Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
Tong Wng, Jie He, Jin Lu, Yi Zhou,b, Zhohui Wng, Ynbo Zhou,b,∗
a State Environmental Protection Key Laboratory of Environmental Risk Assessment and Control on Chemical Process, East China University of Science and Technology, Shanghai 200237, China
b Shanghai Institute of Pollution Control and Ecological Security, Shanghai 200092, China
c Shanghai Key Lab for Urban Ecological Processes and Eco-Restoration, School of Ecological and Environmental Sciences, East China Normal University,Shanghai 200241, China
ABSTRACT Far-ranging and improper uses of pharmaceuticals and personal care products (PPCPs) over the last few decades have led to severe water contamination that imposes serious effects on human beings and the ecological system.Therefore, there is an increasing demand for a highly-efficient and environmentally friendly technology for the removal of PPCPs from aqueous solutions.Adsorption technology is an appropriate technology to solve this issue.Carbon-based composites, ranging from modified activated carbon to functionalized biochar, show great potential for this purpose.This review hence elaborates on the environmental occurrences and risks of PPCPs and summarizes the recent progress in removing PPCPs from water using carbon-based adsorbents.The pore structure, relatively large specific surface area (SSA),abundant surface functional groups, highly aromatic structures and the extra excellent characteristics of the cooperative materials contribute to their outstanding adsorption performance.Furthermore, the biochar-clay material is cost effective and more efficient compared to traditional activated carbon regarding the adsorption of PPCPs.Among the emerging adsorbents, graphene and carbon nanotubes composites show superior adsorption ability.Their adsorption mechanisms, such as electrostatic interactions,hydrogen bonding, and pore filling, are discussed in details.
Keywords:Adsorption Carbon-based composites PPCPs Adsorbent Water treatment
1.Introduction
Pharmaceutical and personal care products (PPCPs) comprise all drugs (such as antibiotics, nonsteroidal anti-inflammatory, and analgesics medicines, diagnostic agents) and personal care products (shampoos, toothpaste, soaps, perfumes, sun-screen agent,skin anti-aging preparations,etc.), which are widely used to treat diseases and improve the quality of daily life [1].There is no doubt that the human-use PPCPs are generally discharged into the sewage treatment plants after use.PPCPs may be converted into by-products and metabolites during conventional biological treatments [2].These residual PPCPs metabolites and their by-products will still be discharged into the environment through various pathways [3].With the development of analytical techniques,PPCPs can be detected at the trace level [4].Although the highest concentration of PPCPs in the environment is only a few thousand ng/L, these PPCPs are characterized by pseudo-persistence, difficult bioactivity, and difficult biodegradation, and their existence will pose a long-term threat to the aquatic environment and human health [5].PPCPs in the aquatic environment can enter organisms through diet, skin contact, or respiration.The exposure of these PPCPs may affect the kidney and reproductive systems of animals such as fish and sheep [6].PPCPs would lead to jaundice, nausea,vomiting, coma, acute pulmonary edema, dyspnea, convulsions,and even sleep time alteration for human beings (Table S1 in Supporting information).Antibiotic overuse is another potential risk,which can lead to the development and reproduction of antibioticresistant bacteria (ARB) and antibiotic-resistant genes (ARG), and finally threaten human health and ecological safety [7–10].Longterm exposure to antibiotics will significantly affect the growth of aquatic organisms [11], and drug-resistant bacteria can also be transmitted through the food chain [12].Its high cytotoxicity affects cell’s growth, survival and reproduction causes gastrointestinal side effects [13,14], damages human immunity, interferes with human hormone secretion, and even weakens the human reproductive mechanism [15].Many of them could gather in the food chain owing to the high logKowand bio-refractory nature.Even some intermediates have more significant toxicity than the parent PPCPs [16,17].The relative information (such as normalized dose,effect) of the widely detected PPCPs is summarized in Table S1.
Some methods have been reported for the removal of PPCPs including coagulation, flocculation, sedimentation [18], biological activated sludge, anaerobic ammonium oxidation [19], ultrasonic treatment [20], advanced oxidation [21–26], reverse osmosis [27],chlorination [28] and adsorption [29,30].However, coagulation,sedimentation, filtration, and biological activated sludge have poor removal performances.Reverse osmosis is not valid owing to the demand for much energy and mass materials during the treatment process [27].Moreover, the low mineralization degree besides high cost restricts the extensive application of chemical oxidation [17,22,31].Among all of these methods, adsorption is considered as the most ideal technology for the removal of PPCPs due to the good operability, less secondary pollution, low cost, and multifunctional attributes [32,33].Amongst different investigated adsorbents used for the eradication of PPCPs, such as clay minerals, resin, zeolite, cyclodextrin-based adsorbents [29] and carbonbased adsorbents [34], carbon-based materials are considered the most effective due to their abundant oxygen-containing functional groups (OCFGs) (hydroxyl, carboxyl, carbonyl, epoxy groups), large specific surface area (SSA), high reaction activity, and cost effi-ciency [34,35].
A growth curve (Fig.S1 in Supporting information) based on Scopus (https://www.scopus.com/) shows that 38,148 papers (including research article and review) about adsorption are published in 2020, in which 12,028 papers about adsorption by carbon-based adsorbents.There are many reviews concerning carbon-based adsorbents, but few have focused on composite adsorbents composed of two or more materials in a certain way[36,37].There is a lack of in-depth comparative analysis on the latest development of PPCPs adsorption by various novel carbonbased composites, significantly cost evaluation.Furthermore, it is known that the integration of the cheap, available minerals and carbon-based adsorbents could lower the expense and realize a much larger adsorption capacity [38,39].Notably, the carbon-based magnetic composites play a significant role in concentrating the organic molecules.These studies provide many promising applications for the utilization of carbon-based composites on the adsorption of trace PPCPs in the water environment.This review aims to offer comprehensive information about the adsorption of PPCPs by different carbon-based composites.The modification methods and adsorption efficiency of carbon-based composites for various PPCPs are summarized.This review not only sorts out the removal mechanism and efficiency of carbon-based composites but also discusses the application potential of several representative composites from the economic point of view.
