Ru(III)-catalyzed construction of variously substituted quinolines from 2-aminoaromatic aldehydes (ketones) and isoxazoles: Isoxazoles as cyclization reagent and cyano sources
Di Hu, Chao Pi, Wei Hu, Xiliang Han, Yangjie Wu, Xiuling Cui
Henan Key Laboratory of Chemical Biology and Organic Chemistry, Key Laboratory of Applied Chemistry of Henan Universities, Green Catalysis Center and College of Chemistry, Zhengzhou University, Zhengzhou 450052, China
ABSTRACT A Ru(Ⅲ)-catalyzed annulation reaction of 2-aminoaromatic aldehydes (ketones) and isoxazoles to afford diverse 3-cyanoquinolines has been developed.Notably, isoxazole acted as a cyclization reagent and nontoxic cyano source via N-O bond cleavage and fragmentation.Variously substituted (especially 6- or 7-substituted) quinolines could be easily afforded.This procedure features wide functional group compatibility, efficiency and avoiding toxic cyano source.Meanwhile, this protocol could be successfully applied to scale-up synthesis.Further chemical transformations of 3-cyanoquinoline could give some valuable skeletons, demonstrating its potential in synthetic application
Keywords:Isoxazoles Cyclization reagent Cyano sources Variously substituted 3-cyanoquinolines
Quinoline is a ubiquitous motif in various natural products [1,2],functional materials [3–5] and medicines [6–8].Copious methods have been explored to construct the quinoline and its derivatives.The representative ones are the Friedländer reaction [9] and the Skraup reaction [10].These protocols access quinoline scaffold through annulation of aniline or its derivatives with carbonyl compounds.When unsymmetrical ketones are used in the Friedländer reaction andmetaor 3,4-disubstituted anilines are used in the Skraup reaction, regioselectivity is a challenging issue [11].Therefore, developing an efficient method for the construction of variously functionalized quinolines have attracted tremendous attention from both academic and industrial community.
Among quinoline derivatives, the diverse substituted (especially 6- or 7-substituted) 3-cyanoquinolines represent an important fragment in the pharmaceuticals and key building block in a tremendous number of clinical medicines [12–16], such as pelitinib, bosutinib, neratinib and pyrotinib (Fig.1).Meanwhile,the nitrile group can be converted into plenty of useful functional groups, such as aldehydes, ketones, amines, amides and carboxylic acids [17–20], even nitrogen-containing heterocycles [21–26].The construction of 3-cyanoquinolines had been explored during the past decades, the traditional methods are Rosenmund-von Braun [27–29] and the Sandmeyer reactions [30–32] in the presence of stoichiometric CuCN starting from diazonium salts or 3-haloquinolines (Fig.2a).Transition-metal-catalyzed cyanation of 3-haloquinolines or 3-quinolineboronic acid with various cyanides(including inorganic cyanides and organic nitriles) provided alternative procedure (Fig.2b) [33–40].Moreover, much success has been achieved by transformations of various C3 functionalized quinolines (e.g., 3-methyl-, carbonyl-, alkynyl- and thiomethylsubstituted quinolines), generally in the presence of the strong
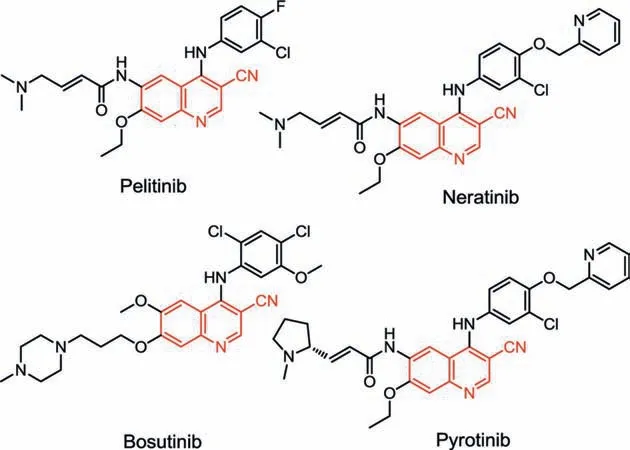
Fig.1.Pharmaceuticals containing 3-cyanoquinolines.
