Local high-density distributions of phospholipids induced by the nucleation and growth of smectic liquid crystals at the interface
Chnjing Yang, Li Chn, Rui Zhang, Dong Chn,, Laura R.Arriaga,David A.Witz
a College of Energy Engineering and State Key Laboratory of Fluid Power and Mechatronic Systems, Zhejiang University, Hangzhou 310027, China
b Zhejiang Key Laboratory of Smart BioMaterials and Center for Bionanoengineering, College of Chemical and Biological Engineering, Zhejiang University,Hangzhou 310027, China
c Department of Theoretical Condensed Matter Physics, Condensed Matter Physics Center and Instituto Nicolás Cabrera, Universidad Autónoma de Madrid,Madrid 28049, Spain
d John A.Paulson School of Engineering and Applied Sciences and Department of Physics, Harvard University, Cambridge, MA 02138, United States
e Department of Physics,The Hong Kong University of Science & Technology, Hong Kong, China
ABSTRACT Amphiphilic molecules adsorbed at the interface could control the orientation of liquid crystals (LCs)while LCs in turn could influence the distributions of amphiphilic molecules.The studies on the interactions between liquid crystals and amphiphilic molecules at the interface are important for the development of molecular sensors.In this paper, we demonstrate that the development of smectic LC ordering from isotropic at the LC/water interface could induce local high-density distributions of amphiphilic phospholipids.Mixtures of liquid crystals and phospholipids in chloroform are first emulsified in water.By fluorescently labeling the phospholipids adsorbed at the interface, their distributions are visualized under fluorescent confocal microscope.Interestingly, local high-density distributions of phospholipids showing a high fluorescent intensity are observed on the surface of LC droplets.Investigations on the correlation between phospholipid density, surface tension and smectic LC ordering suggest that when domains of smectic LC layers nucleate and grow from isotropic at the LC/water interface as chloroform slowly evaporates at room temperature, phospholipids transition from liquid-expanded to liquid-condensed phases in response to the smectic ordering, which induces a higher surface tension at the interface.The results will provide an important insight into the interactions between liquid crystals and amphiphilic molecules at the interface.
Keywords:Liquid crystals Phospholipids Droplet Interface Smectic
Interfaces between two immiscible fluids are generally stabilized by amphiphilic molecules or particles [1,2].Interactions between amphiphilic molecules and liquid crystals (LCs) at the interface have attracted intense attentions, since the first demonstration of highly sensitive biosensors based on the reorientation of LC molecules in response to the change of amphiphilic molecules adsorbed at the interface [3–7].LC molecules possess both crystalline ordering and liquid fluidity [8].In the nematic phase, rodlike LC molecules tend to align their long axes along the director, while the presence of amphiphilic molecules at the interface will impose a either homeotropic or planar anchoring on the LC molecules, thus controlling their orientation [9].In a typical experimental geometry of a LC cell sandwiched between air and water or a LC droplet dispersed in water, the orientation of LC molecules depends on both the types and concentrations of amphiphilic molecules and could reorient in response to the change of amphiphilic molecules adsorbed at the interface [10,11].Highly sensitive LC biosensors have thus been developed by manipulating the interactions between LCs and amphiphilic molecules at the LC/water interface [12–16].These LC biosensors are highly sensitive and highly specific and the results could be read out by direct observation under polarized optical microscope, which greatly enriches the applications of LCs [17–19].
Generally, amphiphilic molecules at the interface could impose either a homeotropic anchoring with LC molecules perpendicular to the interface or a planar anchoring with LC molecules parallel to the interface [20].For example, when sodium dodecyl sulfate(SDS) molecules are adsorbed at the LC/water interface, rod-like LC molecules are perpendicular to the interface and LC droplets adopt a radial configuration [21,22].In contrast, when polyvinyl alcohol(PVA) molecules are used to stabilize LC droplets, LC molecules are planarly aligned at the interface and LC droplets form a bipolar structure [23–25].The changes of LC droplets, for example, from radial configuration to bipolar structure in response to the change of amphiphilic molecules at the interface, could directly be identified under polarized optical microscope (POM) and the underlying mechanisms have thus been applied in different scenarios for the development of biosensors [26–29].
