Self-assembly of reverse micelle nanoreactors by zwitterionic polyoxometalate-based surfactants for high selective production of β–hydroxyl peroxides
Guicong Hu, Wen Chang, Sai An, Bo Qi, Yu-Fei Song
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China
ABSTRACT Surfactants with polyoxometalates (POMs) as polar head groups have shown fascinating self-assembly behaviors and various functional applications.However, self-assembly them into reverse micelles is still challenging owing to the large molecular size and intermolecular strong electrostatic repulsions of POM heads.In this work, a zwitterionic POM-based surfactant was synthesized by covalently grafting two cationic long alkyl tails onto the lacunary site of [PW11O39]7−.With decreased electrostatic repulsions and increased hydrophobic effect, the POM-based reverse micelles with an average diameter of 5 nm were obtained.Interestingly, when these reverse micelles were applied for catalyzing the oxidation of styrene, an unprecedented β–hydroxyl peroxide compound of 2–hydroxyl-2-phenylethan-1–tert-butylperoxide was produced in high selectivity of 95.2%.In comparison, the cetyltrimethylammonium electrostatically encapsulated POMs mainly generated the epoxides or 1,2-diols.A free radical mechanism was proposed for the oxidation reaction catalyzed by the zwitterionic POM surfactants.
Keywords:Polyoxometalate Self-assembly Peroxide Catalysis Zwitterionic surfactant
Reverse micelles (RMs) are nanometer-sized assemblies of surfactants organized around a core of water molecules within a bulk nonpolar solvent.RMs have wide applications in the extraction of protein, enzymatic reaction, nanomaterial synthesis, drug delivery,and catalysis [1,2].With loading catalysts into the special confined water core, RMs have shown many unique catalytic behaviors [3].Several reports indicated that some catalysts, like enzymes or metal nanoparticles, appeared to behave differently when confined in RMs than in bulk solution, because of the conformational changes of reactants, the local concentrated of catalysts and substrates, and the confined water that may lead to an altered hydration state of the active sites [4–6].With the development of RMs, integrating the catalytic activity and amphiphilic properties into one surfactant molecule is an emerging field, which offers more possibilities for novel catalytic systems with excellent performance, high stability, or even new reactions [7].Recently, the multifunctional polyoxometalates, with variable negative charges and notable redox properties, were facilitated as the polar head groups of anionic surfactants.These compounds have shown unusual selfassembly behaviors, interesting photochemical properties, and excellent catalytic activity [8–10].
However, the self-assembly of RMs by POM-based surfactants is extremely challenging, and their further catalytic applications are rarely explored.As the nanoscale size of the POM head group results in a large hydrophilic area, the packing parameters (P) of POM-based surfactants are often less than 1, which favors the formation of micelles, vesicles, or emulsions.Whereas, a largerPis essential for the assembly of RMs.In addition, the multiple negative charges of the POM clusters lead to strong electrostatic repulsions, which prevent the POMs from approaching each other,thus hamper the self-assembly process of RMs.There have been studies for increasing theP, such as fabricating two long alkyl tails modified Keggin-type POMs or using Anderson-type POMs as a smaller head [11–13].However, only liquid crystals or reversevesicular structures were obtained.Beyond the anionic POM-based surfactant, a new type of the surfactant structure is crucial to be synthesized for self-assembly into the RMs.What is more, it is also an opportunity to develop the novel catalytic behaviors of POMs when they are concentrated and confined in the core of RMs.
In this work, we prepared a series of novel POM-based zwitterionic surfactant molecules that two cationic quaternary amine long chains were covalently grafted on the Keggin POM anionviaSi−O−W bonds.For the first time, the POM-based reverse micelle systems were successfully fabricated through the self-assembly of zwitterionic surfactants.On the contrary, the cationic surfactants encapsulated POMs were assembled into the water in oil(W/O) emulsion systems under the same condition.When evaluating their catalytic behaviors in the oxidation of styrene, the reverse micelles highly selective produced aβ–hydroxyl peroxide compound while the emulsions mainly generated the epoxides and 1,2-diols (Scheme 1).
