New flame retardant epoxy resins based on cyclophosphazene-derived curing agents
Zhenwei Mio, Dongpeng Yn, Xiodong Wng, Xinfng Zhng, Wenqi Zhou,Munn Qiu, Fn Yng, Zhnpeng Wu,∗
a State Key Laboratory of Organic–Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, China
b College of Chemistry, Beijing Normal University, Beijing 100875, China
ABSTRACT To obtain high-efficiency flame retardancy of epoxy resins, a cyclophosphazene derivative tri-(ohenylenediamino)cyclotriphosphazene (3ACP) was successfully synthesized and used as a curing agent for the thermosetting of an epoxy resin system.The flame retardant properties, thermal stability, and pyrolysis mechanism of the resultant thermosets were investigated in detail.The experiments indicated that the synthesized thermoset achieved a UL-94 V-0 rate under a vertical burning test as well as a limiting oxygen index (LOI) of 29.2%, which was able to reach V-0 even when a small amount of 3ACP was incorporated.Scanning electronic microscopic observation demonstrated that the char residue of the thermosets was extremely expanded after the vertical flame test.Thermal analysis showed that the samples had a lower initial decomposition temperature when 3ACP was introduced into the epoxy resin systems.This indicates that the carbonization ability of the thermosets was significantly improved at elevated temperatures.In addition, the incorporation of 3ACP can effectively suppress the release of combustible gases during the pyrolysis process, and the decomposition of E-44/DDS-3ACP curing systems also promotes the formation of polyphosphoramides charred layer in the condensed phase.The investigation on the chemical structures of both the gaseous and condensed phase pyrolysis process confirmed the flame-retardant mechanism of the 3ACP-cured epoxy resins.Therefore, the nonflammable halogen-free epoxy resin developed in this study has potential applications in electric and electronic fields for environment protection and human health.
Keywords:Cyclotriphosphazene Curing agent Epoxy resin Flame retardancy Pyrolysis mechanism
Epoxy resin is a prominent thermosetting plastic that has been widely used in many daily and industrial fields of human beings.However, the high flammability of these materials is one of the main drawbacks which greatly hinder the applications and bring potential threats in many areas, particularly involved in electric and electronic industries [1–3].To impart anti-flame properties to epoxy resins, halogen-containing polymeric materials, such as brominated epoxy resins, have been widely exploited to meet the requirement for applications in flammability protection and safe reliability.However, such brominated flame retardants can emit large amounts of smoke corrosive gases, and certain toxic substances during combustion.For the consideration of environmental safety and high flame-retardant efficiency, halogen-free flame retardants for epoxy resins have been attracting increasing attention from both the scientific and industrial communities [3–5].Among them, flame retardants that contain phosphorus and nitrogen elements have been widely applied due to their halogen-free and high flame-retardant features [4,6].Schartelet al.reported the typical 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) and DOPO-based diamino hardener as the additive and reactive flame retardants respectively for epoxy resins.After the addition of these organophosphorus compounds, the epoxy resins achieved an increased LOI value by 13% and the UL-94 rating of V-1 [7].Ionela-Danielaet al.prepared eco-friendly epoxy thermosets through a straightforward and cost-effective approach by adding organophosphorus compounds as flame retardant (PFR).The modified epoxy resin obtained UL-94 V-0 rating when 14.8% PFR was incorporated,and the LOI value also reached more than 30% along with a reduction of peak heat release rate (peak HRR) by 45% [8].Furthermore, Wanget al.developed an intumescent epoxy resin containing ammonium polyphosphate (APP), which showed higher LOI values by adding a 5 wt% total loading of flame retardants [9].After the addition of these halogen-free compounds, the epoxy resins obtained a phosphorus-containing charred layer, and the released non-combustible nitrogenous gas expanded the char layer during the thermal decomposition process [4,10–13].These features endow flame-retardant properties to epoxy resins.
