Defective TiO2 hollow nanospheres as photo-electrocatalysts for photo-assisted Li-O2 batteries
Hiling Jio, Guiru Sun,∗, Yn Wng, Zexu Zhng, Zho Wng, Hirui Wng,Hio Li,∗, Ming Feng,∗
a Key Laboratory of Functional Materials Physics and Chemistry of the Ministry of Education, Jilin Normal University, Changchun 130103, China
b Key Laboratory of Preparation and Applications of Environmental Friendly Materials of the Ministry of Education, Jilin Normal University, Changchun 130103, China
ABSTRACT The large overpotential for conventional Li-O2 batteries is an enormous challenge, which impedes their practical application.Here, we prepare a defective TiO2 (Ov-TiO2) hollow nanosphere as photoelectrocatalyst for photo-assisted Li-O2 batteries to reduce the overpotential.Under illumination, the oxygen vacancies as a charge separation center contribute to the separation of electrons and holes.The generated electrons could promote reducing O2 to Li2O2 during oxygen reduction reaction (ORR) process,while the generated holes are beneficial to Li2O2 decomposition during oxygen evolution reaction (OER)process.Additionally, the proper concentration of oxygen vacancies will decrease the recombination rate between electrons and holes.The photo-assisted Li-O2 batteries with Ov-TiO2-650 exhibit advanced performances, such as the low overpotential (0.70 V), the fine rate capability, and the considerable reversibility accompanied with the formation/decomposition of Li 2O2.We expect that these results could open a new mind to design of highly efficient photo-electrocatalysts for photo-assisted Li-O2battery.
Keywords:TiO2 Hollow nanospheres Oxygen vacancies Photo-electrocatalyst Li-O2 battery
In recent years, rechargeable lithium-oxygen (Li-O2) batteries have attracted considerable attention due to their high theoretical specific energy density (∼3500 Wh/kg), which is considered as a promising candidate to replace Li-ion batteries [1].A typical nonaqueous Li-O2battery consists of a Li anode, an organic electrolyte,and a porous cathode, which operates based on the formation and decomposition of lithium peroxide (Li2O2) in porous cathode during oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) [2].The discharge product Li2O2with insoluble and insulating natures will deposit in the porous cathode, which could clog the mass transport channel and passivate cathode, leading to a large overpotential and then lowering the charge–discharge effi-ciency of Li-O2batteries [3].
As a direct strategy to enhance the reaction kinetics of Li-O2batteries, a number of electrocatalysts such as noble metals (Pt and Pd) [4] and transition metal compound (CoO [5], MoS2[6], TiC [7],and Co2P [8]) have been explored to boost the reaction kinetics of Li-O2batteries.However, the high cost of noble metals and large voltage hysteresis of transition metal compound limit their practical applications [9].Soluble redox mediators (RMs), as another alternative, have been introduced into electrolytes to achieve the low overpotential [10].Unfortunately, the shuttle effect of RMs could lead to the RMs degradation and Li anode corrosion, resulting in poor cycling stability [11].Therefore, it is significant that a new strategy is developed to accelerate the decomposition of Li2O2.
Recently, a photo-assisted Li-O2battery as a new system has been explored to reduce overpotential and improve cycle life [12].In this system, the cathode contains a suitable semiconductor with high light harvesting capability, which can separate electrons and holes under light illumination.The generated electrons and holes are beneficial for Li2O2formation and decomposition, respectively [13].To date, some semiconductors, such as TiO2-Fe2O3[14],WO3@g-C3N4NWA [15], and Co-TABQ [16] have been reported in photo-assisted Li-O2batteries, which remarkably lower the overpotential and enhance the rate capability.Among various semiconductors, titanium dioxide (TiO2) is considered as a promising photocatalyst due to its non-toxicity, cost-effectiveness, good electrochemical stability, and superior photostability [17].Unfortunately,TiO2exhibits poor photo-absorption and low photoconversion effi-ciency in the visible light resulting from its large band gap energy[18].It has been clearly demonstrated that introducing oxygen vacancies into TiO2(i.e., black TiO2) can boost its photoresponse to solar/visible light and its photocatalytic activity [19].However, the bulk oxygen vacancies in TiO2will serve as recombination centers of photogenerated electrons and holes, causing the decrease of photocatalytic efficiency [20].Therefore, it is crucial to control the concentration of oxygen vacancies on the surface of TiO2for improvement of its photocatalytic property.
