A novel therapeutic vaccine based on graphene oxide nanocomposite for tumor immunotherapy
Liming Zhng, Lingfeng Xu, Yi Wng, Jieyu Liu, Gunghong Tn,Fengying Hung, Nongyue He, Zhuoxun Lu,∗
a Key Laboratory of Tropical Translational Medicine of Ministry of Education & Hainan Provincial Key Laboratory of Tropical Medicine, Hainan Medical University, Haikou 571199, China
b State Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, China
c Department of Clinical Laboratory, HwaMei Hospital, University of Chinese Academy of Sciences, Ningbo 315000, China
ABSTRACT With an intensive understanding of the mechanism of immune system, developing a therapeutic tumor vaccine is one of the most perspective strategy of cancer immunotherapy.In this study, we report a facile approach to prepare graphene oxide (GO)-based therapeutic cancer-nanovaccine.The model antigen(ovalbumin, OVA) and adjuvant (CpG ODN), are conjugated with GO-PEI nanosheet through electrostatic interaction.The addition of PEG can improve biocompatibility and prevent nanoparticle aggregation.The prepared GO-based nanovaccine, GO-PEI-OVA-PEG-CpG, exhibits good biocompatibility and low toxicity both in vivo and in vitro. More importantly, it can efficiently induce the maturation of dendritic cells(DCs), the enhancement of antigen cross-presentation ability, and the amplification of cytokine production of immune cells.Impressively, this nanovaccine shows a remarkable therapeutic effect against preestablished B16-OVA-melanoma tumors, which can significantly inhibit tumor growth and prolong the survival time of the OVA-expressed tumor-bearing mice.Moreover, combining GO-PEI-OVA-PEG-CpG with NLG919, an IDO-1 (indoleamine-2,3-dioxygenase) inhibitor which can regulate the tumor microenvironment, displays a synergistic therapeutic effect.These findings indicate the GO-PEI-OVA-PEG-CpG nanovaccine actively induces an antigen-specific antitumor immune response and it combined with NLG919 could achieve better therapeutic outcomes.
Keywords:Nanovaccine Graphene Oxide Adjuvant Tumor-specific antigen NLG919
Malignant tumors are severe diseases in which some cells in the body grow rapidly and uncontrollably and spread to other parts of the body quietly.During the past century, human beings have invested lot of time and money seeking effective therapies for cancer treatment.The subsequent development of surgical treatment, radiotherapy, chemotherapy, and other methods has prolonged the survival time of tumor patients to a certain extent, but still cannot prevent tumor recurrence and transformation.Also, most antitumor drugs are costly.Therefore, it is necessary to develop some new antitumor therapies that effectively prolong patients’survival time and improve patients’quality of life while making them affordable for most people.
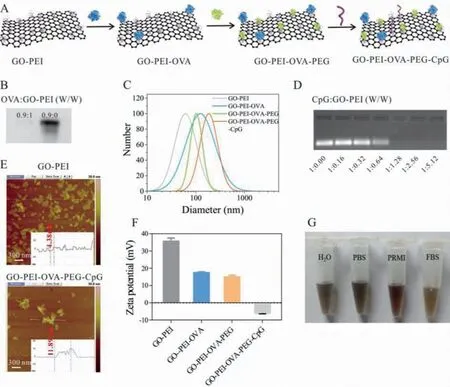
Fig.1.Synthesis and characterization of GO-PEI-OVA-PEG-CpG nanovaccine.(A) Schematic illustration of the formation of GO-PEI-OVA-PEG-CpG nanovaccine.(B) Native-page of GO-PEI binding to OVA, W/W is the mass ratio of OVA to GO-PEI.(C) The hydrated particle size of GO-PEI, GO-PEI-OVA, GO-PEI-OVA-PEG and GO-PEI-OVA-PEG-CpG.(D)Agarose gel of GO-PEI-OVA-PEG binding to CpG ODN, W/W is the mass ratio of CpG ODN to GO-PEI.(E) AFM images of GO-PEI and GO-PEI-OVA-PEG-CpG.(F) Zeta potential of GO-PEI, GO-PEI-OVA-PEG and GO-PEI-OVA-PEG-CpG.(G) The photographs of GO-PEI-OVA-PEG-CpG dispersed in 10% FBS, PRMI, PBS and H2O for 30 days, respectively.
