Interfacial synthesis of crystalline quasi-two-dimensional polyaniline thin films for high-performance flexible on-chip micro-supercapacitors
Tao Zhan, Panpan Zhan, Zhonquan Liao, Faxin Wan, Jinhui Wan,Minchao Wan, Ehrenfrie Zschech, Xiaoon Zhuan, Oliver G.Schmit,Xinlian Fen,∗
a Key Laboratory of Bio-based Polymeric Materials Technology and Application of Zhejiang Province, Ningbo Institute of Material Technology and Engineering, Chinese Academy of Sciences, Ningbo 315201, China
b Faculty of Chemistry and Food Chemistry, Technische Universität Dresden, Dresden 01062, Germany
c Center for Advancing Electronics Dresden (cfaed), Technische Universität Dresden, Dresden 01062, Germany
d Fraunhofer Institute for Ceramic Technologies and Systems (IKTS), Dresden 01109, Germany
e Material Systems for Nanoelectronics, Chemnitz University of Technology, Chemnitz 09107, Germany
f Institute for Integrative Nanosciences, IFW Dresden, Dresden 01069, Germany
g School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules, Shanghai Jiao Tong University, Shanghai 200240,China
h Key Laboratory for Special Functional Materials of Ministry of Education, Henan University, Kaifeng 475004, China
ABSTRACT Quasi-two-dimensional (q2D) conducting polymer thin film synergizes the advantageous features of longrange molecular ordering and high intrinsic conductivity, which are promising for flexible thin film-based micro-supercapacitors (MSCs).Herein, we present the high-performance flexible MSCs based on highly ordered quasi-two-dimensional polyaniline (q2D-PANI) thin film using surfactant monolayer assisted interfacial synthesis (SMAIS).Owing to high electrical conductivity, rich redox chemistry, and thin-film morphology, the q2D-PANI MSCs show high volumetric specific capacitance (ca.360 F/cm3) and energy density (17.9 mWh/cm3), which outperform the state-of-art PANI thin-film based MSCs and promise for future flexible electronics.
Keywords:2D polymers Conducting polymers Interfacial synthesis Flexible electronics Microsupercapacitor
Recently, flexible micro-supercapacitors (MSCs) attracted considerable attention due to their high-power capability, fast chargedischarge rate, and long cycling lifetime that are promising power sources for portable and/or wearable microelectronics [1–4].Conducting polymers have been intensively studied as advanced highperformance electrode materials for flexible MSCs [5,6].In contrast with conventional bulk state, crystalline thin-film of conducting polymers can achieve efficient charge transport at in-plane direction as well as offer a large ratio of charge carriers to volume of active layer in devices [7,8].As such, a simple, scalable and costeffective deposition technique for conducting polymers that produces uniform thin-film morphology and ordered molecular structure is highly desirable [9–11].
As one of the most important conducting polymers, polyaniline(PANI) has exceptional pseudocapacitive properties [12–16], which render it with great potential for the energy storage applications.Unfortunately, the classical interfacial synthetic approaches (e.g.,air-water, liquid-liquid and liquid-solid interfaces) that have been successfully used in the synthesis of two-dimensional (2D) polymers [17–20], only resulted in rough PANI with fibrillary or hierarchical morphologies [21–23], presumably due to the aggregation (via π-πstacking) of aniline oligomers.Here, in this work,we fabricate flexible MSCs based on highly orderedquasi-twodimensional (q2D) PANI thin film, which was prepared utilizing the surfactant-monolayer assisted interfacial synthesis (SMAIS) approach.After transfer onto flexible Kapton® substrate, the fabricated MSCs delivered high volumetric specific capacitance of 370 F/cm3at 1.3 A/cm3, which is superior to the MSCs using other organic electrode materials, such as PANI nanowires [24], azulenebridged coordination polymer framework [25], B/N-enriched conjugated polymer film [26].Moreover, the PI-supported devices exhibited excellent flexibility and stability with different bending angle measurements.
