR electrocatalysts.
(2) Nanocrystals with certain exposed facets are ideal materials for establishing the relationship between atomic arrangement and performance.In principle, for the prepared materials, the facet identification is the first and key step for the sequent mechanism study, especially for building correct models in DFT calculations.However, some literatures used wrong methods in facet identification, in which the observed facet based on the measured lattice fringes in TEM images are believed to be the exposed facet.For the nanocrystals with regular shapes, the exposed facet should be identified by comparing the interplanar spacings and intersection angles with the standard patterns in database.In addition, the crystalline materials with largely exposed facets often show relatively higher intensity for the corresponding diffraction peaks than that of the standard pattern in XRD characterization.This phenomenon could be used as the supplementary evidence for facet identification.
(3) Although high-index facets or metastable crystal phases exhibit higher activities, their poor stability mainly limits the further practical application.If the instability can be overcome by surface modification [119–122], it is very promising to apply highindex facet-based catalysts to CO2RR.
(4) To enhance the selectivity for the desired products, other factors (e.g., electrolytes [20], structure of electrodes [123], and electrolysis devices [120]) should be investigated.For example,it is reported that the activity and selectivity of CO2RR is influenced by the alkali metal cation in the electrolyte.The larger cation size favors the formation of C2and C2+products with suppressing the HER [124].Therefore, it is significant to regulate reaction conditions to achieve better catalytic performance.
(5) Cu-based alloy/intermetallic/composite catalysts often show much better properties for specific products in CO2RR [125].However, the related mechanisms have not been deeply studied.The synergistic effects are often mentioned in the published papers [126–128], however, the detailed mechanism studies are lacking.And how to realize the maximum utilization of the “synergistic effects” is still unclear.Thus, it is important to clarify the related mechanisms for optimizing the performance.
In the future research, more effort should be devoted to addressing these challenges.We believe that emphasizing the role of Cu facets will help to guide the designs of electrocatalysts with better performance for CO2RR in the future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No.92061119), the Beijing NOVA program (No.Z201100006820066) and the Fundamental Research Funds for the Central Universities (No.FRF-DF-20–03, No.06500113, No.06500119).
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