With the in-depth study of malignant tumors, we gradually understand the cause of malignant tumors is that some cancer cells escape from the surveillance of the immune system and grow arbitrarily in the body.Therefore, to treat cancer fundamentally, it is necessary to reactive the human natural defense system, the immune system, which can recognize and kill tumor cells while not harming normal cells.Nowadays, some cancer immunotherapies have been developed which induce the tumor specific-immune response of the tumor bearer [1–6].The tumor vaccine has been one of the hot spots in immunotherapy research in recent years[7–14].Some preventive tumor vaccines have been marketed and widely used, such as the HPV vaccine (not a vaccine against tumor cells, but a virus vaccine used to eliminate tumor-inducing viruses).However, therapeutic tumor vaccines, which activate T cell-mediated antitumor immune responses in various ways, have been challenging to succeed.The weak immunogenicity of tumorassociated antigen, the low antigen loading efficiency, and an immunosuppressive tumor microenvironment are the main obstacles for developing therapeutic tumor vaccines.The arising of nanomaterials provides many new strategies for the development of tumor vaccines [15–19].These nano biomaterials can achieve high loading of antigens and adjuvants through electrostatic interactions, hydrophobic interactions, and covalent binding, and realize the enrichment of tumor-associated antigen in lymph nodes according to their special physical or chemical properties [19–21].In this study, we used graphene oxide (GO), an ideal biological material owing to its unique physiochemical properties [22,23], as a carrier to load model antigens and immune adjuvants to construct a new type of nano-tumor vaccine.The process of preparing a therapeutic vaccine based on GO-nanocomposite is shown in Fig.1A.Firstly, we prepared GO-PEI according to our previous work [24,25].Then, Ovalbumin (OVA), a model antigen, was combined to GO-PEI through electrostatic interaction because OVA is negatively charged(with a zeta-potential of −2.13 mV) [26] and GO-PEI is positively charged (with a zeta-potential of +55.5 mV) [25].When the mass ratio of OVA to GO-PEI was 0.9:1, the OVA was hardly detected by the non-denatured polyacrylamide gel electrophoresis, indicating that OVA is almost completely adsorbed to GO-PEI (Fig.1B).Nanoparticle size was estimated by the dynamic light scattering(DLS).The measured average hydrated particle size of GO-PEI was 63 nm, while GO-PEI-OVA was 143 nm (Fig.1C).This increase in volume may be due to the accumulation of nanomaterials.Because aggregation is not good for loading drugs, we conjugated polyethylene glycol (PEG) to GO-PEI-OVA, which provides a steric barrier to effectively prevent material aggregation and increase the biocompatibility of nanomaterials [27,28].As expected, the measured hydrated particle size of GO-PEI-OVA-PEG was 98 nm, much smaller than that of GO-PEI-OVA, suggesting that PEG effectively prevents nanomaterial aggregation.It had been reported that GO-PEG-PEI has an effect similar to vaccine adjuvants [29], but this ability is not strong enough.Thus, we combined CpG oligonucleotide (ODN),evaluated as an excellent cancer vaccine adjuvant in multiple clinical trials [30,31], with GO-PEI-OVA-PEG through electrostatic interaction to achieve a better therapeutic effect.As illustrated in Fig.1D, when the mass ratio of CpG ODN to GO-PEI is 1:1.28, CpG ODN can be adsorbed entirely through agarose gel electrophoresis.Atomic force microscopy (AFM) analysis shows the height increase of GO-PEI-OVA-PEG-CpG nanovaccine compared to GO-PEI(Fig.1E).The obtained nanovaccine had a hydrodynamic diameter of 186 nm with a zeta-potential of −5.995 mV (Fig.1F).The reduction in zeta potential is due to the binding of negatively charged OVA and nucleic acid to the positively charged GO-PEI.Moreover,to determine whether it can exist stably under physiological conditions, we investigated the stability of this nanovaccine by storing the nanoparticles in deionized water, PBS, RPMI 1640 medium, and FBS (10%) at 4°C.As shown in Fig.1G, the GO-PEI-OVA-PEG-CpG maintains good dispersibility in these solutions for 30 days, suggesting that the obtained nanovaccine is very stable under physiological conditions.