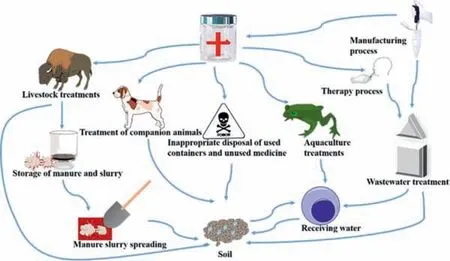
Fig 1.Pathway of PPCPs in environment.
2.Evaluation of PPCPs from the perspective of adsorbate
2.1.Occurrence of PPCPs in water environments
The high consumption of pharmaceuticals and personal care products leads to a significant occurrence of PPCPs in the aquatic environment [40].Sewage treatment plants are the source and sink of PPCPs in the environment.The occurrence, removal and risk assessment of PPCPs in wastewater treatment plants have been extensively studied.Huanget al.investigated 10 antihypertensive drugs and lipid regulators in three municipal wastewater treatment plants in southern China, nifedipine, atenolol, metoprolol,valsartan, and pravastatin were detected in the water, with varying concentrations in other months, and the single drugs in the effluents displayed a low risk to the aquatic environments [41].Benet al.did a risk assessment on the occurrence and removal of 30 PPCPs in 14 municipal wastewater treatment plants.The maximum influent concentration of sulphonamides, tetracyclines, fluoroquinolones, carbamazepine, phenolic estrogenic compounds were recorded as 3930.8, 626.9, 2766.2, 114.7, 4183.4 ng/L and still up to 465.6, 64.5, 831.4, 55, 623.6 ng/L in the effluent, respectively,and based on the data from wastewater treatments (WWTPs), they could pose high risks [42].
The components may migrate into surface water or enter the aquatic environment when the sewage treatment plant’s effluent is used for irrigation, or the sewage-activated sludge is used for agricultural land application [43,44].Veterinary pharmaceuticals would load into the environment by livestock treatments, companion animals, aquaculture and land application of manure and slurry from livestock facilities, and inappropriate disposal of unused and overdue pharmaceuticals.The wastewater produced in the manufacturing and treatment process enters the receiving water after being treated by the sewage treatment plant, and the residual PPCPs migrate and transform in the environment, polluting the water environment and soil environment (Fig.1) [3].Recently 42 kinds of PPCPs were detected in two freshwater lakes of China,with maximum concentrations ranging from 0.04 ng/L to 889 ng/L.Among these detected PPCPs, carbamazepine can pose significant ecological risk, while sulfamethoxazole, caffeine, diethyltoluamide,and carbamazepine are classified as high or intermediate risks[29].The most common and frequently detected PPCPs (such as ciprofloxacin, caffeine, acetaminophen) in water are sorted out as shown in Table S1.
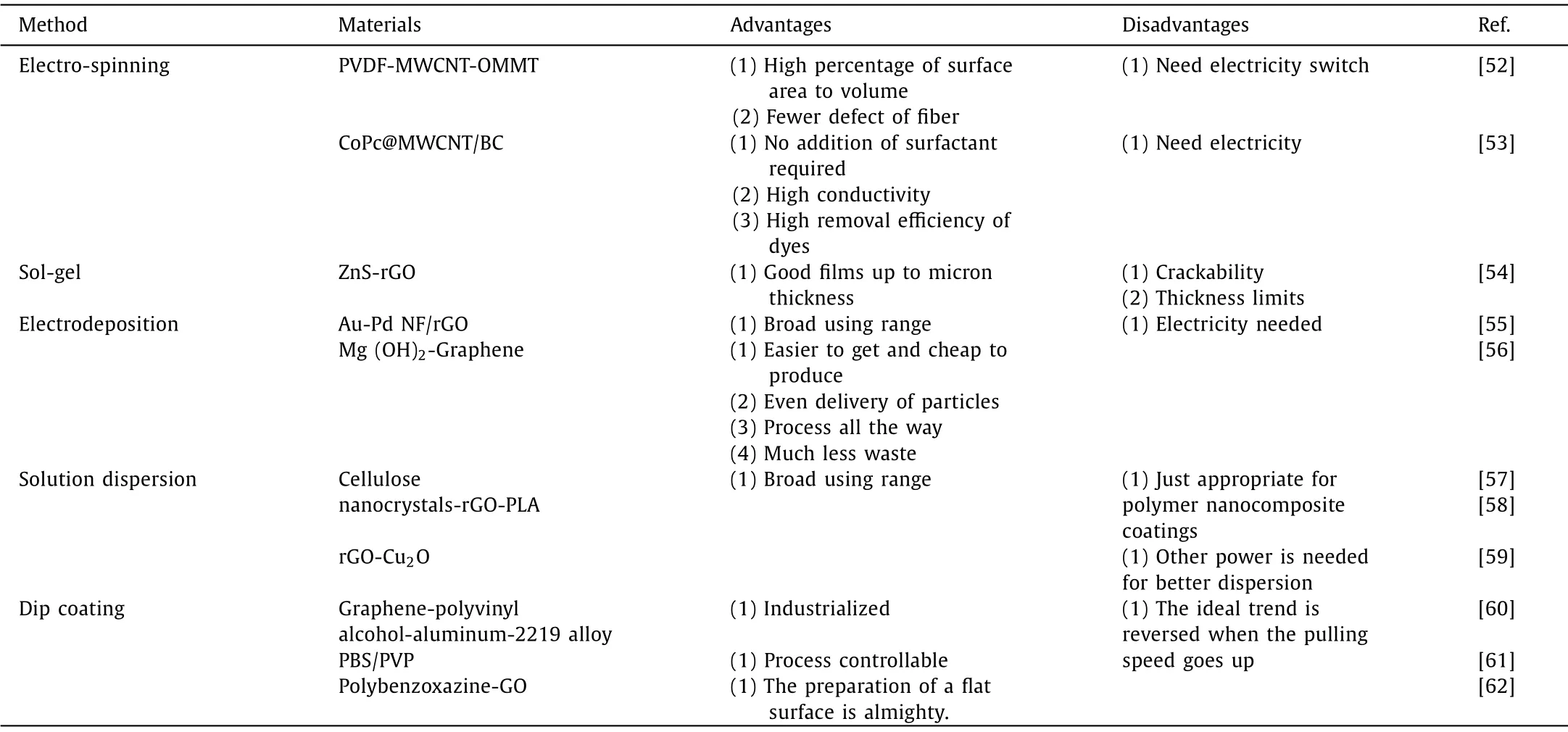
Table 1 Modification of carbon-based composites.