https://doi.org/10.1016/j.cclet.2021.12.072
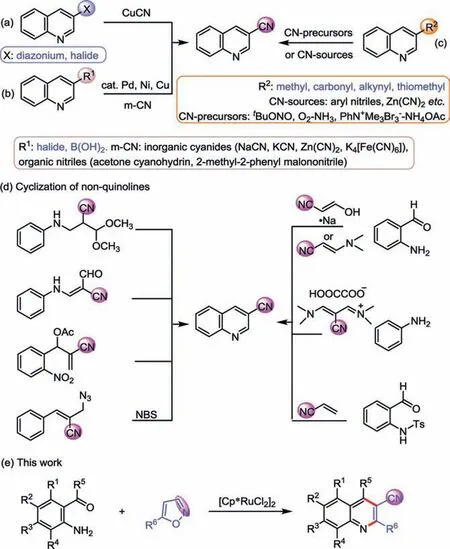
Fig.2.Strategies for the preparation of 3-cyanoquinolines.
1001-8417/© 2022 Published by Elsevier B.V.on behalf of Chinese Chemical Society and Institute of Materia Medica, Chinese Academy of Medical Sciences.oxidants and under high temperature or pressure were required(Fig.2c) [41–45].And these reactions above usually rely on the modification of quinoline skeleton.There are difficulties in the synthesis of diverse substituted 3-cyanoquinolines, especially 6-or 7-substituted ones with problematic preparation.Recently, intermolecular or intramolecular cyclization of cyanides to obtain quinoline ring were developed (Fig.2d) [46–51].For instance, Li’s group documented the Rh(Ⅲ)-catalyzed annulation of N-sulfonyl 2-aminobenzaldehydes with olefins in 2014 [52].Subsequently,Yu’s group disclosed the NBS-mediated radical cyclization reaction of 3-arylallyl azides under visible light irradiation [53].Typically, these approaches were still limited to the narrow scope of substrates, which are not easy availability and safety.Therefore, it is urgent to establish a green, efficient, and high-atom economic method, which is capable of constructing various-locationsubstituted 3-cyanoquinoline derivatives starting from easily available substrates.
Isoxazole proved to be a potential cyclization reagent and cyano source through the N-O bond cleavage [54–58].Most importantly,they are safe, readily available, and highly effective.Guided by its properties and in continuation of our interest on the construction of heterocycles [59–62], we herein disclosed the Ru(Ⅲ)-catalyzed annulation of 2-aminoaromatic aldehydes (ketones) and isoxazoles as a universally general method for the construction of diverse 3-cyanoquinolines (Fig.2e).In this transformation, variously substituted (especially 6- or 7-substituted) 3-cyanoquinolines were easily obtained, and isoxazole acted as a cyclization reagent and nontoxic cyano sourceviathe N-O bond cleavage.Simultaneously, this protocol was successfully applied to scale-up synthesis and various chemical transformations.