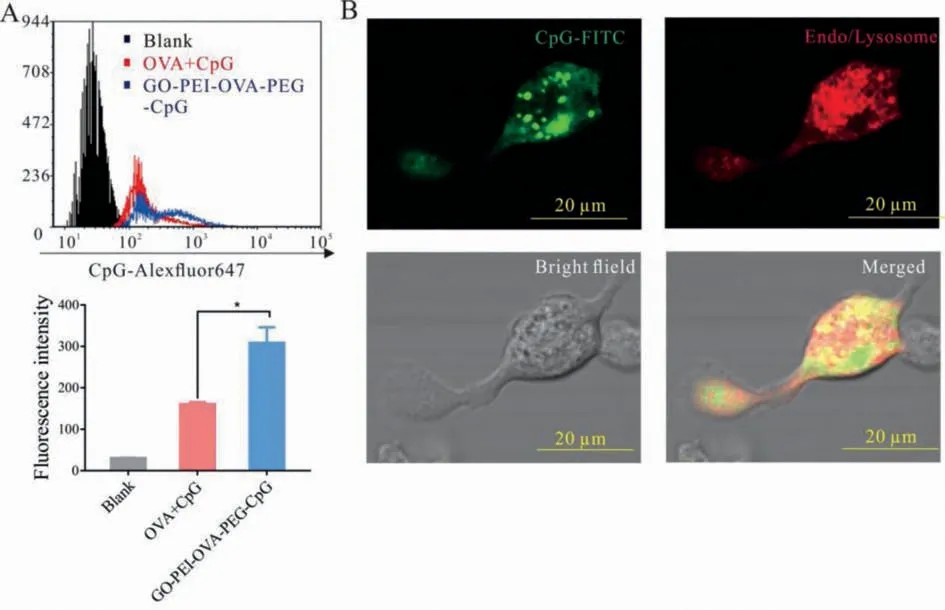
Fig.2.Endocytosis of cells.(A) The OVA+CpG group and GO-PEI-OVA-PEG-CpG containing the same concentrations of OVA and CpG were incubated with the CD11c+ BMDCs for 12 h, and then the fluorescence intensity was measured by flow cytometry.∗P < 0.05.(B) GO-PEI-OVA-PEG-CpG nanovaccine with FITC was incubated with BMDCs for 6 h, then fixed and imaged using the confocal microscope.
Whether nanomaterials can enter cells is essential for playing their functionsin vivo.In general, phagocytic cells, such as macrophages, neutrophils, and DCs, are preferentially take up anionic nanoparticles [32], suggesting that the prepared negatively charged nanovaccine might be more easily taken up by these immune cells.To verify our hypothesis, we investigated the cellular uptake of GO-PEI-OVA-PEG-CpG nanovaccine by using Alexa Fluor 647 labelled-CpG (CpGAlexaFluor647).After incubation with GO-PEI-OVA-PEG-CpGAlexaFluor647and OVA+CpGAlexaFluor647for 12 h, respectively, the fluorescence intensities of Alexa Fluor 647 in CD11c+BMDCs (Bone marrow-derived dendritic cells) were detected.As shown in Fig.2A, the fluorescence intensity of the GO-PEI-OVA-PEG-CpGAlexaFluor647group is twice that of the OVA+CpGAlexaFluor647group.These results demonstrated that the CpG ODN, in the form of nanovaccine, is much easier to be taken up by BMDCs than free CpG ODN.In order to further confirm that the nanoparticles are indeed internalized by BMDCs, we observed the intracellular localization of the nanoparticles through a confocal microscope.BMDCs cultured with the GO-PEI-OVA-PEGCpG Alexa Fluor 647 nanovaccine were fixed, and then imaged after staining with lysosomal dyes to observe the acid lysosomes.As shown in Fig.2B, most of the nanoparticles are localized in the lysosome (demonstrated by the presence of yellow fluorescence in the merged picture).These results confirmed our hypothesis that the negatively charged GO-PEI-OVA-PEG-CpG nanovaccine could be easily taken up by DCs, and it was finally located in the lysosome.