2.2.Properties of PPCPs as adsorbates
The adsorption performance is affected by both adsorbents and adsorbates.As adsorbates, PPCPs are characterized by low concentration, rich molecules and chemical structures [45], coexistence with other pollutants, difficulty in selective removal, low acute toxicity, and high ecological risk.PPCPs in the environment contain various contaminants with different properties, including hydrophobicity, hydrophilicity and amphoteric.The amount of PPCPs adsorbed increase with adsorbate’s hydrophobicity as reported by Zhuet al.[46].On the other hand, an increase in the hydrophilicity of PPCPs reduces their adsorption capacity [47].In actual water, PPCPs often coexist with organic matter (humic acid,etc.), and their competitive adsorption affects the removal effect of PPCPs[48].Although the unique properties of PPCPs as adsorbates may affect the adsorption effect, adsorption is still more economical and applicable than other methods.Carbon-based adsorbents have attracted much attention because of their unique properties.
3.Carbon-based composites and the modification methods
Carbon-based composites refer to the general name of the composite material with carbon as the matrix, and the form is thepowder or blocks non-metallic solid material.It includes activated carbon composites, biochar composites, graphene-based composites, carbon nanotubes (CNTs) composites,etc.Many studies have proved the advantages of carbon-based composites in adsorbing organic pollutants, but their application is limited by a number of factors including reduced porosity, functional groups, poor adsorption, and separation efficiency.
Modification or compounding will significantly improve the adsorption performance of the original carbon materials [49–51].For example, the introduction of magnetic materials into carbonbased materials would enhance the separation efficiency, of which the solid-phase extraction is superior to that of conventional adsorbents.Meanwhile, there is no need for centrifugation or filtration, which simplifies the extraction process and is environmentally friendly (more convenient to recycle and reuse).
Recently, researchers have focused on easy and cost-effective means of operation for the synthesis of composite materials.This review therefore sorts out the most frequently used methods applied during the fabricating process, including electro-spinning,sol-gel, electrodeposition, solution dispersion, and dip-coating.These methods are helpful because they can be used to produce various materials, but they still have some shortcomings.Therefore, the advantages and disadvantages of different methods are shown in Table 1.According to Table 1, the material prepared by electro-spinning has a high specific surface area ratio and high conductivity, but high manufacturing cost [52,53].Sol-gel can produce thin films that are up to micron in thickness, but the material is fragile [54].Electro-deposition is a potential preparation method because it produces less waste and is easy to operate,but it requires a high manufacturing cost [55,56].Solution dispersion is also a widely used method, but it is only suitable for the preparation of polymer nanocomposites [57–59].Each modification method has its own advantages and disadvantages.Compared with other methods, dip coating is the most promising method because it is economical and feasible.This is an industrialized method whose preparation process is effective and controllable, which can be widely applied [60–62].
4.Application of carbon-based composites for the removal of PPCPs
4.1.Adsorption of PPCPs by activated carbon composites
Activated carbon (AC) is manufactured by heating carbonaceous materials at high temperature (above 500 °C) over long periods of time (>10 h) and finally activated with wasteful energy processes[63].AC is widely used as an effective adsorbent for the adsorption of PPCPs owing to its competitive characteristics.First, the amorphous form of carbon is specially treated (usually carbonized at high temperature under oxygen isolation) to produce a highly developed internal pore structure and a large surface area (more than 3000 m2/g), which provide more adsorption sites.Secondly, the raw materials are easily accessible at relatively low prices, which broadens the application of AC to a variety of processes in the adsorption industry [64].Thirdly, activated carbon has outstanding surface chemical characteristics and high surface reactivity, making it an excellent adsorbent for the adsorption of various organic and inorganic pollutants in aqueous solutions [65].However, the existence of dead pores tends to reduce the number of adsorption sites [66].Besides, activated carbon has a high preparation cost, poor separation, regeneration performance, difficult reactivation, and long adsorption process (need to be in contact with pollutants for a long time), which limits its application [67].In addition, enlarging the adsorption capacity, improving adsorption pertinence, and reducing production costs should be considered carefully [68].Therefore, it is indispensable to develop the functionalized composite adsorbents.