The annulation of commercially available 2-aminobenzaldehyde(1a) with isoxazole (2a) was initially chosen as the model reaction.Using DCE as solvent, [Cp∗RuCl2]2as the catalyst, AgNTf2and acetic acid as the additives at 100 °C for 16 h, the desired product 3-cyanoquinoline (3a) was isolated in 36% yield (Table 1,entry 1).To improve the reaction efficiency, other parameters were screened.Firstly, [Ru(p-cymene)Cl2]2was tested as a catalyst, and was found to be inferior to [Cp∗RuCl2]2(Table 1, entry 2).When other transition-metal were examined, such as [Cp∗RhCl2]2,[Cp∗IrCl2]2, Cp∗Co(CO)I2(Table 1, entries 3–5), the target product (3a) was not detected.These results indicated the importance of ruthenium as the catalyst.Next, a set of solvents (DCM, THF,TFE, MeCN, and acetone) were evaluated, and TFE was found to be the optimal (Table 1, entries 6–10), affording product (3a) in 61% yield.Among the silver salts, CF3COOAg gave the best yield of 76% (Table 1, entries 11–15).Then different acids were screened,and the product (3a) could be obtained in 83% yield in the presence of trifluoroacetic acid (TFA) (Table 1, entries 16–20).In addition, the reaction temperature was investigated, and it was shown that the reaction proceeded smoothly at 80 °C, giving 3a in 85%yield (Table 1, entries 21–23).When decreasing the reaction time to 10 h, a similar result was given with 86% yield (Table 1, entry 24).Finally, the optimized reaction conditions were assigned as follows: 2.5 mol% of [Cp∗RuCl2]2, 10 mol% of CF3COOAg, 1.0 equiv.of CF3COOH, and 1.5 equiv.of isoxazole (2a) in TFE under air at 80°C for 10 h
With the optimal conditions in the hand (Table 1, entry 24),we next examined the scope of 2-aminobenzaldehydes and isoxazoles.The results are shown in Scheme 1.The reaction performed well with a wide range of 2-aminoaromatic aldehydes (ketones).In all case (R1-R4), synthetically useful halogens (F, Cl, Br) and methyl, methoxy (Me, OMe) groups were all well tolerated, affording the desired products (3a-3k, 3n-3r, 3u-3y) in moderate to excellent yields (53%−91%).It is worth noting that 6- or 7-substituted3-cyanoquinolines (3g-3t) were easily obtained, which are important intermediates to construct quinoline-containing pharmaceuticals [63,64].Moreover, a variety of functional groups including -CF3(3l and 3s), -COOMe (3m and 3t), -OH (3aa) and -NH2(3ab) were also tolerated.In addition, 2-amino-4,5-dimethoxybenzaldehyde was coupled with 1a to provide the corresponding product 3ac in 70% yield.Interestingly, 2-aminoaromatic ketones, such as 2-aminoacetophenone and 2-aminobenzophenone, were also suitable for this catalytic system (4a and 4b).Furthermore, substitutions at 5-position of isoxazoles were treated with 2-aminobenzaldehyde under the optimized reaction conditions to provide the desired products 5a-5d in 33%−83% yields.Substrates with heterocycles were investigated, such as 4-aminopyridine-3-carboxaldehyde and 2-amino-3-formylchromone, affording the desired products 6a in 60% yield and 7a in 25% yield, respectively.
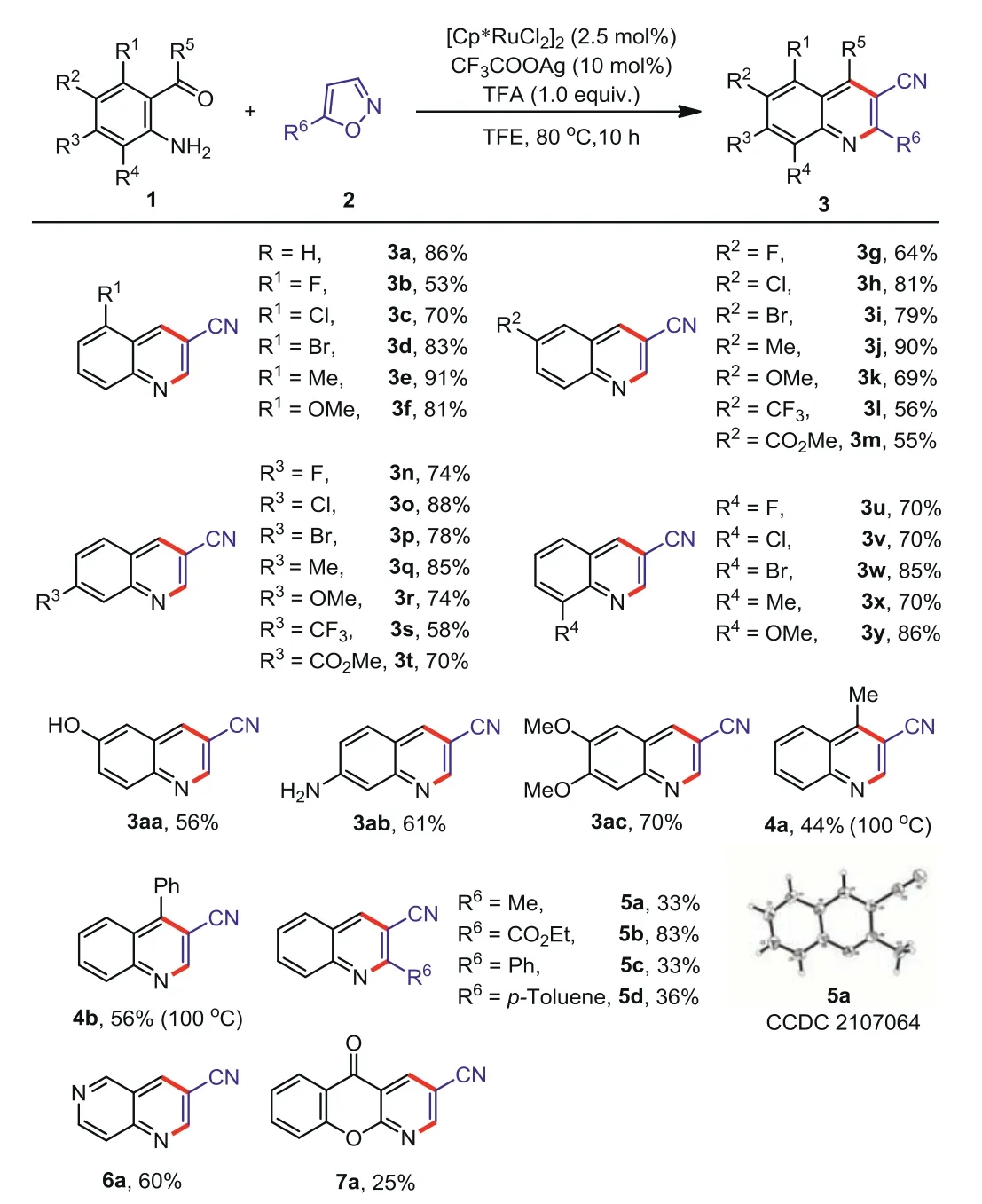
Scheme 1.Substrate scope.Unless specified, reaction conditions are as follows: 1(0.2 mmol), 2 (0.3 mmol), [Cp∗RuCl2]2 (2.5 mol%), CF3COOAg (10 mol%), TFA (1 equiv.) and TFE (1.5 mL), 80 °C, 10 h, air.Isolated yields.

Table 1 Optimization of the reaction conditions.a
To examine the practical utility of this method, a scale-up reaction and further chemical transformations were carried out(Scheme 2).Under the standard reaction conditions, the desired product 3a was obtained with 82% isolated yields in the gramscale.Furthermore, some chemical transformations were investigated.The nitrile group of 3a could be converted into amide in the presence of concentrated sulfuric acid to provide the product 8a in 95% yield.Interestingly, the cyano of 3a could also be converted into nitrogen-containing heterocycles, such as 3-amine-1Hpyrazole (9a, 42% yield), which is an important intermediate in organic synthesis [65,66].
To clarify the reaction mechanism, some control experiments were explored (Scheme 3).When the reaction of 1a with 2a in TFE was carried out in the absence of catalyst, the desired product 3a was not detected (Scheme 3a), which revealed that the N-O bond cleavage of isoxazole might be promoted by the Ru catalyst.The product 3a was obtained in 51% yield in the absence of silver salt (Scheme 3b), suggesting that the silver salt make a promoting effect on this transformation.Furthermore, only trace yield of 3a was detected in the absence of acid (Scheme 3c), so acid played a crucial role in this reaction.