The biosafety of nanomedicine is also very important for clinical use [33,34].In this study, mouse fibroblast cells (BALB/3T3 cell line), macrophage-like cells (Raw264.7 cell line), mouse peritoneal macrophages, and BMDCs were used for evaluating the cytotoxicity of GO-PEI-OVA-PEG-CpG nanovaccine by CCK-8 assay.As shown in Figs.3A-D, the viabilities of all kinds of cells treated with GO-PEI and GO-PEI-OVA decrease with the increase of GO concentration, but the trend is reversed when the cells are incubated with GO-PEI-OVA-PEG and GO-PEI-OVA-PEG-CpG nanovaccine even under the incubation of high concentration.More interesting, it can be observed that the relative cell viabilities of Raw264.7, mouse peritoneal macrophages and BMDCs after incubating with GO-PEI-OVA-PEG-CpG (GO-PEI, 1.6 μg/mL) had risen to 1.63, 1.558 and 1.44, respectively, indicating that GO-PEI-OVA-PEGCpG nanovaccine can promote the proliferation of antigen presenting cell (APCs).
If the immune system is compared to an army, DCs are undoubtedly the potent professional signal soldiers in the army.As illustrated in Fig.4A, immature DCs will transform into mature DCs after phagocytosing tumor antigens, and then mature DCs present antigen peptides through major histocompatibility complex (MHC)molecules to induce T cell activation and differentiation [35–37].To clarify whether the prepared nanovaccine can transform the status of DCs from immature into mature, we measured the expression of costimulatory molecules, which will be up-regulated during the maturation of DCs.As shown in Fig.4B, the percentage of mature BMDCs (CD11c+CD80+CD86+) remarkably increase to 43.11% after treatment with GO-PEI-OVA-PEG-CpG nanovaccine,much higher than those treated with OVA and GO-PEI-OVA-PEG,and slightly higher than those treated by the same dose of CpG(39.1%).Accordingly, the percentage of CD11c+CD40+cells increase to 75.9% for GO-PEI-OVA-PEG-CpG treatment (Fig.4C).Since the interaction between CD40 on dendritic cells and CD154 on T cells leads to T cell activation and CD40 is also thought to lead to the activation of B cells and macrophages [38], the increase in the number of CD11c+CD40+cells induced by the nanovaccine may be beneficial to improving the therapeutic effect.These results confirmed that the nanovaccine could effectively induce the maturation of DCs, and the induction effect is more robust than that of single CpG ODN.DCs present antigens to T cells through the MHC-I pathway, which is essential for activating antigen-specific cytotoxic T cells [39,40].Therefore, we tested the expression of OVA257–264(SIINFEKL) peptide (MHC-I complex on the surface of BMDCs).As illustrated in Fig.4D, the amount of OVA257–264(SIINFEKL) peptide on the surface of BMDC with treatment of GO-PEI-OVA-PEGCpG is almost twice as much as that with treatment of OVA+CpG group, indicating that GO-PEI-OVA-PEG-CpG nanovaccine can enhance antigen presentation.