However, these phosphorus-containing epoxy resins hardly gain a high weight fraction of phosphorus, resulting in a low degree of flame retardancy.Recently, great interest is focusing on the design of both the backbone and the side chains of epoxy resins with more highly flame retarding moieties.Wang’s group [10,14–17]made a progress in this field by developing a series of cyclotriphosphazene derivatives that contained a phosphorus-nitrogen skeletal structure with different substituted side groups.These compounds showed excellent flame retardancy such as higher LOI value (>30%) and self-extinguishing features when incorporated into epoxy resins due to the higher content of phosphorous and nitrogen elements.For example, hexakis(4-hydroxyphenoxy)-cyclotriphosphazene with “-OH” groups were synthesized and applied as a reactive agent to prepare a new phosphazene-based epoxy resin, in which the modified epoxy thermosets obtained UL-94 V-0 rating and 32.9% LOI value[10].Furthermore, 1,1-spiro(ethylenediamino)-3,3,5,5-tetrachlorocyclotriphosphazene, with higher phosphorous and nitrogen contents of 16.5 and 7.4 wt%, can result in excellent flame retardant properties such as UL-94 V-0 classification and higher LOI values[16].Therefore, the synthesis of cyclotriphosphazene-derivativesbased epoxy resins is expected to be feasible and facile [18].
In this work, for the first time, a novel amino-based curing agent tri-(o-phenylenediamino)cyclotriphosphazene (3ACP) was designed and synthesizedvianucleophilic substitution of cyclotriphosphazene (HCCP) witho-phenylenediamine (PDA).The synthesis process of 3ACP is optimized by using a new solvent which leads to a lower reaction temperature and much higher postprocessing efficiency (as shown in the Supporting information).Such a cyclotriphosphazene derivative with 3 substituent side groups has higher element contents of the phosphazene (20.5 wt%)and nitrogen (9.3 wt%), which leads to much higher flame retardancy, and the abundant aromatic rings in 3ACP also effectively enhance the char residue level after combustion [19,20].A complementary study on the curing properties and flammability characteristics of the synthesized epoxy resin was also performed and described.
The synthesis and chemical structure characterization of 3ACP are shown in Supporting information.Fig.S1 (Supporting information) shows the FTIR spectra of PDA, HCCP and 3ACP.It can be observed that two peaks at 3300 and 3325 cm−1appear in the PDA due to the existence of the primary amine bond, which represents N–H stretching vibration in PDA.As for the spectrum of HCCP, the peak at 520 cm−1is related to the P-Cl stretching vibration.This peak disappears in the 3ACP product, while a new peak appears at 1400 cm−1in 3ACP simultaneously.Besides, there is only a single broad peak at 3250 cm−1, representing the N–H stretching vibration of the secondary amine bond in the 3ACP.It can be inferred that all the amino groups in the o-phenylenediamine have reacted with cyclotriphosphazene.The sharp absorption peak at 1608 cm−1is attributed to the stretching vibration of the benzene ring skeleton.These two characteristic peaks were found in both PDA and 3ACP, and the 3ACP product.This means that there is no residual o-phenylenediamine remained in the system, and the desired 3ACP product is obtained.Fig.S2 (Supporting information) represents the mass spectrum of 3ACP product.The relative molecular mass of 3ACP is 453 g/mol through calculating the chemical structure (C6H4(NH2)3)P3N3.3ACP product may combine with H+or H2O in the electrospray ionization environment of the mass test,and the corresponding absorption peak at 454.1001 and 472.1073 can be observed in the FTIR spectra.Fig.S3 (Supporting information) reveals the1H NMR spectrum of 3ACP product, in which the peak at 7.71 ppm belongs to the chemical shift of H (peak c) on the primary amine bond linking to the phosphorous atom.In the enlarged spectrum, it can be observed that the peaks in the range of chemical shifts 6.66 to 6.54 ppm are formed by the superposition of a set of doublets and a set of triplets.Such a distribution of the peak shapes is also in line with the spin coupling and splitting laws of the1H NMR spectrum.These two groups of peaks belong to H (peak a) and H (peak b) with four carbon atoms attaching to the benzene ring.Fig.S4 (Supporting information) shows the31P NMR spectrum of the 3ACP product, the chemical shift of phosphorus in the product is 19.23 ppm.This chemical shift shows a single peak, demonstrating that all of the chlorine atoms in HCCP have been substituted, and each phosphorus atom is connected with two nitrogen atoms on the sameo-phenylenediamine molecule so that the electron cloud of the phosphorus atom is distributed uniformly.All of these results indicate that the 3ACP product has been successfully prepared [21].
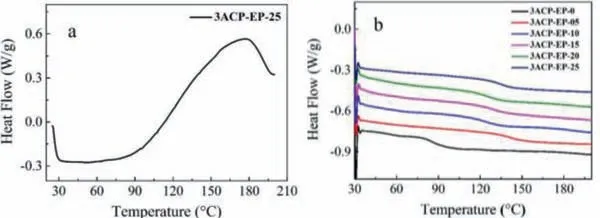
Fig.1.DSC curves of (a) 3ACP-EP-25 before curing and (b) 3ACP-EP-0 ∼3ACP-EP-25 samples after curing process.