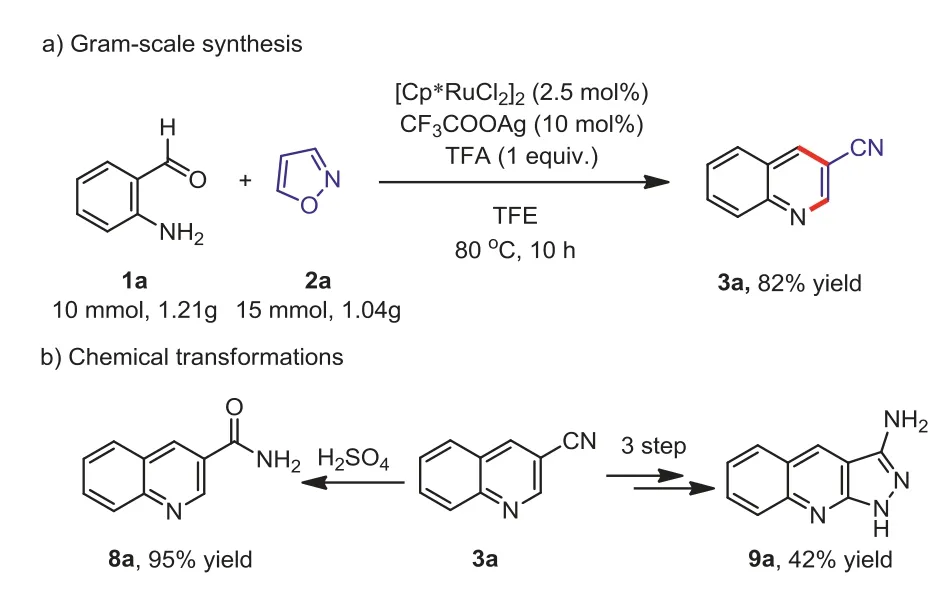
Scheme 2.Large scale synthesis and further transformations.
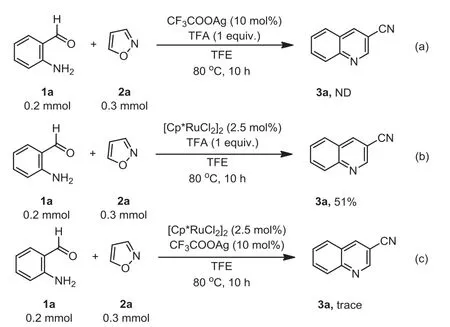
Scheme 3.Control experiments.
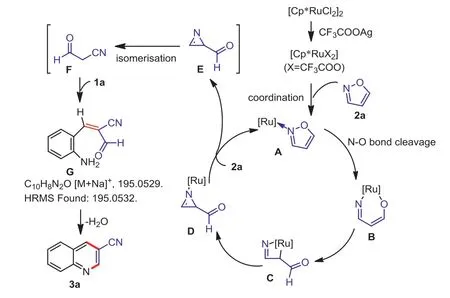
Scheme 4.Proposed reaction mechanism.
Based on the control experiments and literature reports [67–71], a plausible reaction mechanism was proposed (Scheme 4).Firstly, the activated ruthenium complex underwent coordination with 2a (isoxazole) to form isoxazole complex A.Complex A then underwent N-O bond cleavage and directly inserted into the N-O bond to form complex B as a possible intermediate, which subsequently transferred into a four-membered ring C.After a reductive elimination process, azirine complex D was obtained through C-N bond formation and ring reconstruction.Azirine E was released in exchange for the coordination of 2a, which regenerated complex A.Intermediate E directly transferred into 3-oxopropanenitrile (intermediate F) through an isomerization.Intermediate F then reacted with 1a (2-aminobenzaldehyde) to give the intermediate G(observed by HRMS, page S12 in Supporting information)viaAldol reaction.Finally, 3a (3-cyanoquinoline) was obtained through an intramolecular condensation of aldehyde and amine.
In summary, we have developed a novel and efficient method to synthesize 3-cyanoquinolines and its derivatives from easily available 2-aminoaromatic aldehydes (ketones) and isoxazolesviaRu(Ⅲ)-catalyzed annulation reaction.Herein, isoxazole played dual roles as a cyclization reagent and nontoxic cyano sources.In this transformation, variously substituted (especially a series of 6- or 7-substituted) 3-cyanoquinoline derivatives were obtained under the mild reaction, which exhibited wide functional-group compatibility.In addition, a scale-up synthesis, and further chemical transformations of 3-cyanoquinolines enhance its synthetic value.This efficient protocol provides novel strategy for the synthesis of quinoline-containing pharmaceuticals.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We greatly acknowledge partial financial support from the National Key R&D Program of China (No.2016YFE0132600), Henan Center for Outstanding Overseas Scientists (No.GZS2020001),Key Scientific and Technological Project of Henan Province (No.212102311068).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.072.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