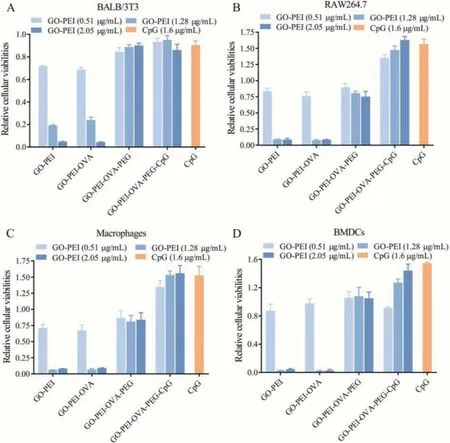
Fig.3.The relative cellular viabilities of (A) BALB/3T3 cells, (B) RAW264.7 cells, (C) macrophages, and (D) BMDCs incubated with different concentrations of GO-PEI, GO-PEIOVA, GO-PEI-OVA-PEG, and GO-PEI-OVA-PEG-CpG for 24 h.The untreated group was used as a blank control group, and the cells were treated with CpG ODN (The amount of CPG ODN is the same as the amount of CPG contained in the nanovaccine with GO-PEI concentration of 2.05 μg/mL.) was used as a positive group.
We further detected the changes in cytokine secretion of BMDCs induced by GO-PEI-OVA-PEG-CpG nanovaccine.As shown in Figs.4E-G, after incubation with nanovaccine, the secretion of proinflammatory cytokines, including TNF-α, MCP-1 and IL-6, increases dramatically.And this tendency to stimulate increased secretion of inflammatory factors also appeared in the peritoneal macrophages and RAW264.7 cells incubated with the GO-PEI-OVAPEG-CpG nanovaccine (Fig.S1 in Supporting information).One of the main functions of IL-6 is to induce the differentiation of CD8+T cells into cytotoxic T cells, so an increase in the concentration of IL-6 in the serum may benefit for enlarging the number of cytotoxic T cells [41].Although TNF-αas a single drug, produced unsatisfactory results, it produced positive clinical results when combined with IFN-γ, IL-2 or cytotoxic drugs [42].MCP-1 induces the recruitment and activation of monocytes and macrophages, and it also stimulates the movement of T cells and natural killer cells[43].Thus, the dramatic increase in the secretion of these cytokines could help the body obtain a strong enough immune response to fight against malignant tumor cells.Moreover, the ability of GO-PEI-OVA-PEG-CpG nanovaccine to stimulate BMDCs to secrete TNF-αand MCP-1 is much stronger than that of free CpG ODN treatment alone.This is probably because CpG ODN as a component of nanovaccine is more easily absorbed by cells than free CpG ODN.The secretion of IL-10, an anti-inflammatory cytokine,somewhat unexpectedly, increased after incubation of the GO-PEIOVA-PEG-CpG or free CpG ODN (Fig.4H).This may be caused by the body’s protective mechanism.Increased regulatory cytokines can prevent excessive production of pro-inflammatory cytokines which may cause damage to normal cells [44].
In order to explore whether the GO-PEI-OVA-PEG-CpG nanovaccine can trigger immune responsein vivo, OVA, OVA+CpG, GOPEI-OVA-PEG-CpG nanovaccine were injected into the thigh of C57 mice by intradermal injection (once per week for 2 weeks), respectively.Firstly, we measured the amount of OVA-specific cytotoxic T cells after treatment with OVA alone, OVA with adjuvant, and GOPEI-OVA-PEG-CpG nanovaccine.The results showed that almost no antigen-specific cytotoxic T cell was detected after treatment with OVA alone or OVA with adjuvant, but a large number of antigenspecific cytotoxic T cells were detected after treatment with GOPEI-OVA-PEG-CpG nanovaccine (Figs.5A and B), indicating that the mature DCs activated by the nanovaccine effectively present antigen to T cells which generate OVA257–264-specific CD8+T cells.Antigen-specific IgG plays an essential role in immunotherapy.Secondly, we tested the relative concentration of antigen-specific IgG expression in the serum of mice after each treatment.The results showed that the relative expression of antigen-specific IgG in mouse serum after GO-PEI-OVA-PEG-CpG treatment was 5 times and 2.5 times of that after OVA treatment or OVA+CpG treatment(Fig.5C).From the above results, we concluded that GO-PEI-OVAPEG-CpG nanovaccine could lead to a strong cell and humoral immune responses.