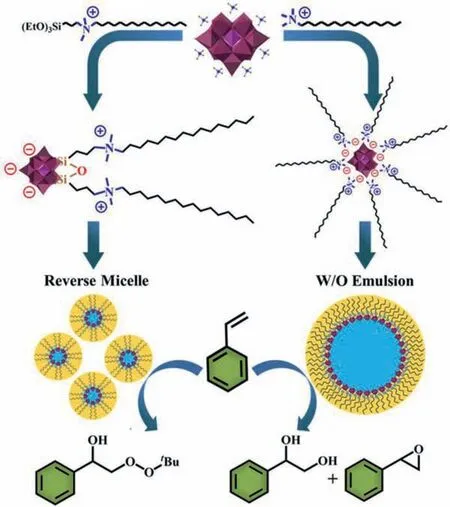
Scheme 1.Illustration for the self-assembly of reverse micelles by zwitterionic POM-based surfactant Na−PW11−NC16 which facilitate the oxidation of styrene to β–hydroxy peroxide (left), and the self-assembly of W/O emulsions by electrostatically bonded POM-cationic surfactant salt CTA + PW11 which favor the epoxides and 1,2-diols products (right).
Initially, a series of cationic organosilane ligands(EtO)3Si(CH2)3N+(CH3)2CnH2n+1were synthesized by a nucleophilic substitution reaction of (3-iodopropyl)triethoxysilane with long-chainN,N-dimethyalkylamines (CH3)2N(CH2)n-1CH3(n= 8,12, 16) [26].The ligands were characterized and denoted as the abbreviation of NCn(Figs.S1−S10 in Supporting information).It has been reported that the neutral organosilane species can be covalently inserted into the pocket of the lacunary Keggin anion in H2O/CH3CN mixed solution [14,15].While for the modification of cationic ligands, unfavorable electrostatically interacted POM salts may be formed and further precipitated out due to their low solubility in water.Therefore, an excess tetramethylammonium (TMA)salt was added into the reaction solution for competing with the cation-anion interaction between ligands and POMs.Besides, a high portion of CH3CN was used as a good solvent for finely dispersing the reactants.With the modified preparation methods, the desired products TMA[PW11O39(SiO0.5(CH2)3N(CH3)2CnH2n+1]2)](TMA−PW11−NCn) were obtained.Subsequently, the counterions were exchanged to sodium ions for increasing the amphiphilicity of POM-based surfactants.

Fig.1.The characterizations of Na−PW11−NC16 with a formula of Na{PW11O39[SiO0.5(CH2)3N(CH3)2C16H33]2} by (a) FT-IR spectrum, (b) 1H NMR spectrum, (c) solid-state 29Si NMR spectrum, (d) 31P NMR spectrum and (e) ESI-MS spectrum.
The resulting POM surfactants were characterized by FT-IR,1H NMR,13C NMR,29Si NMR,31P NMR and electron spray ionization mass spectra (ESI-MS).The characterization results of representative sample Na−PW11−NC16were shown in Fig.1.In FT−IR spectroscopy of Na−PW11−NC16(Fig.1a and Fig.S24 in Supporting information), peaks of the alkyl chains (2924, 2852, 1463, 1078 cm−1) and the POM cluster (1038, 967, 900, 871, 822, 712 cm−1)were visible.The peaks of 1112 and 1078 cm−1represented the vibrations of the Si−O−Si bond and the stretching vibrations of the C−N bonds for quaternary amine cations, which inferred that the cationic ligands were modified onto the POM cluster.It should be noted that the Si−OEt groups were crucial to link the organic ligands with PW11, while they were highly sensitive to the water, which may result in hydrolysis and self-polymerization.In1H NMR spectra (Fig.S25 in Supporting information), signals of the quartet at 3.83 ppm and the triplet at 1.24 ppm (stars signs)suggested that the Si−OEt groups existed in NC16ligands.After the covalent modification, these signals were disappeared, indicating the replacement of Si−OEt to Si−O−Si or Si−O−W groups in TMA−PW11−NC16samples.For TMA−PW11−NC16, the intensity ratio of the protons ofN,N-dimethyl in two ligand chains (3.04,3.06 ppm, 12H, square) and methyl protons of TMA+(3.11 ppm,12H, triangle) was around 1 suggesting that the whole polar head had one negative charge, which was in agreement with the theoretical net charge.The complete counterion exchange from TMA+to Na+was proven by the absence of the signals of TMA+in Na−PW11−NC16(Fig.1b).The modification of the POM cluster can also be confirmed by the unique signals of −13.77 ppm in31P NMR and −51.7 ppm in29Si NMR spectra, which were attributed to the structure of two organosilicate embedded to the lacunary site of the PW11cluster (Figs.1c and d).Moreover, only one signal exists in both spectra indicated that the product was pure.From the ESI-MS spectrum (Fig.1e), the molecule mass of Na−PW11−NC16with a formula of Na{PW11O39[SiO0.5(CH2)3N(CH3)2C16H33]2} was determined as 3372.09m/z, which was consistent with theory.Furthermore, from the analysis of the TG curve (Fig.S43 in Supporting information), the ligand chains were degrade from 372.6°C to 506.5 °C and the POM head was retained even the temperature higher than 700 °C, confirming the considerable thermal stability of Na−PW11−NC16.The co-existence of anionic and cationic centers in Na−PW11−NC16was proven by the dye extraction experiments.With adding one drop of the aqueous solution of cationic dye methylene blue into the chloroform solution of Na−PW11−NC16, the blue color was extracted to the nonpolar phase.The same phenomenon was also observed when the dye was anionic methyl orange.These phenomena suggested that both the cationic dye and anionic dye could be captured by the zwitterionic center of the Na−PW11−NC16surfactant (Fig.S29 in Supporting information).Based on the above characterizations, the structure of the surfactant Na−PW11−NCncould be proposed, in which the nonpolar tail groups were two alkyl long chains and the polar head group was the covalently linked POM anion and quaternary amine cations.For comparison, the cationic surfactants encapsulated POM salts were prepared by directly mixing the K7PW11O39with surfactants like cetyltrimethylammonium bromide (CTAB) in an aqueous solution and collecting the resulted salt precipitates(CTA + PW11).