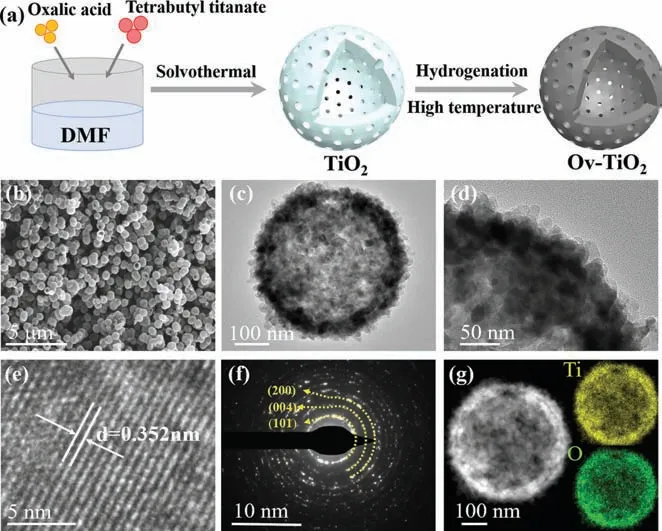
Fig.1.(a) Schematic illustration of the preparation process of Ov-TiO2-T, (T = 550,600, 650 and 700).(b) SEM image, (c, d) TEM images, (e) HRTEM image, (f) SAED image, and (g) HAADF-STEM image and EDX elemental mapping images of Ov-TiO2-650.
In this work, the mesoporous TiO2hollow nanospheres with various concentration of oxygen vacancies (Ov-TiO2) were synthesized at different annealing temperature, which was employed as a photo-electrocatalyst for photo-assisted Li-O2batteries.The oxygen vacancies as charge separation center can promote the separation of electrons and holes with illumination.The photoexcited electrons contribute to the reduction of O2during ORR process,while the photoexcited holes are beneficial to Li2O2decomposition during OER process.Additionally, the proper concentration of oxygen vacancies will decrease the recombination rate between electrons and holes.The photo-assisted Li-O2batteries with Ov-TiO2-650 exhibit a low overpotential of 0.70 V at a current density of 100 mA/g, a superior rate capability, and a good reversibility accompanied with the formation and decomposition of Li2O2.This work represents a promising process in the development of a highly active photo-electrocatalyst for photo-assisted Li-O2battery.
In Fig.1a, the oxalic acid (10.05 g) was slowly poured intoN,Ndimethylformamide (DMF, 90 mL) and stirred at room temperature for 30 min.Afterwards, tetrabutyl titanate (150 μL) was added into the above solution and stirred for 30 min.The obtained solution(30 mL) was then transferred to a Teflon lined autoclave with a capacity of 50 mL for a hydrothermal treatment at 170 °C for 8 h.The formed precipitate was filtered, rinsed and dried in air at 80 °C for 24 h.The obtained white product was named P-TiO2.According to the oxygen vacancies that form under H2reduction, Ov-TiO2-T(T= 550, 600, 650 and 700) were obtained by annealing pristine TiO2at 550, 600, 650 and 700 °C under Ar-H2(volume ratio of 92:8) atmosphere with a heating rate of 5 °C/min for 7 h, respectively.In comparison, TiO2-650 is acquired after calcining at 650 °C in air for 7 h.