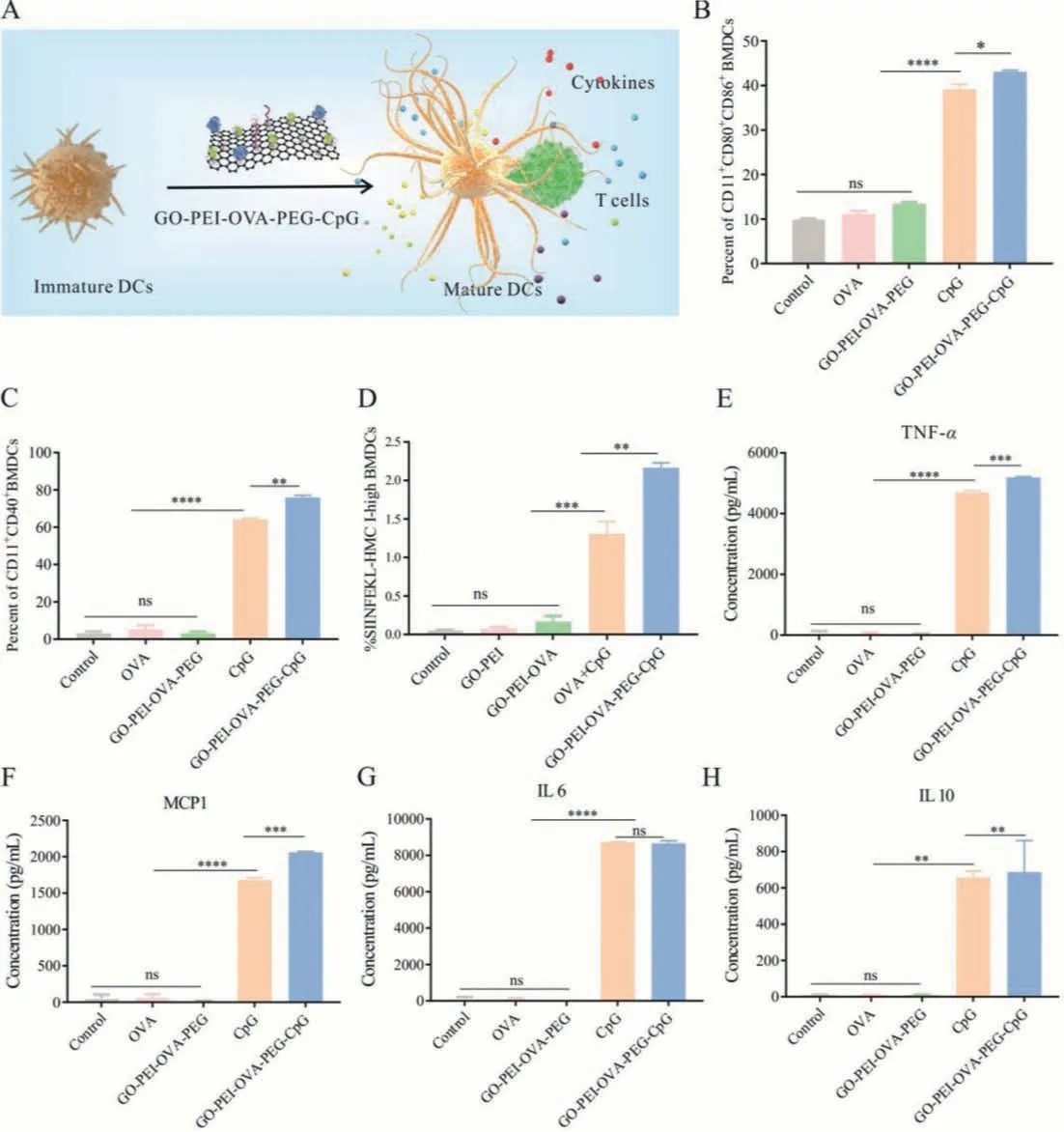
Fig.4.GO-PEI-OVA-PEG-CpG nanovaccine efficiently activated BMDCs.(A) Schematic diagram of GO-PEI-OVA-PEG-CpG nanovaccine stimulating the maturation of BMDCs and promoting BMDCs to present antigens to T cells.(B) The percentage of CD11c+CD80+CD86+ cells was measured by the flow cytometry.(C) The percentage of CD11c+CD40+cells was measured by the flow cytometry.(D) The percentage of presenting-OVA-specific-peptide BMDCs measured by the flow cytometry.(E-H) The expression levels of TNF-α, MCP-1, IL-6, and IL-10 were secreted from BMDCs.∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001, ns: not significant.