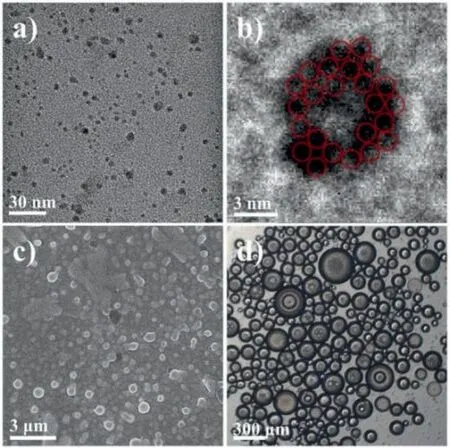
Fig.2.(a) TEM image of the Na−PW11−NC16 assembled reverse micelles with an average diameter around 5 nm.(b) Magnified HR-TEM image of one POM-based reverse micelle showed the aggregation of POM clusters as 1 nm dark spots.(c)SEM image of the emulsions assembled by Na-PW11–NC12.(d) Optical microscope image of W/O emulsions assembled by CTA + PW11.
After determining the structures, the self-assembly behaviors of the Na−PW11−NC16zwitterionic surfactants were developed.It showed that the hydrophilicity of the POM group was significantly decreased after grafting the organic ligands, which was still not improved even after the counterions were exchanged to Na+.Solubility experiments were conducted by preparing 1 mg/mL Na−PW11−NC16in different solvents.The results suggested that the Na−PW11−NC16was highly soluble in DMF, DMSO, acetone,and acetonitrile, while less soluble in water, methanol, toluene,and chloroform.To construct the reverse micelle systems, the Na−PW11−NC16(10 mg) were firstly dissolved in 0.2 mL DMF, and then the above solution was slowly titrated into the chloroform(10 mL) with stirring.A colorless transparent solution was formed after adding 1 mLtert–butyl hydroperoxide (TBHP) 70% aqueous solution.Transmission electron microscopy (TEM) revealed that the assemblies had a spherical appearance in a size range of 3–8 nm(Fig.2a, Figs.S30 and S31 in Supporting information).The mentioned value fits well to the diameter expected for a reverse micellar aggregate constructed from zwitterionic POM surfactants.Taking advantage of the characteristic of high electronegativity and the well-fitted 1 nm molecular size of Keggin clusters, the PW11head groups could be easily observed in these small particles as 1 nm dark spots in HR-TEM images (Fig.2b).Thus, it was believed that the Na−PW11−NC16zwitterionic surfactants were self-assembled into the reverse micelles, and their PW11head groups were compactly located into the nano-sized core of RMs.