DSC test is used to analyze whether the epoxy resins are completely cured.The DSC curve of 3ACP-EP-25 before curing is shown in Fig.1a.There is an obvious increase in heat flow when the sample is heated to more than 60°C, which means the epoxy groups in E-44 have reacted with the secondary amine groups in the 3ACP product.Considering that the 3ACP can act as a single curing agent for the 3ACP-EP-25 sample, the reactivity of 3ACP can improve the flame-retardant properties of epoxy resins.Fig.1b shows the DSC curves of all of the 3ACP-EP samples after the curing process (Table S1 in Supporting information).It can be seen that there is no exothermic peak in all of the six curves, meaning that the curing process was fully accomplished [1].
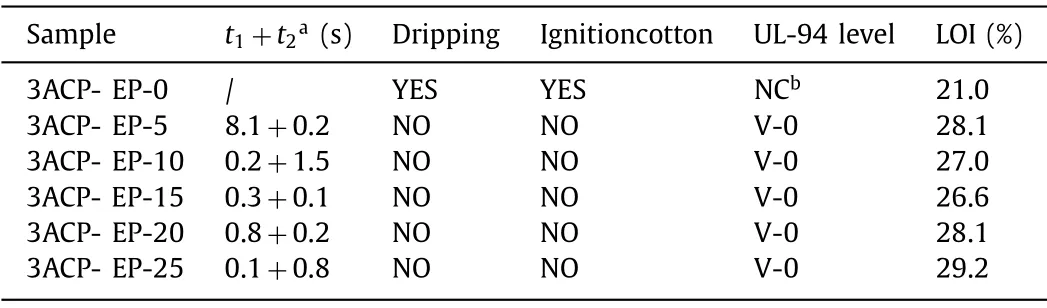
Table 1 Flame retardant properties of 3ACP-EP.
The cured 3ACP-EP samples were then applied to UL-94 and LOI tests to measure their flame-retardant properties.The UL-94 test results are shown in Fig.2 (first ignition) and Fig.3 (second ignition).The LOI values and the UL-94 test are listed in Table 1 that the 3ACP-EP-0 sample is very flammable with an LOI of only 21.0%, and a vertical burn experiment shows that the samples have no rating according to the UL 94 standard.The 3ACP-EP-0 sample burns to clamp after the first ignition, so there is no second ignition for it.There is a dripping phenomenon during combustion,resulting in igniting the cotton wool.Remarkable flame-retardant properties could be achieved when cyclophosphazene-based 3ACP is incorporated into the thermosets.When 5 wt% of the 3ACP was added into the epoxy resin system as a mixed curing agent, the LOI value increased up to 28.3 sharply.The UL-94 test indicates that the 3ACP-EP-05 obtained a self-extinguished feature when the flame was moved at 8.1 s for the first ignition process (Fig.2 and Table 1).There is no dripping phenomenon observed, and the sample reaches a V-0 rating as well.With an increase in the addition amount of 3ACP, the samples all reached V-0 class of UL-94 test and the LOI value of 3ACP-EP-25 reached 29.2 at last.These results indicate that 3ACP exhibits an excellent flame-retardant effect on epoxy resin and can be used as a flame retardant curing agent.
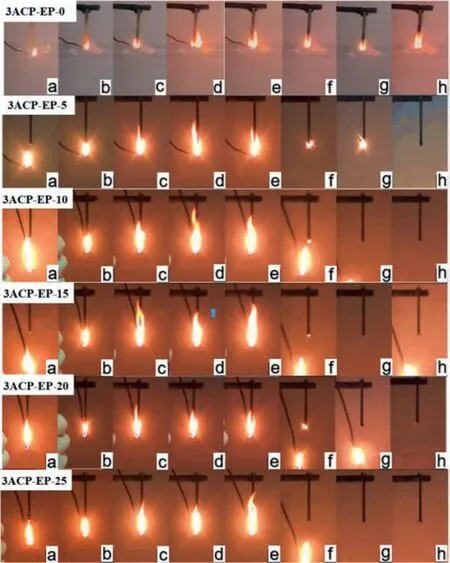
Fig.2.Schematic diagram of UL-94 vertical combustion process of 3ACP-EP products.First ignition process: (a) 1 s before ignition; (b) ignition 1 s; (c) ignition 5 s;(d) ignition 8 s; (e) ignition 10 s; (f) 1 s away from the fire; (g) 5 s away from the fire; (h) 10 s away from the flame.