Thein vivoantitumor performance of GO-PEI-OVA-PEG-CpG nanovaccine was evaluated by using murine melanoma tumor model.Animal experiments in this study were approved by the Animal Ethics Committee of Hainan Medical University.Considering that the immunosuppressive microenvironment may affect the antitumor function of nano-vaccine, we planned to combine drugs that can reverse the immune microenvironment to treat tumors cooperatively.In this study, NLG919, an IDO-1 (indoleamine-2,3-dioxygenase) inhibitor, which specifically blocks IDO-trigged Tcell suppression and restores a strong T-cell response [4,45,46],was selected to synergistically improve the effects of GO-PEIOVA-PEG-CpG nanovaccine on melanoma-bearing mice.C57 mice were inoculated with B16-OVA cells and divided into 5 groups randomly with 6 individuals in each group: (1) control (PBStreated) group; (2) OVA+CpG-treated group; (3) NLG919-treated group; (4) GO-PEI-OVA-PEG-CpG-treated group; (5) GO-PEI-OVAPEG-CpG+NLG919-treated group.As shown in Fig.6A, the average tumor volume of nanovaccine-treated group is obviously lower than those of the PBS-treated group and OVA+CpG-treated group,suggesting that the GO-PEI-OVA-PEG-CpG nanovaccine could significantly inhibit tumor growth.And the smallest tumor average volume is observed in the combination therapy group, indicating that GO-PEI-OVA-PEG-CpG nanovaccine combined with the NLG919 displayed synergistic antitumor effects superior to nanovaccine-treatment alone.Moreover, the survival curve also showed the same trend.The data showed that nanovaccine therapy or combination therapy can significantly extend the survival time of B16-OVA-bearing C57 mice (more than 50% of the mice were alive within 40 days).Instead, all mice in the control (PBStreated) group and the vast majority (83.3%) of the mice treated with OVA+CpG died within 40 days (Fig.6B).The effect of NLG919 treatment alone was not good either, only 33.3% of mice survived within 40 days (Fig.6B).These results confirmed our hypothesis that the drugs which can improve the immunosuppressive tumor microenvironment could effectively improve the antitumor efficacy of GO-PEI-OVA-PEG-CpG nanovaccine.
The earlier cell experiments confirmed that the nanovaccine has no cytotoxicity.To further study whether the GO-PEI-OVAPEG-CpG nanovaccine had a toxic and side effectsin vivo, we observed survival status and monitor the changes of body weight of C57 mice for 30 days.The mice with the treatment of nanovaccine were all alive during one month observation period without abnormal reactions.As shown in Fig.6C, there is no difference between the PBS-treated group and the GO-PEI-OVA-PEGCpG-treated group in terms of the relative weight.Tissue section images also showed that main organs were not damaged after GOPEI-OVA-PEG-CpG treatment (Fig.6D).Moreover, after treatment of nanovaccine, the biochemical indicators of mice including, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CREA), and urea nitrogen (BUN), had no significant changes,proving that the GO-PEI-OVA-PEG-CpG nanovaccine has no obvious side effects on liver and kidney functions (Fig.6E).The above results proved that the GO-PEI-OVA-PEG-CpG nanovaccine has good biosafety.