Then, the self-assembly mechanism of RMs was discussed.The TBHP in solution seemed to play an essential role in the assembly process.Instead of 70% TBHP aqueous solution, 300 μL water was added to trigger the assembly.A cloudier solution was obtained,and some water drops were observed on the inner surface of the glass vial.Thus, it was speculated that the TBHP served as a cosurfactant inserting among the intermolecular of Na−PW11−NC16to strengthen the stability of the interface and promote the solubilization of water.The conformation of TBHP in RMs was proposed thattert–butyl group located in the nonpolar area and hydroperoxide group pointed to the core water area.The corresponding experiments with the CTA + PW11(Fig.2d and Fig.S28 in Supporting information) in the same conditions showed the large size W/O emulsions with a non-transparent milky white appearance.The results suggested that the electrostatically bonded CTA + PW11was incapable of self-assembly into the RMs and the covalent modified zwitterionic head groups contributed to the RMs’formation.In fact, the zwitterionic properties of surfactants could significantly decrease the electrostatic repulsions among the head groups, which favored the compact arrangement of head groups and thus forming the thermodynamically stable RMs.Moreover, considering the assembly was driven by the balance between electrostatic interaction and hydrophobic effect, influence from the chain length of POM surfactant was investigated.The results showed that neither Na−PW11−NC12nor Na−PW11−NC8could assemble into the RMs.Instead, emulsions with ∼0.5 μm size for Na−PW11−NC12(Fig.2c) and large amounts of precipitates for Na−PW11−NC8(Fig.S27 in Supporting information) occurred.It was suggested that the shorter chain length had less solubility in chloroform, and the corresponding lower strength of hydrophobic interaction was insufficient to overcome the electrostatic repulsions.Therefore, the self-assembly of RMs was synergistically driven by the decreased electrostatic repulsions and the increased hydrophobic interaction, as well as the co-surfactant effect from TBHP.
Recently, the synthesis ofβ–hydroxy peroxides by direct oxidation of olefins has gained great attention for the preparation of active 1,2,4-trioxane units, which are present in the anti-malarial drugs artemisinin (Qinghaosu) and play a crucial role in the drug’s mechanism of action [16].The POMs are widely used as catalysts in the oxidation of olefins due to their notable redox properties, strong persistence against oxidants, and environmental compatibility [17-19,23-25].In most cases, the oxidation products are epoxides or 1,2-diols, the peroxy-containing products are rarely reported.
Taking the opportunity of POMs self-assembly into the novel RMs, an unprecedented product 2–hydroxyl-2-phenylethan-1–tertbutylperoxide was obtained by applying the RMs as nanoreactors with styrene as substrate.The structure of the product was determined by the1H NMR,13C NMR and ESI-MS spectra.In the1H NMR spectrum (Fig.S32 in Supporting information), three pairs ofddcharacteristic peaks at 5.34, 4.25 and 4.16 ppm with an integration ratio of 1:1:1 were corresponding to the protons of ethyl groups.After comparing with the characteristic protons in the same position of styrene oxide (3.83, 2.96, 2.71 ppm) and 1-phenylethane-1,2-diol (4.88, 4.13, 3.88 ppm), we confirmed that the C=C bond of styrene was substituted by two functional groups(Fig.S36 in Supporting information).Combined with the fact that thetBu peak (s, 1.28 ppm, 9H) in the1H NMR spectrum and the mass peak at 249.06 (M + K+) in the ESI−MS spectrum (Fig.S33 in Supporting information), it was suggested that the substituted functional groups were hydroxyl andtert–butyl peroxy, separately.For the structure of isomers, it was hard to determine whether the peroxy group was added to theαorβposition of styrene only by1H NMR results, because the shielding effect from the peroxy and hydroxyl group on ortho protons were almost the same.Therefore, the13C NMR spectrum (Fig.S35 in Supporting information)was acquired for the carbon atoms that were more sensitive tothe connected groups.A styrene difunctional compound (1-(tert–butylperoxy)−3,3,3-trifluoropropyl)benzene, in which the peak of thetBuOO group linked carbon atom appeared at 80.83 ppm,was selected as the isomer standard [20].In the13C NMR spectrum (Fig.S34 in Supporting information) of the obtained product,peaks at 76.65 ppm and 81.09 ppm were attributed to the carbons atαandβ, respectively.Thus, thetBuOO group was located at theβposition, the OH group was linked with theαposition of phenylethane.
Then, the reaction conditions, including solvents, temperature,and equivalents of reactant, were optimized, and the peroxide product was obtained with 43.1% yield and 95.2% selectivity (Tables S1–S3 in Supporting information).Catalysts control experiments were carried out to illustrate the critical role of each component on the peroxide reaction.As shown in Table 1 (entries 1 and 2), the reaction could be scarcely possible initiated in the absence of PW11, indicating the active catalytic sites were PW11.As for the K7PW11O39, the clusters were partially dissolved in the polar solvents while most residues remained as solid precipitates.The catalytic result showed a decreased peroxide yield (14.3%) and selectivity (69.6%), which may be due to the poor accessibility of styrene to the active sites (Table 1, entry 3).In addition, the physical mixture of PW11and protonated NC16ligands showed a significantly decreased peroxide yield, and the styrene was mainly transformed into epoxides and 1,2-diols with 75.1% selectivity.When the CTA + PW11salts were applied as catalysts, the epoxides and 1,2-diols were also generated in high selectivity of 77.1%.From the perspective of assembly systems, the surfactants encapsulated POM catalysts formed emulsion systems, in which the POM clusters were highly hydrated and contacted with a relative bulk water environment.On the contrary, POMs in the confined water core of RMs had less hydration states which might be favorable to theβ–hydroxyl peroxide transformation.Moreover, there was no change in the FT-IR and31P NMR spectrum (Figs.S41 and S42 in Supporting information) of the Na−PW11−NC16after the reaction, which confirmed that the structure was not affected by the high concentration of TBHP.Furthermore, the scope of substrates was also expanded and the corresponding peroxide products were detected(Table S4 in Supporting information).