Despite all these advances, interactions between LCs and amphiphilic molecules at the LC/water interface have not been fully explored.Previous studies mainly focus the anchoring of LC molecules by amphiphilic molecules at the interface.The interactions between LCs and amphiphilic molecules at the interface is of great value in exploring how LC ordering could influence the self-assembly of amphiphilic molecules at the interface, thus providing a promising approach for biological sensors and analytical applications [30,31].In a recent study, molecular simulations show that nematic LC ordering in turn could influence the distributions of amphiphilic molecules at the LC/water interface, showing elastic energy-driven phase separation [6,32-34].In the simulations, nematic LC molecules are confined in small droplets of several hundred nanometers, which cause a large elastic deformation of the nematic phase and thus influence the distribution of amphiphilic molecules at the interface.Various patterns showing local high-density distributions of amphiphilic molecules at the interface are then observed in the simulations [5].The interesting results have intrigued intense studies to explore the interactions between LCs and amphiphilic molecules at the interface, since the results predicted by the simulations have been observed in the experiments.
In this paper, we demonstrate that the development of smectic LC ordering from isotropic at the LC/water interface could induce local high-density distributions of amphiphilic molecules.Mixtures of liquid crystals and phospholipids in chloroform are first emulsified in water.Phospholipids spontaneous adsorb at the LC/water interface, imposing a homeotropic anchoring on the LC molecules.Meanwhile, as chloroform slowly evaporates at room temperature,domains of smectic LC layers nucleate and grow from isotropic at the LC/water interface, influencing the distributions of phospholipids.By fluorescently labeling the phospholipids, patterns of high fluorescent intensity, which correspond to regions of local high-density phospholipids, are observed on the surface of LC droplets under fluorescent confocal microscope.Measurements of the surface pressure of phospholipids on molecular density and the surface tension of LC droplets in water on temperature suggest that the development of smectic LC ordering at the interface could induce a local high surface pressure at the interface and thus a local enrichment of phospholipids.The results suggest that phospholipids adsorbed at the interface could affect the orientation of LC molecules while the development of LC ordering at the interface could in turn influence the distributions of phospholipids.
8CB, a typical rod-like LC molecule, consists of a rigid core and a flexible tail.Upon cooling, 8CB undergoes a phase sequence of Iso (40 °C) N (33 °C) SmA (23 °C) crystal.To investigate the interactions between LCs and phospholipids at the LC/water interface, 8CB, POPC and biotinyl-PE are co-dissolved in chloroform and emulsified in water by vortexing, yielding LC droplets stabilized by phospholipids, as illustrated schematically in Figs.1a and b.As chloroform slowly diffuses out of the LC droplets and evaporates in air at room temperature, regions of 8CB smectic layers gradually develop at the LC/water interface from initially isotropic LC droplets, thereby inducing local high-density distributions of phospholipids at the interface, as modeled in Fig.1c.To observe the phospholipids at the LC/water interface, FITC-streptavidin is added to the water phase, which binds to biotinyl-PE and enables the visualization of fluorescent-labeled phospholipids under fluorescent confocal microscope, as illustrated in Fig.1d.To facilitate the image acquisition, the continuous water phase is replaced by 10 wt%PVA solution and the resultant LC droplets are immobilized in the PVA polymer matrix, which forms a polymer film upon the evaporation of water.