One of the improved directions is to develop magnetically activated carbon materials.This composite material has the advantage of high adsorption capacity, easy magnetic separation, and easy solid-liquid separation after removing various organic pollutants.In addition, magnetic composites can improve the adsorption rate and adsorption efficiency [50,69–71].Hydrothermal and co-precipitation methods are widely used to synthesize magnetic composites because of their low cost, high efficiency, and simple operation [72,73].For example, Fröhlichet al.synthesized NiFe2O4/activated carbon by hydrothermal method (Fig.S2 in Supporting information).The adsorption capacity of activated carbon and NiFe2O4/activated carbon (T= 298 K) for ibuprofen were reported as 84.90 mg/g and 97.53 mg/g, and for ketoprofen was 56.5 mg/g and 87.91 mg/g, respectively [50].Wanet al.[71] prepared magnetic activated carbon adsorbents through chemical coprecipitation (Fig.S3 in Supporting information) at different temperature conditions ranging from 25 °C to 150 °C and found that the magnetic composites prepared at 150 °C showed the best magnetic and adsorption capacities (159 mg/g, pH 7.0).In addition, this material resolves the shortcomings of difficulty to separate faced by traditional adsorbents.Danalıo˘gluet al.[74] made magnetic activated carbon/chitosan/Fe3O4by an easy co-precipitation method(Fig.S3), obtaining a high adsorption capacity for ciprofloxacin,erythromycin, and amoxicillin.Maet al.[69] fabricated a chitosaniron/Fe3O4/activated carbon composite by combining powdered activated carbon and the magnetic Fe3O4with chitosan-Fe hydrogel through a simple co-precipitation method (Fig.S3).They found that the modified adsorbent has an ideal adsorption efficiency for a variety of antibiotics.It can remove nearly 99.9% of tetracycline in 30 min.Compared with the research of [74], the additional coating of iron onto the materials could improve the magnetic ability and speed up the adsorption process.However, the coating of magnetic substances onto activated carbon sometimes would decrease the maximum adsorption capacity (MAC).It is reported that Fe2O3/CeO2-activated carbon owns a maximum adsorptive capacity of 1.3 mmol/g while the bare activated carbon owns 2.4 mmol/g (T= 298 K) [75].
The composite of activated carbon and clay minerals is another research direction of modification.Activated carbon-clay minerals composites contain the respective characteristics of activated carbon and clay minerals, and exhibit unique features of high carbon content, multiple-pore structure, and compatibility, making them suitable for regeneration and reuse [76].The mixture of activated carbon and zeolite as a new composite would show their respective hydrophobic and hydrophilic properties, which can be used for the adsorption of the mixture of various contaminants [77].For instance, the aggregation and the solid/liquid separation difficulty can be reduced by the binding of hydroxyapatite and granular activated carbon [78].Khandayet al.[79] prepared a mesoporous high-surface-area zeolite-activated carbon composite by chemical activation and hydrothermal treatment.More information about the adsorption capacity of PPCPs by various activated carbon composites is shown in Table S2 (Supporting information).
4.2.Adsorption of PPCPs by biochar composites
Generally, activated carbon and biochar are two carbonaceous pyrogenic materials that share similar terminologies, fabrication processes, and applications.Although activated carbon has a larger specific surface area and porosity, higher surface activity, and more stable physical and chemical properties, it is one of the most widely used traditional adsorbents.However, its use is limited by preparation cost and regeneration performance.Therefore, many new adsorbents (graphene, carbon nanotubes,etc.) have been developed.Biochar is a kind of carbonaceous material produced by the thermochemical conversion of biomass (such as sawdust, cotton stalk, rice husk, corn stalk, or leaves) under an anaerobic atmosphere without further energy-consuming activated steps.The pore diameter and surface area are determined by the original biomass, heating temperature, activation methods,etc.In addition,the following characteristics make it a superior adsorbent: (1) Low bulk density, high surface area, and variable particle size distribution.(2) Low preparation cost, rich raw materials.These advantages make biochar a favorable competitor and substitute for activated carbon for the adsorption of a variety of pollutants such as PPCPs, volatile organic compounds [80].However, the original biochar has some shortcomings, such as inadequate adsorption capacity and narrow adsorption range [81].Furthermore, the existence of two common toxicant components in biochar, heavy metals, and polycyclic aromatic hydrocarbons, undermines its application.The content of heavy metals in biochar depends on the range of heavy metals in raw materials.In contrast, polycyclic aromatic hydrocarbons were formed during the pyrolysis process no matter what kind of raw materials is used [82].Therefore, it is necessary to modify biochar to improve its applications in wastewater treatment processes.Various modification methods (chemical,physical, magnetic modification) are needed to solve these problems [82].Modified biochar can improve the surface hydrophobicity/hydrophilicity, surface charge, surface area, and pore volume of biochar to improve the adsorption of organic pollutants [83].
Magnetic biochar has been taken into public vision due to its validation on separation and benefits to lower the recycling expense of adsorbents [84].The magnetic materials-loaded biochar has many surface organic functional groups, smaller particle sizes,relatively higher surface area, which would help display higher sorption capacity than original biochar [85].For instance, Weiet al.[86] synthesized the iron-loaded sludge biochar by one-step hydrothermal methods as shown in Fig.S2, and the adsorption capacity of initial biochar and iron-loaded sludge biochar for norfloxacin was 46.21 mg/g and 70.63 mg/g, respectively.The adsorption capacity of raw biochar and Fe-biochar for chlortetracycline was 28.19 mg/g and 89.05 mg/g.The adsorption mechanism mainly relies on hydrogen bonding and inter-particle diffusion.Some researchers also discovered that humic acid-modified magnetic biochar could inhibit the aggregation or auto-oxidation of Fe3O4and optimize the adsorption ability [87].Researchers synthesized the raw biochar through pyrolysis at the temperature of 773 K under anoxic conditions, then coated humic acid onto the magnetic biochar.They found the adsorption capacity of pristine magnetic biochar and humic acid coating biochar (T= 298 K)for ciprofloxacin are 6.94 mg/g and 10.86 mg/g, for norfloxacin are 6.63 mg/g and 9.53 mg/g, for enrofloxacin are 6.83 mg/g and 8.36 mg/g, respectively [88].The adsorption capacity of biochar/chitosan hydrogel beads and humic acid-biochar/chitosan hydrogel beads for ciprofloxacin are 76.86 mg/g and 154.86 mg/g[38].By comparing the two groups of the studies above, we notice that the chitosan coating could increase the adsorption capacity to some extent.All the magnetic biochar modified with humic acid may not have a significant promotion effect on the adsorption of pollutants, which may be related to the types of contaminants and the parent materials of biochar.The magnetic ZnO doped biochar has a tremendous adsorption capacity (449.40 mg/g)and stable and fast PPCPs adsorption (the adsorption capacity of ciprofloxacin increased in the first 2 h and reached to a balance at 4 h ultimately) when comparing with other works [89].Nowadays,Liyanageet al.[90] synthesized Fe3O4/biochar for the adsorption of caffeine, ibuprofen and acetylsalicylic acid.They found that biochar loaded with Fe3O4significantly improve the removal efficiency of ibuprofen and acetylsalicylic acid.In addition, they inferred that the high adsorption rate might be owing to the parent material(Douglas fir byproduct) [90,91].