The morphology of P-TiO2, TiO2-650, and Ov-TiO2-T (T= 550,600, 650 and 700) were characterized by scanning electron microscope (SEM) and transmission electron microscope (TEM).In Fig.1b and Fig.S1a (Supporting information), the P-TiO2, TiO2-650 and Ov-TiO2-T (T= 550, 600, 650, and 700) are uniform nanospheres with diameter of ∼500 nm.The as-prepared TiO2nanospheres present a hollow structure (Fig.1c and Fig.S1b in Supporting information).In Fig.1d and Fig.S1c (Supporting information), it can be observed that the as-prepared TiO2nanospheres exhibit porous structures [21].The N2adsorption/desorption isotherms indicate that as-prepared TiO2nanospheres are mesoporous structure with a pore size distribution from 5 nm to 20 nm(Fig.S2 in Supporting information).Note that the morphology and size of Ov-TiO2-T (T= 550, 600, 650 and 700) are similar with PTiO2, implying that the introduced oxygen vacancies do not alter the microstructure of TiO2.The high-resolution transmission electron microscopy (HRTEM) images of as-prepared TiO2show the lattice fringes with distances of 0.35 nm, which corresponds to the (101) lattice plane of anatase TiO2(JCPDS #PDF No.21-1272)(Fig.1e and Fig.S1d in Supporting information).Interestingly, with the increasing of temperature, the distance of lattice plane is increased, which might be caused by the regrowth of grain [22].As shown in Fig.1f and Fig.S1e (Supporting information), the selected area electron diffraction (SAED) of as-prepared TiO2display (101),(004), and (200) planes, proving the polycrystalline nature [23,24].The high-angle annular dark-field scanning TEM (HAADF-STEM)images and the corresponding energy dispersive X-ray (EDX) elemental mapping images for as-prepared TiO2are exhibited in Fig.S1f (Supporting information) and Fig.1g, which clearly confirms the existence and homogeneous distribution of Ti and O elements.The atomic ratios of O and Ti are 1.88, 1.57, 1.54 and 1.56 for Ov-TiO2-T (T= 550, 600, 650 and 700), respectively, suggesting a larger concentration of oxygen vacancies for Ov-TiO2-650 than that of others (Table S1 in Supporting information).
Fig.2a displays the X-ray diffraction (XRD) patterns of asprepared P-TiO2, TiO2-650, and Ov-TiO2-T (T= 550, 600, 650 and 700).The detected diffraction peaks at 25.2°, 37.8°, 48.0°, 53.9°,55.0°, 62.7°, 68.7°, 70.3° and 75.0° are assigned to the (101), (004),(200), (105), (211), (204), (116), (220), and (215) crystal planes of anatase TiO2(JCPDS #PDF No.21-1272) [25].Raman spectra of asprepared TiO2show five peaks at 167, 198, 396, 518 and 636 cm−1,which are attributed to the Eg1, Eg2, B1g, A1gand Eg3Raman active modes of the anatase TiO2, respectively (Fig.2b).With the increases of hydrogen reduction temperature, the peak of Eg1shift to smaller wavenumbers (Fig.S3 in Supporting information), resulting from the charge transfer of surface disorder between O2−and Ti4+,further suggesting that the oxygen vacancy and Ti3+exists in the as-prepared Ov-TiO2[26].Note that the Ov-TiO2-700 is an exception, which mainly because when the temperature rises to 700 °C,more energy separated electrons from oxygen vacancies and transferred to Ti4+[27].