The textures of 8CB LC droplets at room temperature are observed under polarized optical microscope (POM), as shown in the leftmost panel of Fig.2a.The observed textures show typical four dark brushes without any director fluctuation under crossed polarizers, suggesting a radial configuration of smectic layers in the droplets.Interestingly, the distributions of fluorescent-labeled phospholipids at the LC/water interface of the LC droplets are not homogeneous, as shown in the fluorescent confocal microscope images of Fig.2a.When chloroform is evaporated at 25 °C in the absence of PVA, phospholipids at the LC/water interface impose a homeotropic anchoring on LC molecules and patchy regions of high fluorescent intensity are observed at the interface, suggesting local high-density distributions of phospholipids, as shown in Fig.2a.In contrast, when chloroform is evaporated at 25 °C in the presence of PVA, PVA molecules at the LC/water interface impose a competing planar anchoring on LC molecules and phospholipids at the interface form a highly-connected fractal network of high fluorescent intensity, as shown in Fig.2b.The appearance of local highdensity phospholipids is attributed to the nucleation and growth of smectic LC domains on the droplet surface, which have been directly observed under POM as chloroform gradually evaporates, as shown in Fig.2c.
The observations of patch-like or fractal-like patterns of local high-density phospholipids are attributed to the different nucleation and growth of smectic layers under different anchoring conditions.In the absence of PVA molecules, phospholipids adsorbed at the LC/water interface impose a homeotropic anchoring on the LC molecules and the initial growth of smectic layers are parallel to the interface with the layer normal perpendicular to the interface, leading to the formation of patch-like patterns.In contrast, PVA molecules impose a competing planar anchoring on the LC molecules, under which LC molecules prefer to be parallel to the interface, and the initial development of smectic layers at the interface tends to form fractal-like patterns, which are consistent with previous studies [10,35].
To confirm that local high-density distributions of phospholipids at the LC/water interface are caused and locked by smectic layers that develop at the interface, different sets of control experiments are carried out.When the LC droplets, which are prepared by evaporating chloroform at 25 °C and show local highdensity phospholipids, are incubated at 38 °C for 2 h, smectic LC droplets transition to nematic LC droplets, showing typical four dark brushes with director fluctuation under crossed polarizers, as shown in the leftmost panel of Fig.3a.Interestingly, local highdensity distributions of phospholipids essentially disappear after incubation, showing a relatively homogeneous coverage of phospholipids on the surface of nematic LC droplets, as shown in the fluorescent confocal microscope images of Fig.3a.Compared with smectic LCs, nematic LCs have a much larger molecular mobility as evidenced by the director fluctuation.Therefore, local high-density distributions of phospholipids developed and locked in the smectic phase could be relaxed in the nematic phase at high temperature.Similarly, when the LC droplets are incubated at 65 °C for 2 h, LC droplets transition from smectic to isotropic, in which LC molecules also have a large molecular mobility, and relatively homogeneous distributions of phospholipids are observed on the surface of isotropic LC droplets, as shown in Fig.3b.
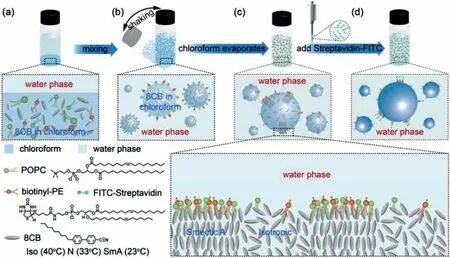
Fig.1.Interactions between liquid crystals and phospholipids at the LC/water interface.(a, b) 8CB, POPC and biotinyl-PE are co-dissolved in chloroform, which are emulsified in water by shaking to form LC droplets.(c) As chloroform slowly evaporates, phospholipids tend to anchor at the LC/water interface and smectic LCs gradually develop at the interface, inducing regions of local high-density phospholipids.(d) The distribution of phospholipids at the interface is directly visualized under fluorescent confocal microscope by binding biotinyl-PE with FITC-Streptavidin.