The pretreatment of biochar with clay minerals can produce ultrafine silica particles on the surface of biochar substrate and increase the number of oxygen-containing surface functional groups(OCSFG).Finally, the silica particles and OCSFGs can act as sorption sites for containment in aqueous solutions and heavily enhance the adsorption efficiency of the biochar [92].Modified biochar through impregnation with clay minerals might worsen the problems such as the electrostatic repulsion (if the pollutants are ionic in nature) [93].For biochar-clay composites, in which the biochar would supply space for the distribution of the clay particles and thus enhance the adsorption ability of the raw biochar [94].In addition, the introduction of clay minerals would strengthen the active sites, thus improving the interaction between ionizable PPCPs molecules and composites adsorbents, giving high sorption efficiency.The MAC of biochar and biochar-montmorillonite composite for norfloxacin is 122.16 mg/g and 167.36 mg/g, respectively [95].The biochar-attapulgite composites own a maximum adsorption of 5.02 mg/g (T= 298.15 K) while the raw biochar is 2.72 mg/g [92].The MAC of biochar, biochar-silica and biocharzeolite composite for chlortetracycline are 28.19 mg/g, 45.57 mg/g and 30.42 mg/g, respectively [83].The adsorption properties of both magnetic-biochar composites and clay mineral-biochar composites are better than those of original biochar.In addition, we found that iron-loaded biochar had a better adsorption capacity to clay-loaded biochar.However, in the previously published papers,the contribution ratio of each component to the adsorption capacity of the composite material was unknown, and the adsorption mechanism was not thoroughly studied.More information about adsorption capacity of PPCPs by various biochar composites can be seen in Table S2.
4.3.Adsorption of PPCPs by graphene-based composites
Graphene is a two-dimensional monolayer honeycomb carbonbased nanomaterial synthesized by bonding sp2hybrid carbon atoms [96].Comparing with plat structured activated carbon, ball structured graphene owns a relatively large specific surface area.However, a particular surface area of activated carbon would approach or even exceed graphene.Some researchers found it is impossible to increase the adsorption capacity by increasing pore diameter to increase the specific surface area.Usually, compared with biochar, graphene shows higher removal efficiency due to its transparency, electrical conductivity, mechanical strength, and chemical stability [97].Graphene as an adsorbent owns fantastic characteristics for the adsorption of PPCPs.First, the single-layered carbon structure ensures that all the atoms contained in the materials are exposed from both sides to the aqueous solutions and are accessible to touch with PPCPs (mainly byπ-πinteraction).Secondly, graphene has aroused great attention compared with primary adsorbents due to its high SSA (2630 m2/g), excellent active adsorption sites, great delocalizedπ-electron systems, and excellent chemical stability, which renders it proper for the adsorption of PPCPs [36,98].However, the graphene layer has the characteristics of easy aggregation and hydrophobic surface, which will reduce the material transport speed and reduce SSA, and its application in ecological protection is limited [37,99].Therefore, it is of great necessity to make functionalized graphene to get over these drawbacks.
The composites of magnetic substances and graphene are a great idea to get good solid-liquid separation.The commonly used magnetic substances, such as Fe3O4,contain many superiorities such as particular size and morphology-dependent physical and chemical characteristics, biocompatibility, and favorable magnetic characteristics [100].Combining magnetic materials and graphene could avoid the reuniting of magnetic substances and eliminate the stacking of graphene and its derivatives [101].For example, the preparation of mesoporous silica-magnetic graphene oxide nanocomposite, which combines the superiority of these substances (graphene oxide, magnetic, and mesoporous silica) as an adsorbent and the advantages of nanomaterial such as small particle and high surface/volume ratio for adsorption application was effective [102].The composites were synthesized to enhance the interfacial characteristics of graphene oxide, improve the dispersity, realized PPCPs adsorptionviapore structure [103].Miaoet al.synthesized magnetic graphene oxide by one-pot solvothermal method as shown in Fig.S2 at 190 °C.The MAC of tetracycline, chlortetracycline, and oxytetracycline were 142.0 mg/g,286.5 mg/g, and 292.4 mg/g under 313 K, respectively [104].Linet al.prepared graphene oxide functionalized with magnetic nanoparticles by two-step method.The MAC of tetracycline, oxytetracycline, chlortetracycline, and doxycycline were 39.1 mg/g, 45.0 mg/g,42.6 and 35.5 mg/g, respectively [105].Penget al.synthesized NrGO/Fe3O4by N modifying reduced graphene oxide (rGO) with Fe3O4.The MAC of N-rGO/Fe3O4for norfloxacin and ketoprofen are 158.1 mg/g and 468.0 mg/g [106].However, because the preparation process is more complex than the general synthesis process,the cost of preparation processes should be considered carefully.
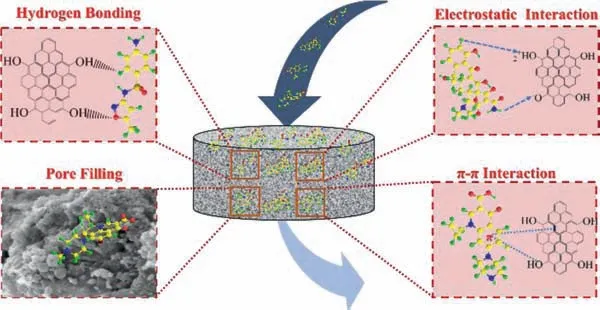
Fig 2.Summary of possible mechanisms for the adsorption of PPCPs by carbonbased composites.