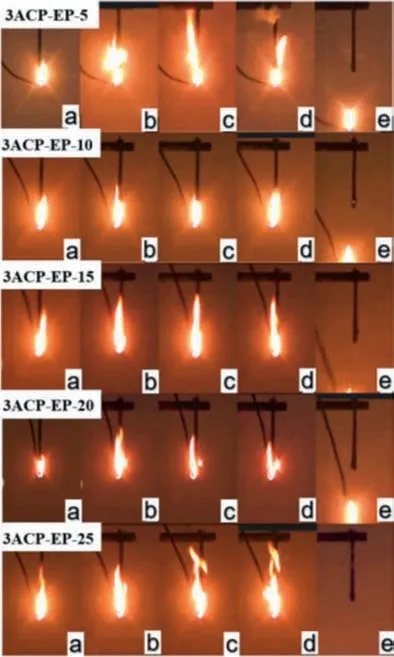
Fig.3.Schematic diagram of UL-94 vertical combustion process of 3ACP-EP products.Second ignition process: (a) ignition 1 s; (b) ignition 5 s; (c) ignition 8 s; (d)ignition 10 s; (e) 1 s away from the flame.
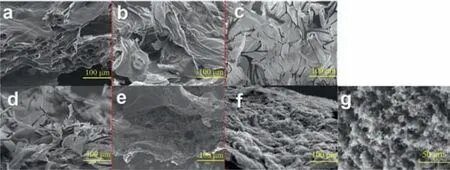
Fig.4.SEM micrographs of the inner char residues of 3ACP-EP epoxy thermosets after UL-94 test, (a) 3ACP-EP-05, (b) 3ACP-EP-10, (c) 3ACP-EP-15, (d) 3ACP-EP-20,(e) 3ACP-EP-25 and (f, g) the surface char residues of 3ACP-EP-25.
Scanning electron microscopy (SEM) is applied to measure the morphology of char residues of the 3ACP-EP samples.Figs.4a–e shows the SEM photographs of the inner chars residues (formed from the areas which are not directly contracting with the flame)obtained from 3ACP-EP samples after UL-94 tests.It shows that the inner char residue of 3ACP-EP samples are all expanded sheet structures which indicates that the char residue tends to expand due to the improved carbonization process and the release of inert gas when 3ACP was added.The charred layer is extremely important for the epoxy resins system to obtain flame retardant properties.Besides, an expanded char residue can prevent molten drops and result in the extinguishment of flame during combustion.The formed char can also largely impede the transfer of heat and the contact between flammable gases and oxygen.
It is interesting to note that a large number of bright spots are attached to the surface char (the char residue formed from the area which is directly contacting with the flame) of 3ACP-EP-25 sample as shown in Figs.4f and g.It is speculated that these white spots are phosphate crystals that were formed by the decomposition of phosphorous compounds during combustion.The phosphate and charred layer formed a good eutectic system, and these phosphates promoted charred layer shrinkage due to the fast cooling rate after melting.The molten phosphate is more likely to form on the surface of formed char residue that may be caused by the direct contact to the flame and higher temperatures.All the SEM results confirm the improved carbonization process of 3ACP-EP products, and the expanded char layer is beneficial to obtaining high flame-retardant properties.
Besides, to further confirm the formation of expanded char residue when 3ACP is incorporated into the epoxy resin system,the cured 3ACP-EP samples are heated in a muffle furnace for 2 h at 800°C, and the morphology of the char residues are further detected.As shown in Fig.S5 (Supporting information), the 3ACP-EP splines after high-temperature treatment have been significantly expanded compared to the 3ACP-EP-0 sample, and the residual surfaces show an obvious metallic lustre phenomenon.The appearance of this kind of expansion and metallic lustre effect results from the reinforced release process of inert gases and formed phosphate crystals on the surface of char residue, and this phenomenon becomes more obvious along with the increasing of the amount of introduced 3ACP product.It thus confirms great improvement of the carbonization facilitates the flame-retardant ability of the 3ACP modified epoxy resin systems at elevated temperatures.