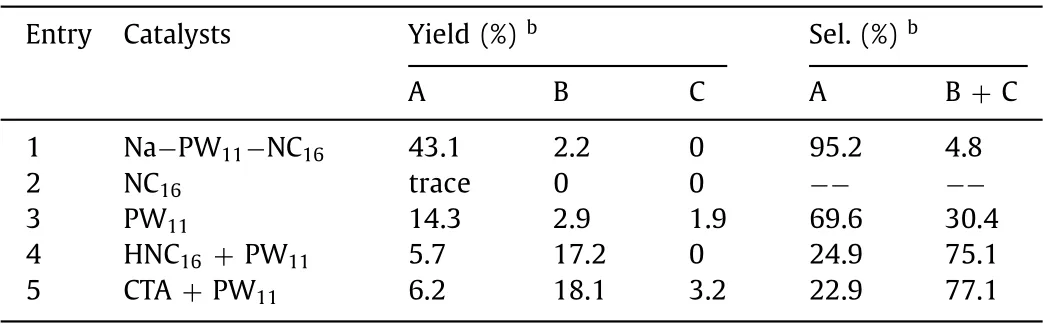
Table 1 Catalysts control experiments of styrene oxidation reactiona.
To investigate the mechanism of this peroxide reaction, styrene oxide was used as a substrate in the same reaction conditions.The catalytic results showed that no peroxides were produced.Thus,the possibility can be ruled out that styrene was firstly oxidized to styrene oxide then ring open by the TBHP to theβ–hydroxy peroxide product.Subsequently, 2,2,6,6-tetra-methyl-1-piperidinyloxy(TEMPO) was employed in the reaction system as a radical scavenger [21].When adding 6 equivalents of TEMPO, the reaction was completely inhibited without any peroxide products.In addition, when adding a hydroxyl radical scavenger KBr [22], a 1,2–tertbutylperoxy-phenylethane was obtained (Figs.S35 and S36).Based on the above results, a free radical pathway was proposed (Fig.S39 in Supporting information).The distinct oxidation pathway may be due to the oxidation state variation of POMs after the organic modification.X-ray photoelectron spectroscopy (XPS) was performed to support the hypothesis.W 4f spectra (Fig.S26 in Supporting information) showed W 4f5/2(37.7 eV) and W 4f7/2(35.5 eV) of PW11,attributing to W6+.As expected, the binding energy of W (36.2 and 38.4 eV) in Na−PW11−NC16shifted to a higher energy than that in PW11.The results indicated that the electron structure of W was affected by cationic quaternary amine long chains leading to a higher oxidation state of W in the Na−PW11−NC16.
In summary, a new type of zwitterionic POM surfactant was prepared by covalently modifying two cationic long alkyl chains onto the mono-lacunary site of [PW11O39]7−.Under the cooperation with co-surfactant TBHP, the Na−PW11−NC16could selfassemble into the challenging POM-based reverse micelles with diameters around 5 nm.The mechanism studies demonstrated that the self-assembly was synergistically driven by the decreased electrostatic repulsions among zwitterionic head groups and the increased hydrophobic effect from long alkyl chains.Furthermore,by applying the reverse micelles into the oxidation of styrene,a new compound, 2–hydroxyl-2-phenylethan-1–tert-butylperoxide was obtained with 95.2% selectivity.On the contrary, the W/O emulsion systems, which were assembled by the cationic surfactants encapsulated PW11, generated epoxides and 1,2-diols with high selectivity of 77.1%.We hope this work will facilitate the further development of POM-based supramolecular assemblies and expand the scope of catalytic applications in fields of new reactions and high value-added peroxide products.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Beijing Natural Science Foundation (No.2202039), the National Natural Science Foundation of China.(Nos.22178019, 22101017, U1707603, 21625101, 21521005,21808011), the National Key Research and Development Program of China (No.2017YFB0307303) and the Fundamental Research Funds for the Central Universities (Nos.XK1802–6, XK1803–05, XK1902,12060093063).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.006.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