Some small organic molecules are usually coated onto graphene or graphene oxide surfaces.It is easy for these molecules to bind to graphene materials because their rich hydrophilic groups can give more adsorption sites for PPCPs.For example, Liet al.[107] prepared nitrilotriacetic acid-functionalized magnetic graphene oxide,utilized to remove tetracycline and ciprofloxacin.The MAC of synthesized materials was 263.78 mg/g (T= 298 K).Liet al.synthesized diethylenetriaminepentaacetic acid-functionalized magnetic graphene oxide, whose MAC of tetracycline and ciprofloxacin were 1230.83 mg/g and 1750 mg/g (T= 293 K) [108].Maybe the abundant amid and carboxyl groups contributed to the excellent adsorption performance.Chitosan and alginate have received extensive attention owing to their biocompatibility and nontoxicity.Wanget al.prepared the magnetic chitosan grafted graphene oxide for the adsorption of ciprofloxacin, and the MAC is 282.9 mg/g [109].Feiet al.prepared sodium alginate hydrogel, sodium alginate aerogel, graphene oxide-sodium alginate hydrogel, and graphene oxide-sodium alginate aerogel.Their MAC are 8.66 mg/g, 4.23 mg/g, 86.12 mg/g, and 55.55 mg/g, respectively.As reported, the adsorption capacity increased nearly 10 times for the adsorption of ciprofloxacin [110].The unique molecular structure is competitive for binding with PPCPs to the graphene sheets.Nevertheless, expensive and toxic assist agents are needed during the preparation process, restricting such composites’industrialization[111].
By modifying the structure of the original graphene to synthesize magnetic graphene composites and introducing suitable functional groups, aerogels, and other excellent composites, agglomeration can be avoided, surface-active sites can be increased, structural properties can be improved, and adsorption efficiency can be improved [112].However, the preparation process of composite materials is complex and toxic auxiliaries are needed.Therefore, the cost of adsorbent, biodegradability, and toxicity of raw materials should be considered for industrial application.The current study on the adsorption mechanism of composite materials is not clear, and further research on the mechanism is needed.More information about the adsorption capacity of PPCPs by various graphene composites as shown in Table S3 (Supporting information).
4.4.Adsorption of PPCPs by CNTs composites
Carbon nanotubes (CNTs), a kind of nanomaterial with a hexagonal network structure, have the advantages of solid hydrophobicity, large specific surface area, high porosity, and hollow layered structure been successfully applied in the field of water treatment[34,113].Carbon nanotubes (CNTs), including both the single-wall carbon nanotubes (SWCNTs) and multiple wall carbon nanotubes(MWCNTs), were first found in 1991 [32].Comparing with activated carbon, biochar, and graphene, smaller specific surface area and larger pore diameter reduce CNTs’ adsorption capacity.The adsorption performance of hollow CNTs is lamed when comparing with ultra-flake graphene.Abundant surface functional groups,sharp curvatures, and chemical stability are conducive to the adsorption and removal of organic pollutants [114].Larger inner volumes and accessible inner surfaces also play an essential role in adsorption than activated carbon and biochar [115].In addition,CNTs has a strong affinity for organic compounds, which made the adsorption of PPCPs more favorable [116].However, CNTs are easily aggregated owing to the strong van der Waals interactions [114].Particles released into the environment by accident would harm organisms.Therefore, the functionalized CNTs are indispensable for the removal of pollutants and avoiding the drawbacks.
Functionalization by polymers enhances both solubility and dispersion characteristics of CNTs, which contributes to the increase of the adsorption capacity of CNTs in aqueous solutions[117].For example, Azqhandiet al.synthesized MWCNT/poly ethylene glycol (PEG)/beta-cyclodextrin (β-CD) for the adsorption of triclosan.The MAC is 420 mg/g (T= 298 K, sonication time: 0.5–30 min) [117].Assembly of graphene or graphene oxide into three-dimension graphene-based macrostructures has been regarded as a prospective method to protect graphene oxide nanosheet from aggregation, promote mass transport behavior,and reduce environmental risk of nanoparticles release [118,119].Shenet al.prepared the three-dimension macrostructures by mixing one-dimension oxidized CNTs and two-dimension graphene oxide nanosheets through the ice template method [119].The saturation uptake capacities of three-dimension macrostructures towards oxytetracycline and diethyl phthalates are 1729 mg/g and 680 mg/g, respectively.Huet al.synthesized multiwall carbon nanotubes/iron oxides/β-cyclodextrin composite by using the plasma-induced grafting method.The MAC of 1-naphthylamine on MWCNTS/iron oxides and MWCNTs/iron oxides/β-cyclodextrin are 153.8 mg/g and 200.0 mg/g (T= 293.15 K), respectively [120].However, this adsorbent exhibited a lower adsorption rate: it attained the adsorption saturation at 80 h In addition, it is demonstrated that the immobilization of CNT onto a polymer membrane is an effective way to improve the adsorption behavior and better antifouling properties.The membrane with CNT uniformly scattered in the rGO layer performed better than other membranes regarding the PPCPs removal efficiency (73%−100%) [121].
The cooperation of CNTs and magnetic materials would be beneficial for providing active adsorption sites and thus increase the adsorption capacity.The stability would be improved and thus reduce the aggregation [122].For example, Xionget al.synthesized MWCNT/MIL-53(Fe) by dispersing MWCNT intoN,Ndimethylformamide, and other mixture solution.The MAC of tetracycline hydrochloride (TCN), oxytetracycline hydrochloride (OTC)and chlortetracycline hydrochloride (CTC) by MWCNT/ MIL-53(Fe)are 364.37 mg/g, 325.59 mg/g and 180.68 mg/g, respectively [123].Xionget al.[124] prepared the MWCNT/NH2−MIL-53(Fe) composite by dispersing purified MWCNT in precursor mixtures of the NH2−MIL-53(Fe).The MAC of TCN and CTC by MWCNT are 287.91 mg/g and 54.01 mg/g, while MWCNT/NH2−MIL-53(Fe) are 368.49 mg/g and 254.04 mg/g.Wanget al.[125] synthesized CNTs/CoFe2O4composite by using carboxylic CNTs and amino CNTsviathe hydrothermal method and realized a satisfied adsorption capacity.The MAC of sulfamethoxazole (SMX) and 17β-estradiol(E2) by CNTs-C/CoFe2O4are 33.7 mg/g and 30.8 mg/g; The MAC of SMX and E2 by CNTs-N/CoFe2O4are 68.4 mg/g and 32.6 mg/g.In the modification process, we should not only consider how to improve the adsorption performance of the adsorbent, but also carefully consider the application of reagents and the cost of the fabrication process.The above studies all demonstrated the improvement of the adsorption capacity, but the contribution to the adsorption capacity of each material is still unclear.More information about adsorption capacity of PPCPs by various CNTs composites as shown in Table S4 (Supporting information).
