Chloro-free synthesis of LiPF6 using the fluorine-oxygen exchange technique
Jin Liu, Yunli Ci, Hun Png, Bin Co, Chengzhi Luo, Zhengho Hu,Choqun Xio, Hu Zhng, Fulu Lv, Yito Co, Lei Yu,∗
a School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002, China
b Tianzhu Hongfu Lithium Industry Technology Development Company Limited, Wuwei 733200, China
c Royal Holloway, University of London, Egham TW20 0QR, United Kingdom
ABSTRACT A hydrogen fluoride-free and chloro–free method for synthesizing LiPF6 was developed.Employing CaF2 as the direct fluorinating reagent instead of hydrogen fluoride made it much safer and more environmentfriendly than conventional methods and reduced the metal residues in product owing to the relatively low-acid reaction conditions less corrosive to equipments.The use of P2O5 as phosphorus source instead of traditionally employed PCl5 significantly reduced the chloro residue in product.Ca(H2PO4)2, the only by-product of the process, could be easily converted into Ca3(PO4)2, a best-selling chemical.The above advantages not only reduce the production costs by ca. 20%, but also significantly improve the product purity.The fluorine-oxygen exchange reaction is a completely new technique for LiPF6 production and may bring about technological revolution in the related industry.
Keywords:Lithium Fluorine Electrolyte High purity Green chemistry Process
LiPF6is a fundamental material in lithium-based battery production [1–9].It constitutesca.11%–16% weight of the battery electrolyte and accounts forca.40%−60% of the raw material cost of the electrolyte in lithium-ion batteries.In line with the rapid development of the lithium battery industry, the market requirement of LiPF6is growing very quickly.In 2020,ca.48,000 t of LiPF6was produced.Although the production capacity is rapidly increasing,the supplied LiPF6is still insufficient for the strong market demand, resulting in the significant price rising of the chemical (from$10,000/t to $69,000/t).In the conventional synthesis of LiPF6, PCl5is usually employed as the phosphorus source for its high reactivity, while HF is used as the fluorinating agent (Fig.1, method a)[10].During the processes, the reaction of PCl5with HF initially generates PF5, which then reacts with LiF to produce LiPF6.The disadvantages of this method are obvious.First, because HF is a highly corrosive and toxic chemical, the synthetic technology involving HF is not friendly to the environment and may pose a potential harm to the health of workers.Moreover, processes dealing with HF put forward high requirements to the equipments, and may inevitably bring impurities into the products due to the corrosion of the equipment metal.The use of PCl5may lead to chloro impurities in product, which results in high corrosion to the components in battery [11].Thus, improvement of the synthetic technology for LiPF6is urgently demanded not only for resolving the environment-protection and safety issues, but also for the purpose to enhance the product purity, which is a very important parameter in for electronic chemicals determining their grade and price.
In 2019, we reported a groundbreaking non-HF process using CaF2as the direct fluorinating agent instead of the hazardous HF(Fig.1, method b) [12].The method not only provides a relatively environment-friendly and safe access to LiPF6, but also greatly reduces the cost of production by 30% for simplifying the process from four steps (containing the HF generation steps) to only two steps.Moreover, the non-acidic reaction conditions can reduce the metal impurities in product being caused by equipment metal corrosion and significantly enhances the purity of the product.The by-product CaCl2is much easier to treat than the corrosive HF generated in conventional method, and it can be sold as a snowmelt to increase the income of the production line.However, since PCl5is employed as the phosphorus source, chloro ion residues cannot be avoided in product preparedviathe above non-HF method, and this issue still brings great challenges to the production of high-purity LiPF6.Recently, we found that, by using P2O5as the phosphorous source instead of the irritant PCl5, the chloro–free synthesis of LiPF6could be achieved (Fig.1, method c).The method not only further improves the production process from the environment-protection aspect, but also provides an access to high quality LiPF6and the related downstream products free of chloro.Herein, we wish to report our findings.

Fig.1.Comparison of the methods producing LiPF6.