Thermal resistance is one of the most important properties of epoxy thermosets because it establishes the service environment for epoxy-based functional materials.TGA measurement is applied to investigate the thermal stability of 3ACP-EP epoxy thermoset curing system.Fig.5 shows the TG and DTG curves of all epoxy thermosets which are heated from 30°C to 800°C under a nitrogen atmosphere with a heating rate of 10°C/min.Table 2 showsthe temperature at the mass loss rate reaching 5% (Tonset) of the epoxy samples and the temperature at the mass loss rate (Tmax).Besides, the maximum mass loss rate (Vmax) and the char residue of the samples at 600 and 800°C have also been studied.
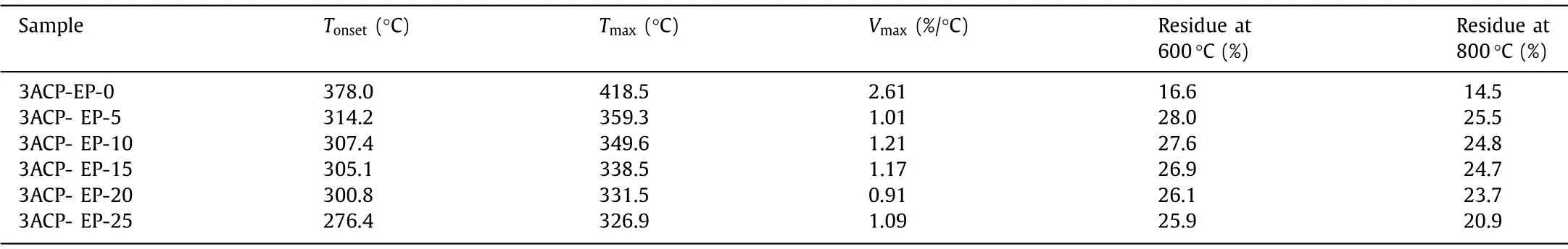
Table 2 Flame retardant properties of 3ACP-EP.
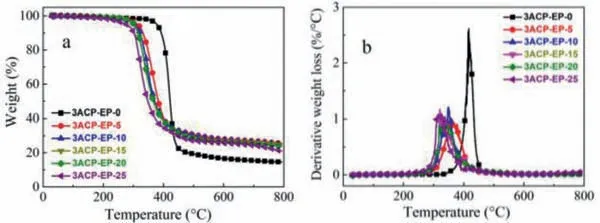
Fig.5.(a) TG and (b) DTG curves of 3ACP-EP product under nitrogen atmosphere.
The TG and DTG curves reveal that the 3ACP modified epoxy samples decompose at relatively lower temperatures than that of the DDS cured epoxy sample.TheTonsetof 3ACP-EP samples is lower to 276.4∼314.2°C when 3ACP are incorporated, and theirTmaxalso decreased from 418.5°C (3ACP-EP-0) to 326.9∼359.3°C(3ACP-EP-5∼3ACP-EP-25).The lower decomposition temperatures are believed to be caused by the corporation of 3ACP.At lower temperatures, the 3ACP is pyrolyzed to phosphoric acid substances.Therefore, this promotes the epoxy systems to form an expanded char layer and further suppresses the spread of oxygen, heat, and flammable gases resulting from the degradation process.The char residue sharply increases from 14.5% (3ACP-EP-0) to 25.5% (3ACPEP-5), and all of the samples show lower maximum thermal decomposition rates (Vmax, Table 2) compared to the 3ACP-EP-0 sample.TheVmaxvalues decrease from 2.61 (3ACP-EP-0) to 0.91%/°C(3ACP-EP-20).A much lower Vmaxmay also be caused by the formed expanded char layer, which hinders the transfer of heat and oxygen and further prevents the thermal decomposition of the modified 3ACP-EP samples.Therefore, the decomposition at lower temperatures is not a defect but necessary for the achievement of flame-retardant properties imparting to 3ACP-EP modified epoxy samples [6].

Table 3 XPS data of 3ACP-EP-0 and 3ACP-EP-25.