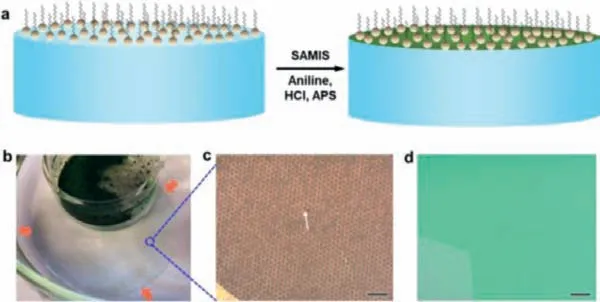
Fig.1.(a) Schematic illustration of the synthetic procedures via SAMIS.(b) Photograph of a 2D PANI thin film floating on water surface, which is highly homogeneous and transparent.(c) Freestanding q2D-PANI thin film on a copper TEM grid.The white arrow points to a hole in the q2D-PANI thin film, which is in contrast to surrounding freestanding film.(d) The q2D-PANI thin film on 300 nm SiO2/Si wafer visualized by optical microscopy.Scale bars: (c, d) 100 μm.
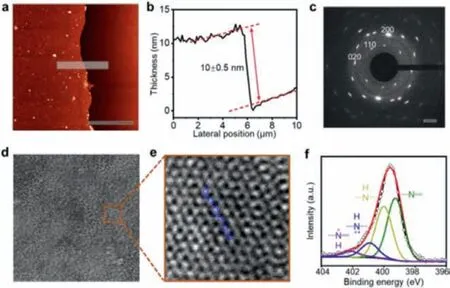
Fig.2.(a) AFM topographic image and (b) corresponding height profile of the q2DPANI thin film prepared in 48 h.(c) SAED pattern of q2D-PANI along [001] axis.The 200 and 020 reflections are at 2.96 nm−1 and 2.70 nm−1, respectively.(d) ACHRTEM image of q2D PANI along [001] axis.(e) A close-up of (d).(f) XPS highresolution N1s core level spectrum of q2D-PANI.The fitting in (f) was performed with a set of Voigt peaks.Scale bars: (a) 10 μm; (c) 1 nm; (d) 2 nm; (e) 0.3 nm.
The SMAIS synthesis of q2D-PANI was achieved with an anionic surfactant monolayer (e.g., sodium oleyl sulfate, SOS) on water surface to control the preorganization of subsequently added aniline monomers (0.13 mmol/L) (Fig.1a) [11,27-30].Hydrochloric acid (0.5 mol/L) and ammonium persulfate (APS, 0.05 mmol/L)were then added into the subphase to initiate the polymerization.The reaction was kept at 1°C under ambient conditions for 72 h to produce q2D-PANI thin films on the water surface (Fig.1b).The assembling and polymerization of aniline was guided underneath surfactant monolayer due to the electrostatic interactions and hydrogen bonds, thus ensure the polymerization of aniline in a more controlled way than classical interfacial synthesis approaches[31,32].
The resulting q2D-PANI thin film can fully float on water surface with more than 28 cm2(Fig.1b), or suspend over large holes of ∼20 μm on a copper grid (Fig.1c), suggesting high mechanical strength of the q2D-PANI.The resultant film was fished using a 300 nm SiO2/Si wafer and visualized under optical microscopy(Fig.1d), which shows a large-area continuous morphology with excellent uniformity.After cleaning with chloroform, atomic force microscopy (AFM) measurements at edges by random sampling revealed a thickness ofca.10 nm (Figs.2a and b).In order to prove the existence of long-range order within q2D-PANI, the film was suspended over copper grid and characterized by selectedarea electron diffraction (SAED) that gives a clear and very reproducible diffraction spots of single crystal structure (Fig.2c).The nearest reflections revealed a rectangle unit cell with lattice parameters ofa=6.8andb=7.4[11].Further characterization by aberration-corrected high-resolution transmission electron microscopy (AC-HRTEM) shows that the linear polymer chains align parallel to each other (Figs.2d and e), packing into a q2D molecular sheet.Unlike polymers obtained by solution synthesis [33,34],the PANI chains in the molecular sheet exhibit long-range order,showing no chain folding or any entanglement.X-ray photoelectron spectroscopy (XPS) reveals that the q2D-PANI film contains carbon, nitrogen, chlorine, sulfur, and oxygen in a ratio of 63.4%,7.0%, 1.2%, 0.2% and 28.1% (Fig.S1 and Table S1 in Supporting information), respectively.The two prominent peaks of N1s signal at 401.9 eV and 399.8 eV can be attributed to two types of nitrogen(-NH- and =NH-) in q2D-PANI (Fig.2f).The four peaks of C 1s signal can be assigned to C 1s of C=C at 284.5 eV, C-C at 285.0 eV,C-N at 285.9 eV, and C-O at 288.2 eV (Fig.S2 in Supporting information), respectively.The peak at 197.7 eV belongs to the Cl−counterions of acid dopant (Fig.2e).The infrared spectroscopic data of the q2D-PANI film showed that the characteristic bands at 1563 and 1488 cm−1, attributable to the C=C a stretching deformation mode of the quinoid and benzenoid rings (Fig.S3 in Supporting information).Band at 1287 cm−1arises from the C–N stretching of the secondary aromatic amine and C–N stretching vibration in the polaron structures, respectively.