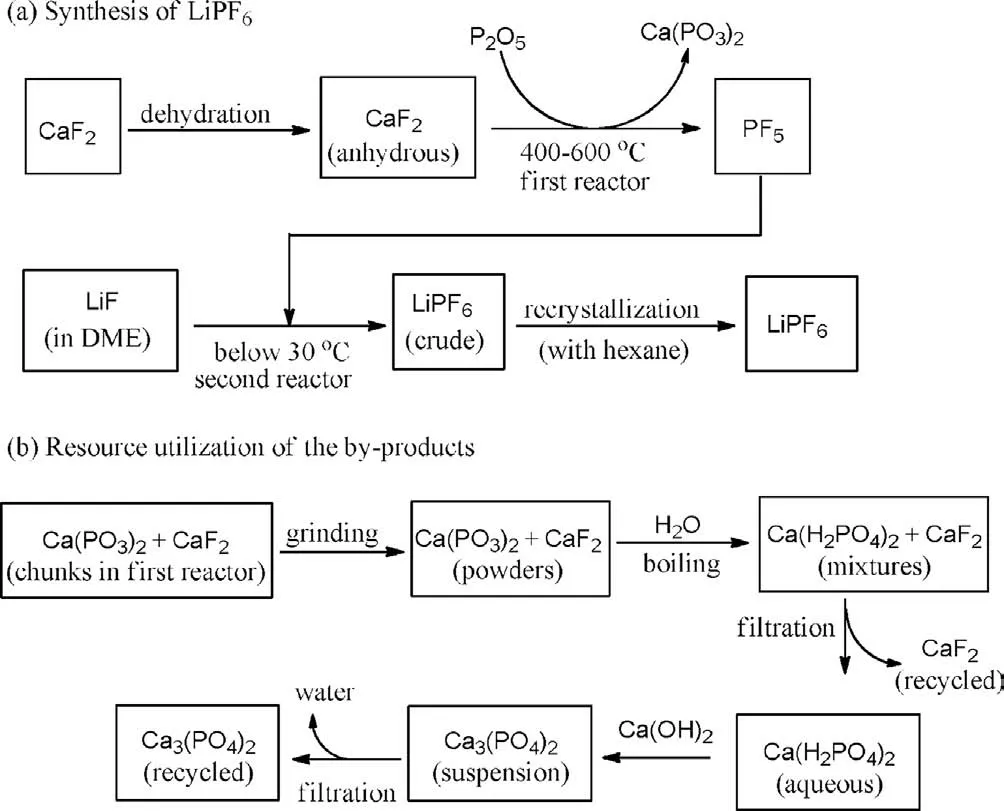
Fig.2.Diagram of the CaF2-P2O5 process.
The process of the new technique was very similar to the CaF2-PCl5method reported by us previously, but P2O5was used as the phosphorus source instead of PCl5.In the new process shown in Fig.2a, dehydration of CaF2was initially conducted to produce the anhydrous material.It then reacted with P2O5at 400–600 °C to generate the PF5gas in the first reactor, which was introduced into another reactor for the next step of reaction, while Ca(PO3)3,the generated by-product, was remained as the residue.The subsequent reaction of PF5with LiF in DME (i.e., dimethyl carbonate)in the second reactor at the temperature below 30 °C led to crude LiPF6, and it could be refinedviathe recrystallization with hexane.The mixtures of by-product Ca(PO3)2and unreacted CaF2in the first reactor existed as chunks and could be recycled (Fig.2b).They were initially ground into powders and heated in boiling water.The unreacted CaF2could be recycled by filtration and reused in the next run of production, while Ca(PO3)2was converted into Ca(H2PO4)2and dissolved in filtrate.Neutralization of the filtrate with lime cream led to the Ca3(PO4)2suspension, which was then separated by filtration.Ca3(PO4)2is an abundantly used chemical in the manufacturing of glass and ceramic wares [13–18].It is also used as additives in fertilizer and feed production.It has very broad application scopes and the market requirements are very large.Therefore, the by-product Ca3(PO4)2generated in the process could be easily sold without overstocking.The whole process produces no wastes other than water.The reaction was successfully performed in 10 kg of LiF scale, and it produced 53.9 kg of LiPF6, yielding 92% on the basis of the employed LiF amount.
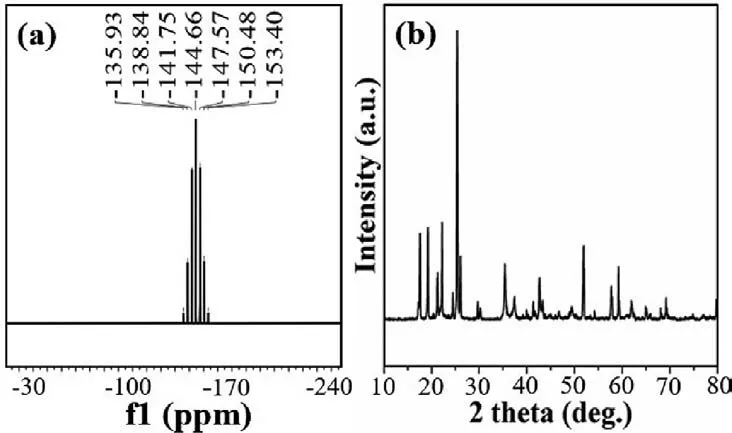
Fig.3.Comparison of the methods producing LiPF6.
31P NMR analysis in methanol-D4 solution on the 162 MHz frequency equipment indicated that there were no other phosphoruscontaining impurities in the product (Fig.3a).The signal of phosphorus in the prepared sample was reflected by the peak at−144.66 ppm, which divided into seven sub-peaks with theJvalue = 471.7 Hz.These results were in accordance with the characterization data of LiPF6in literature reports [19].Moreover, the powder X-ray diffraction (XRD) pattern of the sample also indicated the existence of the LiPF6crystals by comparison with the standard PDF#82–0784 card (Fig.3b).The above characterization data demonstrated that the pure LiPF6had been successfully synthesizedviathe chloro–free method using P2O5as the phosphorus source.