To further evaluate the pyrolysis mechanism of 3ACP-EP systems, the chemical components of the residual chars for the 3ACPEP-0 and 3ACP-EP-25 samples (heat in a muffle furnace for 10 min at 600°C) were investigated by XPS.As shown in Fig.6, three bands are observed from C 1s spectra.The peak at around 284.6 eV is attributed to C–H and C–C in aliphatic and aromatic species, the peak at around 286.0 eV is assigned to C–O (ether and/or hydroxyl group), and another peak at around 287.2 eV corresponds to carbonyl groups [22].There are minor changes in the C 1s peaks.For the N 1s spectra, 398.4 eV can be assigned to the C–N bond in the pyridinic group.The peak at 399.3 eV is attributed to N–H structure in the char residual.The peak at 400.3 eV is assigned to C–N in imide groups which are formed by the condensation of small molecules.The peak at 401.2 eV is observed only in 3ACP-EP-25,which can be assigned to the P–N in imide groups [23,24].The obviously enlarged C–N peak, and the P–N peak in imide groups of 3ACP-EP-25 sample indicate that the 3ACP promotes epoxy resin to form imide compounds during the thermal decomposition process.There are three peaks in O 1s spectrum at around 532.7, 533.4 and 531.2 eV.The first one at 532.7 eV can be assigned to the C–O or P–O groups, and another signal is observed at 533.4 eV, which is ascribed to C-O-C, C–OH and -COOR groups.The last one at 531.2 eV is determined as the effect of the C=O or P=O groups.In P 2p spectrum, two peaks at 133.8 and 134.7 eV are assigned to phosphorous atoms in phosphate and phosphoramide.The phosphoryl structure is calculated to account for about 60%.The results show that the 3ACP-EP produces phosphoric acid-like substances (Figs.4f and g) during the combustion process, which effectively catalyzes the formation of an expanded char residue of epoxy resins during combustion.This further achieves the purpose of imparting flame-retardant properties to epoxy resins [11].
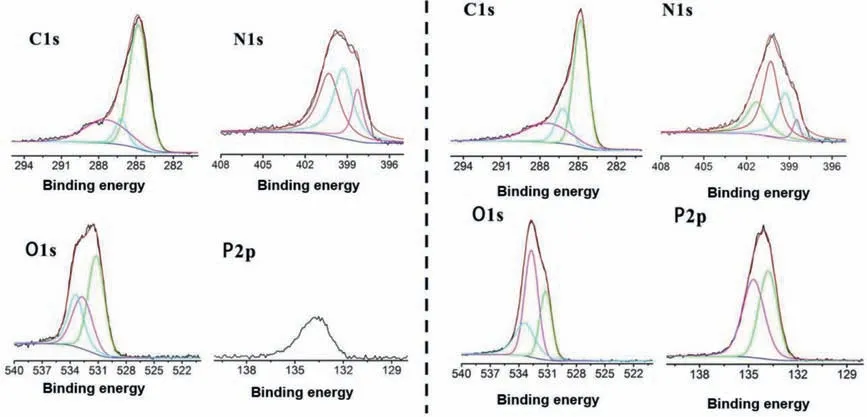
Fig.6.C 1s, O 1s, N 1s and P 2p XPS spectra of the char residue of 3ACP-EP-0 (left) and 3ACP-EP-25 (right) after heated at 600°C.
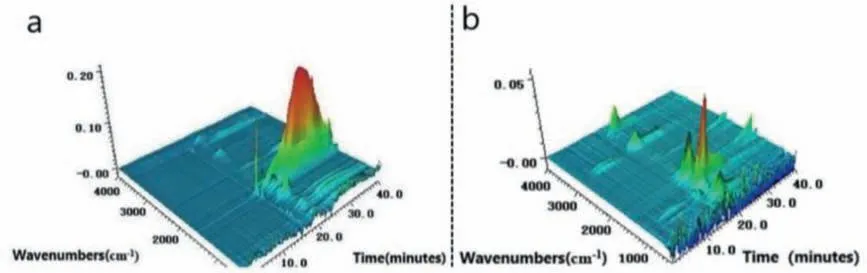
Fig.7.3D TG-FTIR spectrum of the gas phase in the thermal degradation of (a) 3ACP-EP-0 and (b) 3ACP-EP-25.
The percentage of carbon atoms in the char residues of 3ACPEP-0 and 3ACP-EP-25 are 76.44% and 52.10%, respectively (Table 3).Besides, the percentage of oxygen and phosphorous in char residues of 3ACP-EP-25 is much higher than that of the 3ACP-EP-0 sample.It reveals that the incorporation of 3ACP to epoxy resins will promote the formation of a new series of high heat resistant compounds which contain elements of phosphorous and nitrogen.The effect of 3ACP on the combustion behaviors of epoxy thermosets could be described as follows: The 3ACP-cured epoxy resin led to form higher content of charred residue during combustion.A series of melting polyphosphoramides have been formed after the decomposition of cyclophosphazene groups at elevated temperatures, and the polyphosphoramides will catalyse the formation of char residue at the same time.The charred layer covers the underneath epoxy thermosets tightly and insulates the transformation of heat and oxygen into the underlying polymeric substrate.Furthermore, the formed char also hinder the release of volatile products from the underlying polymeric matrix, and the nitrogen element in the phosphazene rings acts as a dehydrogenation reagent to form nitrogen-containing gases which will dilute the concentration of combustible gases.