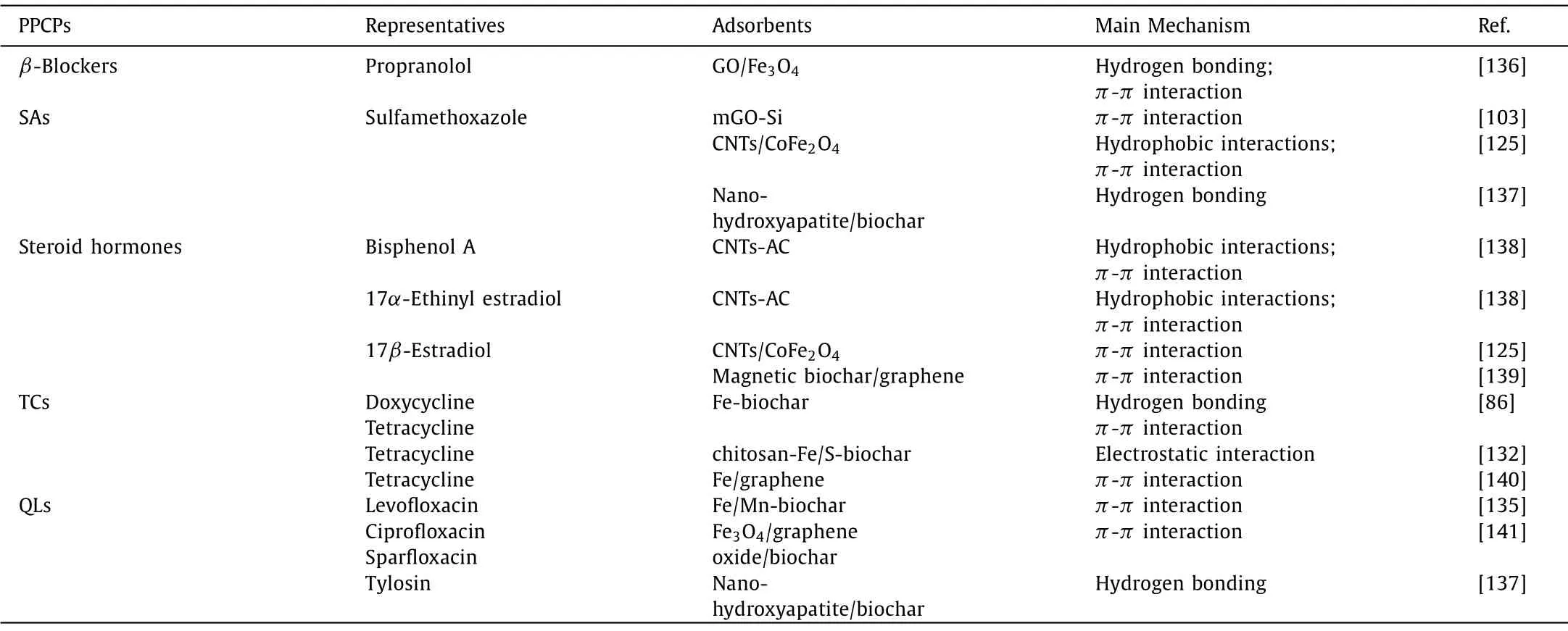
Table 2 Main mechanism of carbon-based composites for the adsorption of representative PPCPs.
In all the systems mentioned above, researchers just considered the single pollutant in the water ideal.However, the current research still shows a low adsorption performance in the multi pollutants exit system.The existence of a variety of pollutants in the complex sewage system may affect the adsorption performance of the adsorbent.For example, Zhouet al.[126] synthesized the cyclodextrin polymer for bisphenol A, chloroxylenol, and carbamazepine adsorption.They found the apparent competitive adsorption between these pollutants in the mixture solution according to the adsorption kinetics.Carbon-based composites have excellent adsorption properties.Although the research of most new adsorbents is limited to the laboratory level and is difficult to be applied in industry due to the impact of actual water quality and cost, the vigorous development of adsorbents provides more options for the removal of PPCPs in the future.
5.Adsorption mechanisms
To clarify the adsorption mechanism associated with carbonbased composites, mechanisms includingπ-πinteractions, electrostatic interactions, and hydrogen bonding interactions between adsorbates and adsorbents have been highlighted.The concrete adsorption mechanisms as shown in Fig.2.
5.1.π-π interaction
π-πinteraction, as a significant weak interaction, majorly exists in the aromatic rings system.Functional groups on the adsorbents act asπ-electron-acceptor/donor, while the benzene rings of pollutants act asπ-electron-donor/acceptor.Zhouet al.used the alkaliacid modified magnetic biochar for the adsorption of tetracycline and found thatπ-πinteraction is the main force.It is inferred that the tetracycline acted asπ-electron-donor, while the functioned adsorbents were seen asπ-electron-acceptor [127].Ninwiweket al.[103] inferred that SMX was seen as theπ-electron-acceptor owing to the amino-functional group and oxygen-contained rings.Delhirajaet al.[128] made the graphene oxide-activated carbonchitosan composites for the adsorption of PPCPs.In the presence of activated carbon, theπ-πinteraction between the rings of the molecules and the composites is much stronger.This is owing to the large number of active centers of PPCPs.Denget al.[129] synthesized the carbon dot-modified magnetic carbon nanotubes for the removal of carbamazepine.The adsorption mechanism was inferred according to the Raman spectra.In their study, the blue shift of the G-band illustrated the existence ofπ-πinteraction.