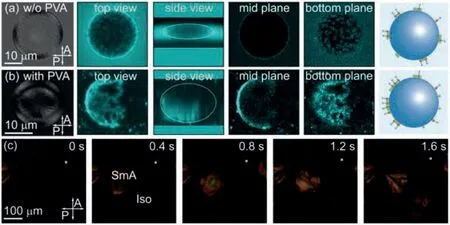
Fig.2.Local high-density distribution of fluorescent-labeled phospholipids at the LC/water interface induced by smectic LC ordering.Textures of smectic LC droplets and distributions of phospholipids are shown in the polarized optical microscope(POM) and fluorescent confocal microscope images, respectively.(a) Patch-like patterns of high fluorescent intensity are observed at the LC/water interface, when chloroform is evaporated at 25 °C in the absence of PVA.(b) Fractal-like patterns of high fluorescent intensity are observed at the LC/water interface, when chloroform is evaporated at 25 °C in the presence of PVA.All images of LC droplets are observed in dried PVA films.(c) Snapshots showing the formation of smectic LC domains on the surface of a LC droplet as chloroform gradually evaporates.
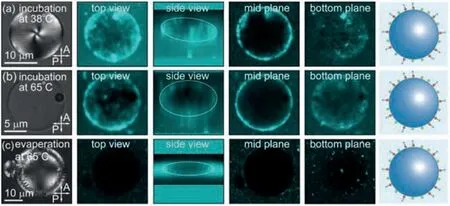
Fig.3.Relaxation of local high-density phospholipids in the nematic or isotropic phases.POM and fluorescent confocal microscope images of LC droplets prepared by (a) evaporating chloroform at 25 °C and then heating the sample up to 38 °C for 2 h, (b) evaporating chloroform at 25 °C and then heating the sample up to 65°C for 2 h, and (c) evaporating chloroform at 65 °C and then cooling the sample down to 25 °C.In all the three cases, phospholipids show a relatively homogeneous distribution at the LC/water interface.The twist of the four dark brushes at the center of the nematic LC droplet in (a) is attributed to the oblate shape.
To further verify the influence of smectic layers that nucleate and grow at the LC/water interface on the distributions of phospholipids, chloroform is evaporated at 65 °C, at which 8CB is isotropic.After the complete removal of chloroform, LC droplets are cooled down from isotropic to nematic and then to smectic at 25°C.As expected, no regions of high fluorescent intensity are observed on the droplet surface in this case, as shown in Fig.3c.This is because local high-density phospholipids are induced by the nucleation and growth of smectic layers directly from isotropic instead of transition from nematic.
The observed local high-density patterns of phospholipids,which are caused and locked by smectic layers, are different from the results reported in the simulations [6].The differences are attributed to the different conditions between the experiments and the simulations: (1) In the experiments, phospholipids undergo a thermal equilibrium of adsorption and desorption on the droplet surface, while phospholipids are fixed on the surface in the simulations.(2) In the experiments, phospholipids are overdosed to cover the whole droplet surface.POPC molecules roughly have a project area of 702/molecule and a length of 2.5 nm and thus the amount of POPC molecules is large enough to cover all LC droplets.In the simulations, phospholipids only partially cover the droplet surface.(3) In the experiments, the droplet size is tens of micrometers and the local high-density phospholipids are caused and locked by smectic layers that nucleate and grow at the interface.In contrast, the droplet size is several hundred nanometers in the simulations and elastic deformation of nematic LC within the droplet plays the dominant role in forming the local high-density patterns of phospholipids.
Generally, phospholipids at the interface are in thermal equilibrium, which adsorb and desorb from the interface driven by thermal energy, and the density of phospholipids are expected to be proportional to the surface tension.In a traditional Langmuir experiment of a monolayer of phospholipids at the air/water interface, the measured surface pressure isotherm,Π=γw–γl, whereγwis the surface tension of water in air andγlis the surface tension of water with phospholipids in air, shows that the surface pressure of phospholipids increases as the molecular density increases, as shown in Fig.4a.Meanwhile, the elasticity modulus calculated from the numerical derivative,KA=−, whereΠis the surface pressure andAis the molecular area, increases as the surface pressure increases, as shown in Fig.4b.The relation between surface pressure and molecular density suggests that the local high-density distributions of phospholipids are associated with the surface tension of smectic domains, which nucleate and grow at the interface.