TG-FTIR test is performed to analyze the gas products evolving during the thermal degradation of 3ACP-EP-0 and 3ACP-EP-25 (Fig.7).The 3D TG-FTIR spectra of the gas phase of 3ACP-EP-0 sample during thermal degradation are shown in Fig.7a.The presence of evolved gases can be clearly identified by the strong FTIR characteristic peak positions, such as methane (3015 cm−1), carbon dioxide (2360 cm−1), and water (3650 cm−1) [25].The intensive peaks at 1607 and 1433 cm−1correspond to the evolution of a large number of aromatic rings due to the severe thermal decomposition process caused by the fracture of the epoxy structure(Fig.7a).However, further decomposition is not observed after the completion of the first decomposition cycle (at about 17∼20 min,Fig.7b), besides the release of a small amount of aromatic ring is observed from 3ACP-EP-25 sample.
Fig.8 demonstrates the presence of various pyrolysis gaseous compounds of the 3ACP-EP-0 and 3ACP-EP-25 curing systems at the maximum decomposition rate, such as H2O and phenol (3500–3600 cm−1), CO2(2360 cm−1), carbonyl (1730 cm−1), aromatic ethers (1260 cm−1), hydrocarbons (C–H stretching at 1172 cm−1),and aromatic rings (1607, 1433 cm−1).An obvious difference between the gas products of 3ACP-EP-0 and 3ACP-EP-25 is the amounts of CO2and N–H (stretching at 3450 cm−1) bonds.In addition, a large amount of hydrocarbons compounds can be detected in 3ACP-EP-25 compared to 3ACP-EP-0.After 3ACP is added, the epoxy resin releases less CO2, and gases contain N–H bonds such as ammonia.The nitrogen tends to form stable phosphoramidite compounds and imide compounds during thermal decomposition.This further promotes the flame retardant properties of 3ACP-EP products.
The FTIR absorbence spectra of pyrolysis products for 3ACPEP-0 and 3ACP-EP-25 as a function of time were measured (Fig.9) to further evaluate the gaseous phase flame retardant mechanism.It can be clearly seen that the gaseous product compounds such as N–H (Fig.9b), CO2(Fig.9d), and carbonyl (Fig.9e), the aromatic rings (Fig.9f) during decomposition of 3ACP-EP-25 are hardly observed.However, N–H, CO2, carbonyl and aromatic rings are obviously detected in the case of 3ACP-EP-0 sample.3ACPEP-0 may be decomposed into amides (3450, 1730 cm−1), CO2(2310 cm−1) and aromatic compounds (1610, 1510, 1433 cm−1).With the introduction of 3ACP into epoxy resins, the amides are turned into thermal stable phosphoramidite, and the 3ACP product together with the decomposition product also catalyzes the aromatic rings to become carbon char.This also causes the absence of amides (3450, 1730 cm−1), CO2(2310 cm−1), and aromatic rings(1610, 1433 cm−1) during the decomposition process of 3ACP-EP-25.Some of the amino compounds are pyrolyzed into ammonia and other nitrogen oxides.It is well documented that combustible gases such as aromatic compounds, hydrocarbons, and amides released during thermal decomposition can lead to the combustible features of epoxy resins.Consequently, the addition of 3ACP into epoxy resins can effectively hinder the release of combustible gases and further increase the flame-retardant properties of epoxy resins.
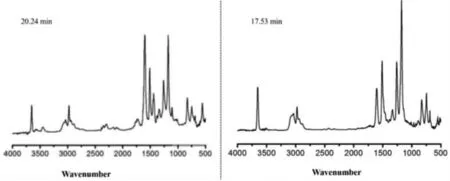
Fig.8.FTIR spectrum of pyrolysis products for 3ACP-EP-0 (left) and 3ACP-EP-25 (right) at the maximum decomposition rate.