The chemical state of the TiO2-650 and Ov-TiO2-650 are characterized by X-ray photoelectron spectroscopy (XPS).The survey spectra exhibit that TiO2-650 and Ov-TiO2-650 consist of Ti and O elements (Fig.S4 in Supporting information), which agrees with the results of the above EDX mapping.The Ti 2p spectra of TiO2-650 and Ov-TiO2-650 are displayed in Fig.2c.The peaks at 458.82 and 464.58 eV represent Ti4+.While the peaks at 458.05 and 463.75 eV are a characteristic of Ti3+.In Fig.2d, the O 1s spectra of TiO2-650 and Ov-TiO2-650 show two peaks at 532.25 and 530.13 eV, which is characteristic of the lattice oxygen (O-Ti)and hydroxyl group and/or surface adsorbed oxygen, respectively[28,29].Note that the ratio of adsorbed oxygen significantly enhances by H2reduction, demonstrating that oxygen vacancies is beneficial to improving surface adsorption of oxygen species [30].Equally, the atomic ratios of O and Ti are calculated, as shown in Table S2 (Supporting information).The results show that the Ov-TiO2-650 possesses highest concentration of oxygen vacancies,which is consistent with the EDX mapping results.The electron spin resonance (ESR) spectra of TiO2-650 and Ov-TiO2-650 are obtained at room temperature to determine unpaired electrons in oxygen vacancies [31].As shown in Fig.2e, the TiO2-650 only presents a baseline noise signal, while the Ov-TiO2-650 exhibits an unpaired electron signal atg= 2.0014, revealing the existence of oxygen vacancies in Ov-TiO2-650.The Ti L-edge X-ray absorbtion spectrum (XAS) profiles of TiO2-650 and Ov-TiO2-650 are shown in Fig.2f, which display four well-defined peaks (labeled as A, B,C and D), representing the transitions from Ti 2p to Ti 3d levels of 2p3/2→3d t2g, 2p3/2→3d eg, 2p1/2→3d t2g, and 2p1/2→3d eg, respectively.The peak of B is split into two signals due to distortion of the octahedron formed by the ligands in TiO2[32–34].Generally,the intensity of characteristic peaks relates the Ti valence state.TheI(L2)/I(L3) intensity ratio for TiO2-650 and Ov-TiO2-650 is 1.266 and 0.692, respectively, further suggesting a high concentration of Ti3+ions in Ov-TiO2-650.
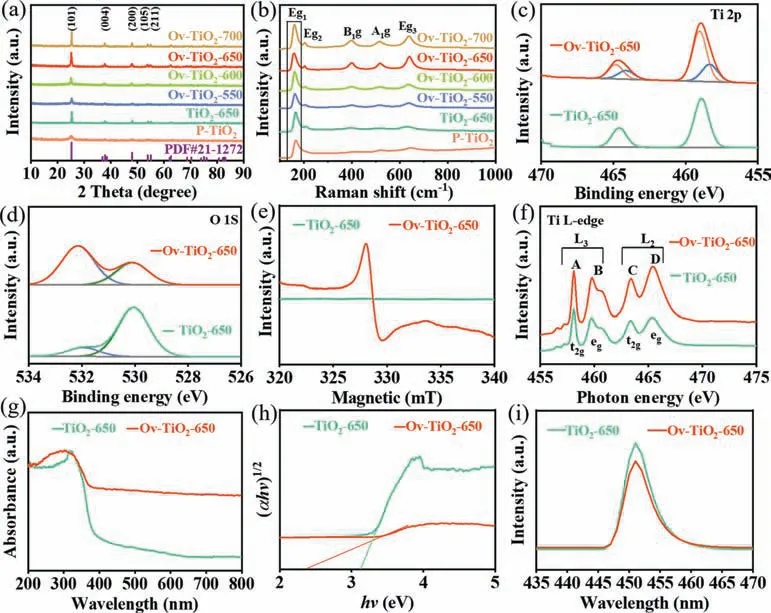
Fig.2.(a) XRD patterns and (b) Raman spectra of as-prepared P-TiO2, TiO2-650, and Ov-TiO2-T (T = 550, 600, 650 and 700).XPS core-level spectra of (c) Ti 2p and (d) O 1s of TiO2-650 and Ov-TiO2-650.(e) ESR spectra, (f) Ti L-edge XAS profiles, (g) UV–vis absorption spectra, (h) the corresponding Kubelke-Munk transformed diffuse reflectance spectra, and (i) PL spectra of TiO2-650 and Ov-TiO2-650.