Quality of the produced LiPF6was then analyzedviathe standard methods, and the experimental details were summarized in Table S1 (Supporting information).As shown in Table 1, all of the parameters of the product met the required standard.The product purity was over 99.99% (Table 1, entry 1), with moisture less than 12.0 ppm (Table 1, entry 2).Because the material was produced via non-HF method, the HF residue in LiPF6was far less than the required value (Table 1, entry 3).Contents of the insoluble matter and sulfate were also well restrained (Table 1, entries 4 and 5).Notably, chloride ion, which might significantly affect the properties of electrolyte materials [11], was reduced to only 0.2 ppm(Table 1, entry 6), and this value is far less than the same product preparedviaPCl5-method (vs.1.5 ppm in Ref.[12]).Thus, it can be concluded that the chloro–free synthesis of LiPF6not only brings an environment-friendly production method, but also significantly enhanced the quality of the product for reducing the chloride ion content to ensure the applications of the related materials (i.e.LiPF6and its downstream products) in high-end batteries.Other metal impurities, such as Na, K, Ca, Fe, Pb, Cu, Mg, Cr, Ni, Cd and Zn were all less than the permit (Table 1, entries 7–17).For details, please see Supporting information.
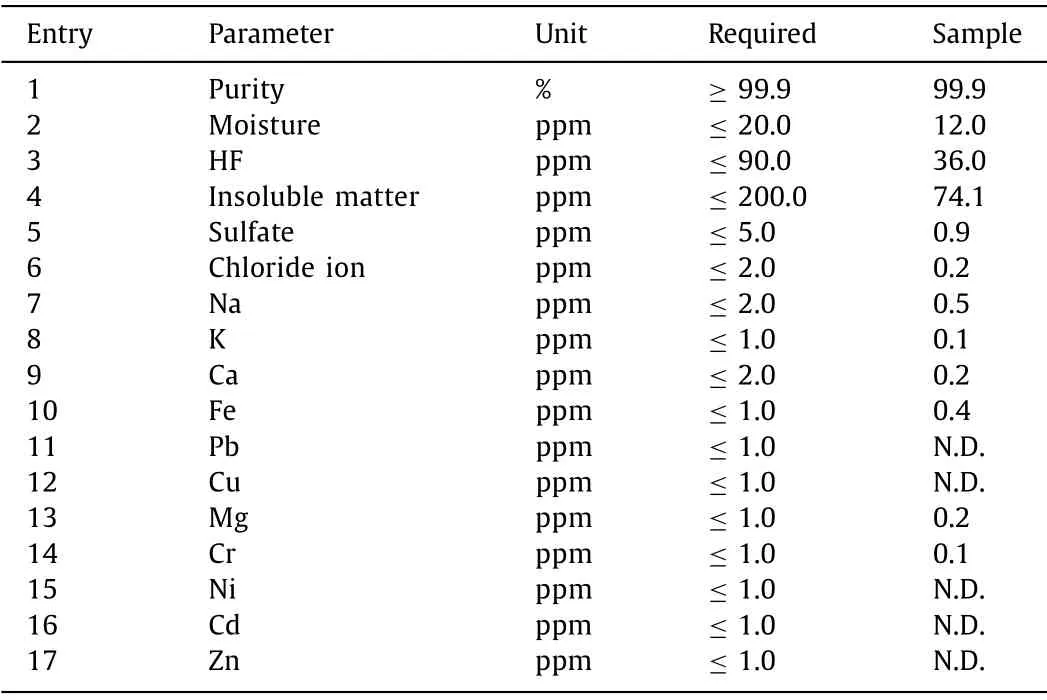
Table 1 Product quality analysis.
In conclusion, we have developed a novel method for producing LiPF6.From the point of view of element transfer reaction [20],the technique employed P2O5and CaF2as the phosphorus and fluorine sources respectively, and the generation of PF5gas that can be continuously removed from the first step reaction system endowed sufficient driving force to push forward the reaction equilibrium, in regardless of the break of the strong P=O bond.It is a HFfree method and the unique fluorine-oxygen exchange technique can significantly reduce the chloro ion impurites in downstream proudct.Besides the environment-friendly and safety advantages,this method leads to an access for the synthesis of high purity fluorine-containing lithium salt in lithium battery manufacturing industry.It may also be used in the electrocatalytic synthesis in future, which is just unfolding recently [21–24].In our cases, the LiPF6produced via the chloro–free method has been successfully applied as the starting materials to produce LiPO2F2, which is of even higher added value [25,26].It was also sold to the customers as the reagents for experiments.Investigations on magnified reactions are ongoing to convert this technique into real industrial production and the related Huichang production base in Jiangxi Province is under construction.The new technique may bring out the adjustment of the related industry pattern.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Yangzhou University-Hongfu Lithium joint-laboratory project, Jiangsu Provincial Six Talent Peaks Project (No.XCL-090), the Special Funds for Industrial Transformation and Upgrading and Information Industry Development of Gansu Province in 2018 (the First Phase Construction of Lithium Battery-Related New Material Industrial Park-the LiPF6 Production Project), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.068.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