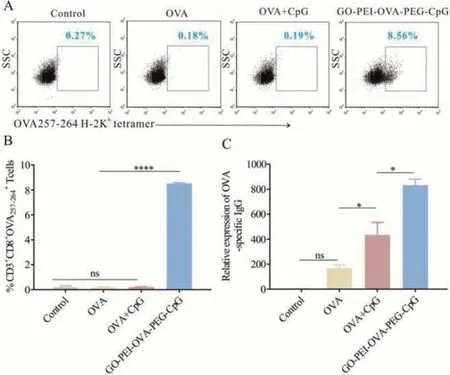
Fig.5.Activation of immune response in vivo.(A) the percentage (cells previously gated by CD3+ and CD8+) and (B) data analysis of OVA257–264-specific CD8+ T cells measured by the flow cytometry after different treatment.(C) Relative expression of IgG concentration in serum after treatment with OVA, OVA+CpG, GO-PEIOVA-PEG-CpG, respectively.∗P < 0.05, ∗∗∗∗P < 0.0001, ns: not significant.
In summary, we developed a novel graphene oxide-based nanovaccine, GO-PEI-OVA-PEG-CpG.In vitroexperiment proved that the nanovaccine is easily taken up by BMDCs, stimulates the maturation of BMDCs, and induces BMDCs and macrophages (peritoneal macrophages and Raw264.7 cells) to secrete a large amount of proinflammatory cytokines.In vivoexperiments indicated that the GO-PEI-OVA-PEG-CpG nanovaccine can effectively induce DC maturation and activated DCs effectively present antigen to lymphocytes to generate OVA257–264-specific CD8+T cells and OVAspecific IgG.Moreover, GO-PEI-OVA-PEG-CpG nanovaccine exhibited good biosafety and evident antitumor activityin vivo, which can effectively inhibit tumor growth and prolong the survival time of mice.And the prepared vaccine could achieve a better therapeutic effect when it is combined with NLG919.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
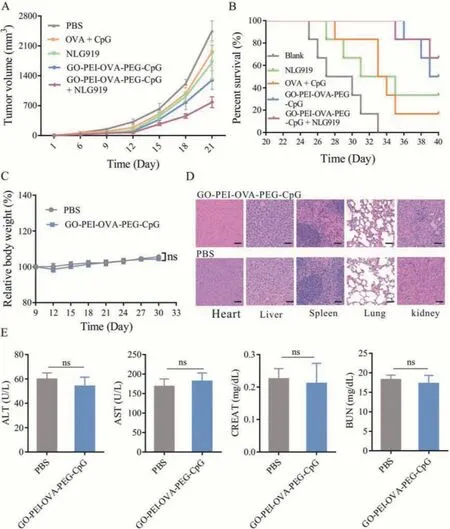
Fig.6.The in vivo therapeutic efficacy and toxicity analysis of GO-PEI-OVA-PEG-CpG nanovaccine.(A) The tumor growth curves, and (B) the survival curves of mice after different treatments.(C) The changes of relative body weight after GO-PEI-OVA-PEG-CpG nanovaccine-treatment.(D) HE staining images of main organs from mice after GOPEI-OVA-PEG-CpG nanovaccine-treatment (Scare bar: 50 μm).(E) Biochemical indexes, including ALT, AST, CREAT and BUN after GO-PEI-OVA-PEG-CpG nanovaccine-treatment.
Acknowledgments
We acknowledge the financial support from Basic and Applied Basic Research Program of Hainan Province (Nos.2019RC209 and 820RC646), the National Natural Science Foundation of China(No.81860037) and China Postdoctoral Science Special Foundations(Nos.2015T80488 and 2014T70459).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.071.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