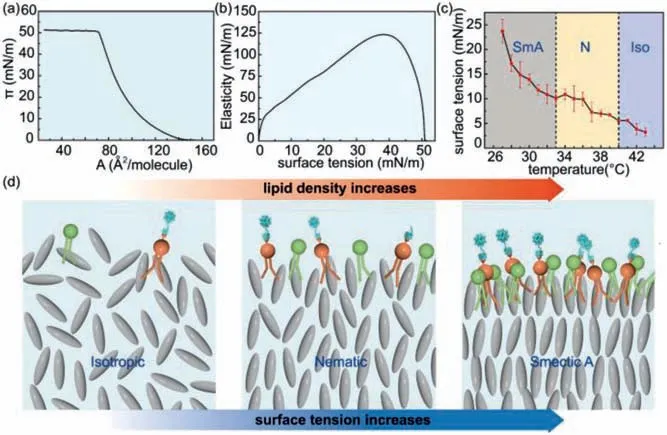
Fig.4.Correlations between smectic LC ordering, phospholipid density and surface tension.(a) Dependence of surface pressure of phospholipids at the air/water interface on molecular area [35].(b) Dependence of elasticity of phospholipids at the air/water interface on surface pressure [35].(c) Dependence of surface tension of LC droplets in water on temperature.(d) Models showing the increase of phospholipid density at the LC/water interface as the surface tension increases.
To explore the correlation between phospholipid density, surface tension and smectic LC ordering, the surface tensions of 8CB LC droplets in water without any surfactants are measured by pendant drop experiments, which are shown as a function of temperature in Fig.4c.In the absence of surfactants, LC droplets in water adopt a planar alignment at the LC/water interface.As 8CB LC droplets are cooled down from isotropic to nematic and then to smectic, their surface tension steadily increases, especially in the smectic phase where LC molecules are densely packed.Corresponding to the increase of surface tension as 8CB transitions from isotropic to nematic and then smectic, the thermal equilibrium density of phospholipids at the LC/water interface is expected to increase correspondingly, as modeled in Fig.4d.Therefore, as chloroform gradually evaporates, domains of smectic LC layers gradually nucleate and grow from isotropic at the LC/water interface, thus inducing local high-density distributions of phospholipids.Different from isotropic and nematic phases, which have a lower surface tension and a larger molecular mobility, local highdensity phospholipids are irreversibly caused and locked by smectic layers that initially develops at the interface.
The interactions between 8CB LC molecules and POPC phospholipids at the LC/water interface are investigated by directly visualizing the distributions of FITC-labeled phospholipids under fluorescent confocal microscope.Regions of high fluorescent intensity suggesting local high-density distributions of phospholipids are observed on the surface of LC droplets.The phenomena only occur when 8CB smectic layers nucleate and grow directly from isotropic as chloroform evaporates.Investigations on the correlation between phospholipid density, surface tension and smectic LC ordering suggest that the initial development of smectic LC ordering at the LC/water interface, which has a higher surface tension than that of isotropic phase, induces the transition of phospholipids from liquid-expanded to liquid-condensed phases.The results confirm that while phospholipids adsorbed at the interface could affect the orientation of LC molecules, the development of LC ordering at the interface could in turn influence the distributions of phospholipids, providing an important insight into the interactions between LCs and amphiphilic molecules at the interface.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is supported by Zhejiang Provincial Natural Science Foundation of China (No.LY20B060027) and National Natural Science Foundation of China (No.21878258).L.R.Arriaga acknowledges the Spanish Ministry of Economy MINECO for a Juan de la Cierva–Incorporacion Fellowship (No.IJCI-2014–22461).This work is also supported by the National Science Foundation (No.DMR1310266) and the Harvard Materials Research Science and Engineering Center (No.DMR-1420570).
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