In Fig.2g, the ultraviolet visible spectrophotometer (UV–vis) absorption spectra are used to identify the optical absorption properties of the TiO2-650 and Ov-TiO2-650.In comparison with the TiO2-650, the Ov-TiO2-650 exhibits a clearly shift in the onset of absorption from the UV to visible light region.In Fig.2h, the bandgap of Ov-TiO2-650 is about 2.38 eV, which is smaller than that of TiO2-650 (3.16 eV), indicating that the solar-driven photocatalytic activity of TiO2can be enhanced by introducing oxygen vacancies [35].Equally, the photoluminescence (PL) spectra are carried out to evaluate the recombination behavior of the photogenerated carriers in the TiO2-650 and Ov-TiO2-650 (Fig.2i).the emission peak for the Ov-TiO2-650 is much lower than that for TiO2-650, demonstrating that the introducing oxygen vacancies into TiO2contributes to decrease the electron-hole recombination rate [36,37].
The catalytic activity of as-prepared TiO2is studied by evaluating the electrochemical performance of photo-assisted Li-O2batteries with TiO2-650 and Ov-TiO2-T (T= 550, 600, 650 and 700).In Fig.3a, under illumination, the Li-O2cell with Ov-TiO2-650 delivers highest discharge and charge specific capacities at 500 mA/g,which reaches 9390 and 9853 mAh/g, respectively.Note that the cells show two platforms during the charging process.The first platform at ∼3.75 V is attributed to the decomposition of Li2O2.The second platform located at ∼4.40 V, corresponding to the decomposition of electrolyte.Fig.S5a (Supporting information) displays the first discharge-charge profiles of Li-O2cells with TiO2-650 and Ov-TiO2-T (T= 550, 600, 650 and 700) at 500 mA/g under the fixed capacity of 1000 mAh/g with illumination.The discharge and charge voltage platform for a cell with Ov-TiO2-650 are 2.80 and 3.75 V, respectively, leading to an overpotential of 0.95 V,which lower than that of Li-O2cells with TiO2-650 and Ov-TiO2-T(T= 550, 600, 700).Therefore, the Ov-TiO2-650 could be considered to contain the proper concentration of oxygen vacancies on its surface in this work, which could decrease the recombination rate between electrons and holes [38,39].
The Li-O2batteries based on the Ov-TiO2-650 are tested to study the impact of the illumination on cell performance in the discharge process.The first discharge-charge curves for the Li-O2batteries at 100 mA/g under a capacity limit of 1000 mAh/g with and without illumination are presented in Fig.3b.Under illumination, the discharge potential increases from 2.72 V to 2.86 V and the charge potential decreases from 3.8 V to 3.56 V.The reduced discharge and charge overpotentials with illumination are attributed to the photo-energy [40].Additionally, the rate performance was measured at different current densities, as shown in Fig.3c and Fig.S5b (Supporting information).In Fig.3c, the discharge–charge voltage plateau for a cell cycled with illumination are slightly changed from 2.86/3.56 V at 100 mA/g to 2.82/3.77 V at 500 mA/g.In contrast, the discharge voltage plateau for a cell cycled without illumination significantly decreases to 2.70 V, and the charge voltage increases to 4.01 V, at a high current density of 500 mA/g (Fig.S5b).Moreover, the cycle abilities of the cells were investigated at current density of 500 mA/g with a capacity limit of 1000 mAh/g.As shown in Fig.S6 (Supporting information), the cell shows a good cyclability of 100 cycles with illumination.For the cell without illumination, it only sustains 62 cycles.These results imply that the Ov-TiO2-650 as photo-electrocatalysts in photo-assisted Li-O2battery not only contributes to the Li2O2formation at a high discharge voltage but also promotes its decomposition at a low charge voltage [41].