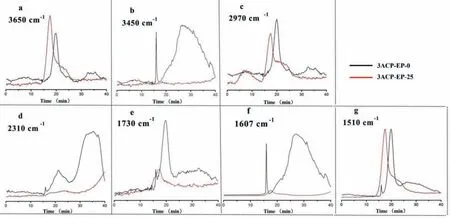
Fig.9.Absorbence of pyrolysis products for epoxy thermosets vs. time: (a) H2O, (b) N–H, (c) hydrocarbons, (d) CO2, (e) carbonyl, (f) aromatic rings, (g) substituted aromatic compounds.
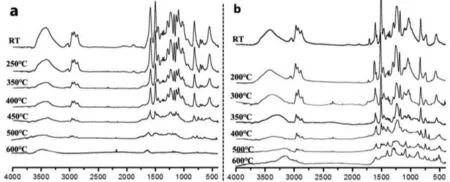
Fig.10.Series FTIR spectra of (a) 3ACP-EP-0 and (b) 3ACP-EP-25 at different pyrolysis temperatures.
The FTIR spectra of the 3ACP-EP-0 at different degradation temperatures are shown in Fig.10a.It can be noted that the characteristic absorptions peaks at 2930, 1607, 1510, 1456, 1107 and 1036 cm−1are corresponding to the epoxy, while peaks originate at 3392, 1275, 1250, 1200, 1175 cm−1are indexed to the characteristic absorptions of DDS and curing bonds.Below 400°C, there is no obvious change in the relative intensities of the characteristic peaks.With increasing the temperature to 450°C, the intensity of absorption peaks at 2930, 1607, 1510, 1456, 1275, 1250, 1200,1175,1107 and 1036 cm−1remarkably decreased and weakened,indicating that the main decomposition representing the whole epoxy curing system occurs at 400–450°C.These findings are consistent with the results of TGA and FTIR-TGA tests (Figs.5 and 7).The FTIR spectra of the 3ACP-EP-25 at different degradation temperatures are shown in Fig.10b.Herein, we use a series of temperatures that are suitable for the decomposition process according to the TGA results.It can be seen the same peaks about epoxy as those appearing in Fig.10a, in which the peaks at 1250, 1200, 810,750 cm−1are the characteristic absorptions of 3ACP and curing bonds.The relative intensity of epoxy characteristic peaks changed greatly by increasing the temperature to 300–400°C, while no obvious change is observed in the relative intensity of characteristic peaks of 3ACP.It can be easily interpreted that the epoxy bonds have been broken down, while 3ACP bonds mostly remain intact during decomposition.As a result, the catalytic effect by the carbon char residue of 3ACP can be fully implemented.When the temperature approaches 500°C or is even higher (600°C), some new peaks are originated at 1275, 1070, 860 cm−1, corresponding to P=O and P–O bonds.This represents the dominant production of phosphoramides.Therefore, it can be concluded that the incorporation of 3ACP product into the thermosets might catalyse the formation of a charred layer and finally turn into phosphoramides,which are more thermal stable at higher temperatures.
In summary, a novel spiro-cyclic amino-cyclophosphazene derivative tri-(o-phenylenediamino) cyclotriphosphazene (3ACP)has been successfully synthesized.The chemical structures of 3ACP were characterized by FTIR spectroscopy,1H NMR,31P NMR spectroscopy and mass spectroscopy.New thermosetting material was obtained by incorporating the 3AC into the E-44/DDS epoxy resin system, in which 3AC can act as a new type of high-functional halogen-free flame retardant curing agent.The 3ACP-modified epoxy thermosets obtained excellent flame-retardant properties of a V-0 rating during UL-94 tests even with the addition of 5 wt%3ACP.The LOI value reached 29.2 at the same time.The incorporation of 3ACP into the epoxy resin increased the char residue values significantly due to the effective accelerating of the carbonization process at elevated temperatures.The investigations of char residue and gaseous products indicated that the improved flame retardancy performance of the epoxy resin was proved by the effective synergistic flame-retardant action that occurred in both the condensed and the gaseous phases.The introduced 3ACP can effectively suppress the release of combustible gases during combustion.The follow-up decomposition of E-44/DDS-3ACP curing systems promoted the formation of polyphosphoramides charred layer in the condensed phase.Finally, the less release of the combustible gases in the gaseous phase can inhibit the development of flame.In addition, own to the significant high char formation efficiency and multi-functional characteristics of 3ACP, the new epoxy curing system possessed excellent flame retardant properties.Therefore, this work supplies a facile way to obtain new nonflammable halogen-free epoxy resin for environmental protection and human health.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (No.51773010) and the Fundamental Research Funds for the Central Universities (No.XK1802–2).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.003.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