PANI has been widely used as the electrode material for electrochemical energy storage due to its variable oxidation states and excellent doping-dedoping characteristic, which contributes to a very high specific pseudocapacitance [12-14,22,24,35,36].Due to the unique morphology and ordered molecular structure, the q2DPANI thin films are expected to serve as a novel class of promising electrode materials for electrochemical energy storage [37–39].As such, in the next part, we evaluated the electrochemical properties of the synthesized q2D-PANI thin films as electrodes in flexible MSCs.
Fig.3a illustrates the fabrication process of the MSC based on a q2D-PANI thin film supported on substrates (e.g., Kapton®foil or SiO2/Si wafer), and a photograph of the as-prepared MSC is presented in Fig.3b.This device was constructed with 20 interdigitated microelectrodes (10 positive and 10 negative microelectrodes).Cyclic voltammetry (CV) and galvanostatic chargedischarge (GCD) measurements were then performed to investigate the electrochemical performance of the fabricated MSCs.It is clear that the CV curves exhibit the typical pseudocapacitive behavior of PANI with strong redox peaks in the range of 0 to 1 V at various scan rates (Fig.3c and Fig.S4a in Supporting information).The planar architecture of q2D-PANI allows to reduce the ionic diffusionpathway as well as maximize the accessible surface area, and thus results in the fast charge/discharge rates observed with the MSCs as confirmed by the GCD curves (Fig.3d and Fig.S4b in Supporting information).
Among the MSCs based on q2D-PANI thin films of various thicknesses (10, 20 and 40 nm) (Fig.S5 in Supporting information), the q2D-PANI10 (∼10 nm in thickness) MSC delivered the highest volumetric specific capacitance ofca.370 F/cm3at 1.3 A/cm3(i.e., 0.37 mF/cm2; Fig.3e), superior to the state-of-the-art MSCs based on other PANI electrode materials (e.g., PANI nanowires: 105 F/cm3)[24], and other organic thin-film electrode materials, such as B/Nenriched conjugated polymer films (f-3BNF: 20.9 F/cm3) [26] and azulene-bridged coordination polymer frameworks (PiCBA: 34.1 F/cm3) [25].The achieved volumetric specific capacitance is also largely higher than the most carbon-based electrodes (usually less than 100 F/cm3; Table S2 in Supporting information), which implies that the ultra-thin 2D structure contributes to the fast ion diffusion and high-charge storage behavior.Even at a higher scan rate of 1000 mV/s, the q2D-PANI10 MSC still presented a significant volumetric specific capacitance of 92 F/cm3.To further evaluate the overall performance of the MSCs, the volumetric power and energy densities were calculated.The q2D-PANI10 MSCs exhibited a high energy density up to 17.9 mWh/cm3(at 1.3 A/cm3) and power density ofca.20 W/cm3(Fig.4a and Table S2), which are comparable to those of reported on-chip MSCs [24,25,36,40-42].