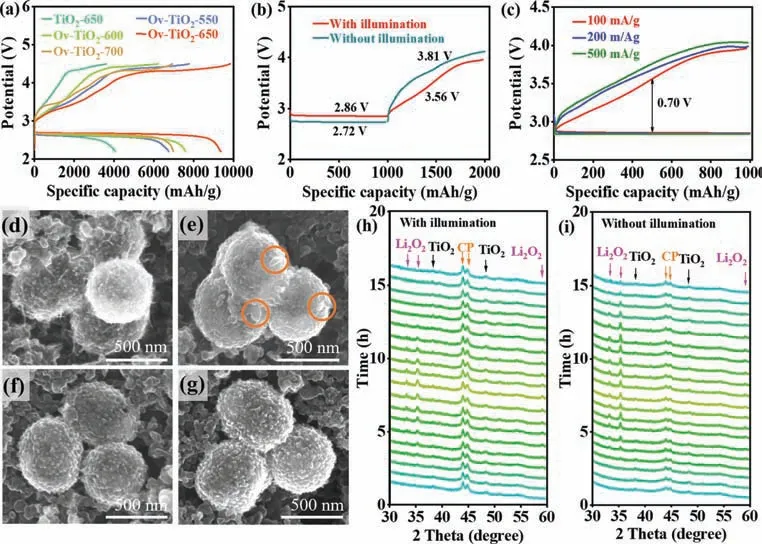
Fig.3.(a) The discharge–charge curves of photo-assisted rechargeable Li-O2 battery with TiO2-650 and Ov-TiO2-T (T = 550, 600, 650 and 700) at 500 mA/g within a potential range of 2.2–4.4 V vs. Li/Li+.(b) The first discharge-charge profiles of Li-O2 cells with Ov-TiO2-650 with and without illumination at 100 mA/g.(c) The first discharge–charge profiles of photo-assisted Li-O2 cell with Ov-TiO2-650 with at different densities.SEM images of Ov-TiO2-650 after discharge (d) without and (e) with illumination.SEM images of Ov-TiO2-650 after recharge (f) with and (g) without illumination. In situ XRD patterns of Li-O2 cell (h) with and (i) without illumination.
The reversibility of Li-O2cells using Ov-TiO2-650 catalyst with and without illumination is investigated through characterizing the cathodes at different electrochemical states by SEM andin situXRD.In Fig.S7 (Supporting information), the super P (SP) particles and Ov-TiO2-650 nanospheres are distributed on the surface of cathode.After discharge, the film-like discharge product deposits on the surface of Ov-TiO2-650 without illumination (Fig.3d).Note that the large film-like products on the surface of catalyst limit the contact between electrolyte and active sites, which hinders the further discharge–charge reactions [14].In contrast, under illumination, toroidal-like discharge product forms on the surface of Ov-TiO2-650 (Fig.3e).After recharge, some residues of film-like product remain without illumination (Fig.3f), while the toroidal-like products totally disappear with illumination (Fig.3g), suggesting a good reversibility of Li-O2cells with Ov-TiO2-650 under illumination.Equally, the morphology of discharge products was studied after deep discharge.In Fig.S8 (Supporting information), the morphology of the discharge products remained unchanged with and without illumination.In Fig.S9 (Supporting information), after discharge, the cathodes were characterized by Raman.The peaks of Li2O2at 788 cm−1are observed for cathodes cycled with and without illumination, indicating that the toroidal-like and film-like discharge products are Li2O2.Figs.3h and i display thein situXRD patterns of Li-O2cells using Ov-TiO2-650 catalyst cycled with and without illumination, respectively.The new diffraction peaks corresponding to Li2O2appear and gradually increase with the discharge reaction for both cells, which proves the film-like and toroidal-like products are Li2O2.During recharge process, the intensity of Li2O2diffraction peaks gradually decreases until it disappears for a cell cycled with illumination (Fig.3h).In contrast, the Li2O2diffraction peaks always exist after recharge for a cell cycled without illumination (Fig.3i) [42,43].The results further confirm the photo-assistance could improve the reversibility of Li-O2cells with Ov-TiO2-650, which are consistent with the above SEM results.Additionally, the Nyquist plots of the cells using Ov-TiO2-650 catalyst before and after cycling without and with illumination are shown in Figs.S10a and b (Supporting information).In Fig.S10c(Supporting information), the corresponding impedances are obtained by fitting using the equivalent circuit.After 2nd cycles, the total resistance value is 154.12Ωfor a cell cycled without illumination, which significantly higher than that of a cell cycled with illumination (108.89Ω).These results reveal that a cell using Ov-TiO2-650 catalyst with illumination exhibits a better reversibility than without illumination, providing an evidence of the high ORR and OER actives of Ov-TiO2-650 induced by both photo-assistance and oxygen vacancies [39].