5.2.Hydrogen bonding
The hydrogen bond is the electrostatic force between the hydrogen nucleus on the strong polar bond (A-H) and the highly electronegative and partially negative atom B.The oxhydryl on the adsorbents would bind with the OCFGsviahydrogen bonding [130].Delhirajaet al.[128] produced graphene oxide-activated carbon-chitosan composites for the adsorption of PPCPs.Hydrogenbonding played an essential role during this adsorption process.Fuet al.[131] fabricated biochar-montmorillonite composites for the adsorption of atenolol.Various OCFGs of biochar and hydroxyl groups of montmorillonites make it easy to form hydrogen bonds with atenolol.Lianget al.[130] synthesized the magnetic biocharmontmorillonite composites and demonstrated that the OCFGs on the surface of adsorbents would bond with OTCviahydrogen.
5.3.Electrostatic interaction
The electrostatic interaction is always evaluated under various pH values [132].Pollutant has many ionizable functional groups under different pH values.For example, tetracycline (TC) is positively charged when the solution pH is below A, non-charged or negatively charged when the solution pH ranges from A to B, and negatively charged when the solution pH is beyond B.Meanwhile,the surface charge of the adsorbents is affected by the solution pH.The surface of adsorbents would be positively charged when the solution pH is below the pHpzc(pHpzc>A).So, when the solution pH is below A, both of TC and surface of adsorbents are positively charged.Thus, electrostatic repulsion worked.When the solution pH ranged from A to pHpzc, the positively charged surface of adsorbents would attract neutral and negatively charged TC due to the electrostatic attraction.However, electrostatic repulsion worked again due to negatively charged TC and adsorbents when the solution pH is beyond pHpzc[132].
5.4.Other mechanisms
Pore filling is regarded as the primary mechanism.However, it always occurred on the microporous-contained adsorbents[133–135].The size of molecules is a significant factor for the diffusion process, which plays a much more critical role than the active sites presented in the PPCPs [128].Xianget al.[135] proposed that pore filling was the primary physical sorption mechanism.In addition, ion exchange reaction is also one of the more common adsorption mechanisms.Lianget al.[130] inferred that montmorillonite coated onto the magnetic biochar composites promoted the adsorption process through cation exchange reaction.Mechanisms between different adsorbents and PPCPs as shown in Table 2[86,103,125,132,135–141].
6.Conclusion and outlook
This review concludes the modification methods of carbonbased composites and the application of carbon-based composites,along with the mechanisms for the adsorption of PPCPs from aqueous solutions.Electrostatic interactions, hydrogen bonding, pore filling and especiallyπ-πinteractions, are the most common mechanisms during adsorption.These composites present both the functional groups of carbon family materials and excellent characteristics of the cooperative materials.The developed pore volume and considerable SSA help carbon-based adsorbents gain a competitive edge in the adsorption field due to the large adsorption capacity and fast adsorption rate.The graphene-based composites and CNTs based composites are relatively outstanding.However, most current researches focus on evaluating the removal efficiency under a laboratory scale, so the performance under the real wastewater condition should be investigated in depth combined with the cost analysis to determine the application prospects.There are some ideas we can consider to render the process more cost effective.
First, simplifying complexity, although the compounding of multiple substances increases the adsorption capacity of pollutants,the chemicals and energy consumed during the synthesis of these adsorbents may be greater than the economy brought about by the increased adsorption capacity benefit.The evaluation of adsorbents by their adsorption capacity is inappropriate.Secondly, reuse of ‘the waste’.The organics adsorbed on the adsorbents are diffi-cult to deal with and use directly.So, this ‘waste’can be used as the raw materials of graphene or CNTs by a chemical vapor deposition method, which can lower the cost and make full use of pollutants.Thirdly, make full use of the energy and materials.For example, the gas produced during the chemical vapor deposition process can be burned to produce heat for desorption.The highly recyclable CNTs or graphene should be regenerated with the appropriate method to extend their lifespan.
Some researches only investigate the adsorption ability of one of the original materials and the composite, and the simple addition of the two materials for the adsorption of pollutants also needs further investigation to verify the synergy of the composites.Furthermore, much more attention and efforts should be paid to natural low-cost carbon family materials (such as biochar).Regeneration of adsorbent materials is an important factor to evaluate the adsorption performance of adsorbents.Solution elution is the most used method for regeneration of carbon-based composites after adsorption.The most used elution methods include acid washing (hydrochloric acid, sulfuric acid, nitric acid), alkaline washing (NaOH, dilute ammonia water), organic solution (methanol,ethanol) elution,etc.The elution process will lead to the quality loss of adsorbent and poor regeneration performance, and consume a lot of water and electricity, the biggest disadvantage is that the elution solvent is harmful to the environment.Future researchers should focus on developing green, low energy consumption, simple operated and good regenerative performance industrial methods.
In addition, the current studies on adsorbents focus more on the adsorption performance but not on the disposal of waste adsorbents.Even though some of the adsorbents are biodegradable,no degradation experiments have been done in the actual soil, and the whole life cycle assessment of the adsorbents is lacking.In addition to the cost and practical application of adsorbents, future research should pay more attention to adsorbents’whole life cycle assessment to realize the real ‘green’adsorption.With the efforts of the researchers, it is expected that the carbon-based composites would be soon used in the area of wastewater treatment in an actual application.
Declaration of competing interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.51778230) and Program of Shanghai Outstanding Technology Leaders (No.20XD1433900).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.09.029.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
- Crystal facet-dependent electrocatalytic performance of metallic Cu in CO2 reduction reactions