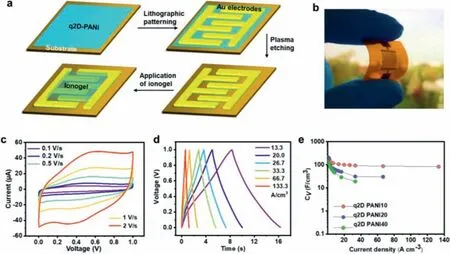
Fig.3.Electrochemical performance of q2D-PANI thin film-based MSCs.(a) Schematic fabrication of q2D-PANI thin film-based MSCs.(b) Digital photo of the fabricated MSC on a Kapton® foil.(c) CV profiles for a q2D-PANI thin film (ca. 10 nm) MSC at various scan rates.(d) Charge-discharge curves of the MSC at various current densities.(e)Evolution of the volumetric capacitances as a function of current density of MSCs with various thicknesses.
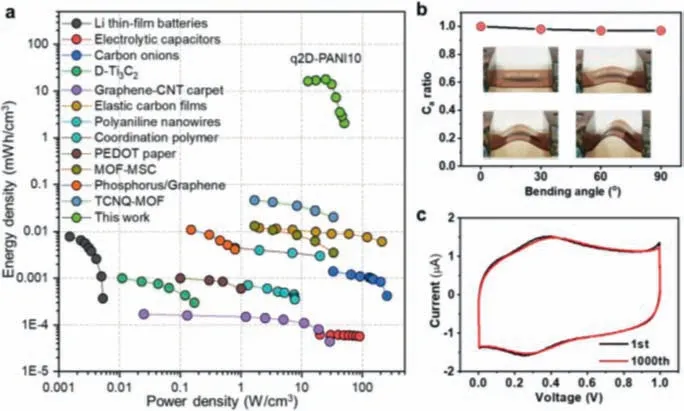
Fig.4.(a) Ragone plots for q2D-PANI10 based MSC in comparison to commercial Li-thin-film batteries, electrolytic capacitors, MSCs based on carbon onions, D-Ti3C2,CNT-graphene carpets, elastic carbon films, PANI nanowires, coordination polymer,PEDOT paper, MOF-MSC, phosphorus/graphene, TCNQ-MOF.(b) Capacitance ratio(Ca) for the q2D-PANI10 MSCs at different bending angles between 0° and 90°.(c)CV profiles for the q2D-PANI10 MSCs at 1st and 1000th cycles.
Since flexibility is crucial for portable and wearable energy storage devices, we examined it under various bending angles of 0°,30°, 60°, and 90° (Fig.4b).The MSCs devices showed only slight changes of the CV curves at different bending angles (Fig.S6 in Supporting information) and 97% of initial capacitance was kept for bending even at 90° (Fig.4b), highlighting exceptional flexibility.The high flexibility of the q2D-PANI-MSCs could be attributed to the following two reasons: (1) The abundant micro/nano wrinkles on q2D-PANI surface induced by substrate-effect (mechanical mismatch) improve its flexibility (Fig.S7 in Supporting information)[43]; (2) The high crystallinity and molecular ordering enhance mechanical stability of q2D PANI film [17].Furthermore, q2D-PANIMSCs showed good cycling stability under flat and constant bending states, maintaining the initial performance after 1000 cycles(Fig.4c).This superior performance can be attributed to the ultrathin electrode that facilitates the transport of ions and electrons and provides abundant surfaces for charge-transfer reactions [7],ensuring a great utilization of active materials.
In summary, we have developed a robust interfacial approach for the preparation of crystalline q2D-PANI thin film as advanced electrode materials for flexible on-chip MSCs.The fabricated onchip MSCs delivered high specific capacitances and energy densities, fast charge/discharge rates as well as excellent flexibility.We can expect that these results will inspire the applications of q2DPANI thin film in flexible electronics.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the ERC Grant 2DMATER, ESF Young Researcher Group ‘GRAPHD’and the EC under the Graphene Flagship (No.CNECTICT-604391).The German Excellence Initiativeviathe Cluster of Excellence EXC1056 “Center for Advancing Electronics Dresden” (cfaed) is gratefully acknowledged.T.Zhang acknowledges the Excellent Youth Foundation of Zhejiang Province of China (No.LR21E030001).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.052.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