The ORR and OER performances with and without illumination are studied to verify the discharge/charge reaction mechanisms for photo-assisted Li-O2batteries with Ov-TiO2-650.The cyclic voltammetry (CV) curves for the Li-O2cells using Ov-TiO2-650 catalyst at 0.5 mV/s with and without illumination are exhibited in Fig.4a and Fig.S11 (Supporting information).Compared with a cell without illumination, a higher onset reduction potential and a larger cathodic current are found for a cell under illumination.The rate capability of the Li-O2cells with Ov-TiO2-650 from 50 mA/g to 1000 mA/g are studied, as shown in Fig.4b.The cell maintained a high discharge voltage (2.71 V) at a large current density of 1000 mA/g, which clearly exceed the cell without illumination (2.51 V at 1000 mA/g).These results indicate Ov-TiO2-650 with a higher activity toward ORR under illumination, which could be attributed to the photogenerated electrons [44,45].Additionally, the kinetic properties of Ov-TiO2-650 with illumination during the charge process are assessed by executing a Li2O2contained experiment.The preloaded Li2O2cathodes are made based on the commercial Li2O2and Ov-TiO2-650.Fig.4c presents the linear sweep voltammetry (LSV) curves for the Li-O2batteries utilizing preloaded Li2O2cathodes with and without illumination at a scan rate of 0.2 mV/s.It can be observed that both cells show the anodic peak related to decomposition of Li2O2[14].Significantly, the anodic peak for the cell with illumination is higher than the cell without illumination, confirming that the Ov-TiO2-650 is beneficial to promote the decomposition of Li2O2under illumination, which are consistent with the CV results (Fig.S11).This impact is further demonstrated by the constant current charging,as shown in Fig.4d and Fig.S12 (Supporting information).The charge voltage of Li-O2batteries with preloaded Li2O2are 3.34,3.85, and 3.88 V at 100, 500, and 1000 mA/g, respectively with illumination, outperforming the cell without illumination.These results provide an evidence that the photo-assistance contributes to enhancing the reaction kinetics of Ov-TiO2-650 in OER process,which could be contributed by the photogenerated holes [46,47].In Fig.4e, based on above experimental results, the ORR and OER mechanisms for a photo-assisted Li-O2batteries with Ov-TiO2-650 are proposed.The Ov-TiO2-650 will promote the separation of electrons and holes under illumination.During discharge process, the photoexcited electrons are uniformly distributed on surface of Ov-TiO2-650, which could accelerate the rate of LiO2generation, thus facilitating the growth of Li2O2.During charge process, the photogenerated electrons migrate to Li anode by external circuit due to the force of the electric field, leading to the reduction of Li+to Li and deposition on surface of Li anode [48].As a result, the photoexcited holes are separated to the surface of Ov-TiO2-650, which helps to the decomposition of Li2O2at a low charge voltage.Therefore, the Li-O2batteries with Ov-TiO2-650 exhibit superior electrochemical performance with illumination, ascribing to appropriate a concentration of oxygen vacancies of Ov-TiO2-650, which could provide a proper band gap and abundant active sites for the high efficiency separation of electrons and holes.
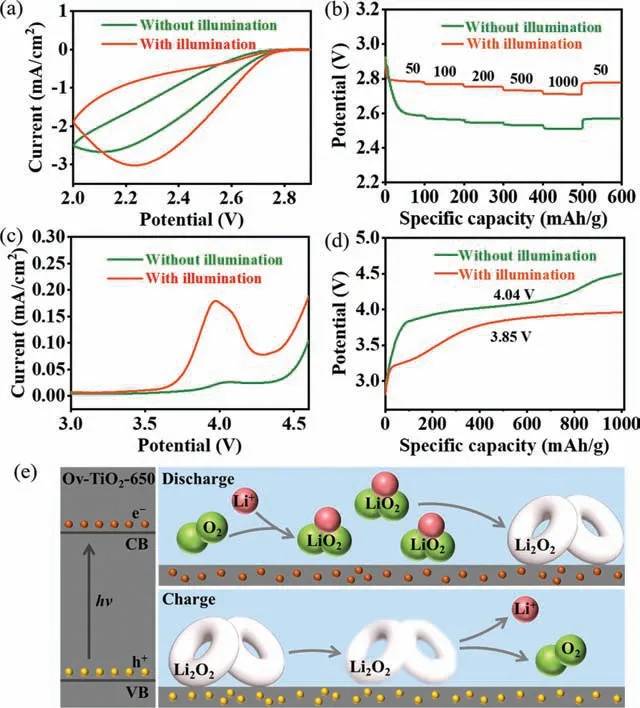
Fig.4.(a) CV curves of Li-O2 cells with and without illumination at 0.5 mV/s with 2.0–2.9 V vs. Li/Li+.(b) Rate capability of Li-O2 cells during the discharge process with and without illumination, (c) the LSV curves for the Li-O2 cells with preloaded Li2O2 cathodes with and without illumination at 0.2 mV/s.(d) Charge curves of the Li-O2 cells with preloaded Li2O2 cathodes with and without illumination at 500 mA/g.(e) Schematic diagrams of the reaction mechanisms for the photo-assisted Li-O2 batterie with Ov-TiO2-650.
In summary, we successfully prepared the Ov-TiO2-650 as a photo-electrocatalyst for photo-assisted Li-O2batteries.The Ov-TiO2-650 presents a microstructure with abundant mesoporous channels, which could provide paths for the diffusion of Li+and O2, as well as enough space for the deposition of Li2O2.The oxygen vacancies are as charge separation center, which contribute to the separation of electrons and holes with illumination.The photogenerated electrons are beneficial to reducing O2to Li2O2during discharge process.Equally, the photogenerated holes could promote the decomposition of Li2O2during charge process.Consequently,the photo-assisted Li-O2batteries with Ov-TiO2-650 exhibit advanced performances, such as the low overpotential (0.70 V), the fine rate capability, and the considerable reversibility accompanied with the formation/decomposition of Li2O2.We believe that the work could offer an insight into the design of highly efficient photo-electrocatalyst for photo-assisted Li-O2battery.
Declaration of competing interest
The authors declare no conflicts of interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21978110, 51772126, and 52171210), the Jilin Province Science and Technology Department Program (Nos.20200201187JC, 20190201309JC, and YDZJ202101ZYTS047), the“13thfive-year” Science and Technology Project of Jilin Provincial Education Department (Nos.JJKH20200407KJ and JJKH20210444KJ)and the Jilin Province Development and Reform Commission Program (No.2020C026-3).Allocation of beamtime at 4B9B, BSRF, Beijing, China, is gratefully acknowledged.The authors would like to thank Dr.Jiaou Wang and Dr.Kaiqi Nie for the help in soft-X-ray absorption spectrum.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.086.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
