N, O co-doped porous carbon with rich pseudocapacitive groups exhibiting superior energy density in an acidic 2.4 V Li2SO4 electrolyte
Xio Wng, Kixing Zou, Weijing Wu, Yunfu Deng,b,∗, Guohu Chen
a The Key Laboratory of Fuel Cell for Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, China
b Electrochemical Energy Engineering Research Center of Guangdong Province, South China University of Technology, Guangzhou 510640, China
c Department of Mechanical Engineering, The Hong Kong Polytechnic University, Hong Kong, China
ABSTRACT Designing a carbon material with a unique composition and surface functional groups for offering high specific capacity in a wide voltage window is of great significance to improve the energy density for the supercapacitor in a cheap and eco-friendly aqueous electrolyte.Herein, we develop an efficient strategy to synthesize a N, O co-doped hierarchically porous carbon (NODPC-1.0) with moderate specific surface area and pore volume as well as rich heteroatoms using a deep eutectic solvent (DES) as an activator.It is found that NODPC-1.0 with a large proportion of pseudocapacitive functional groups (pyrrole-N, pyridine-N and carbonyl-quinone) can work stable in an acidic 2 mol/L Li2SO4 (pH 2.5) electrolyte, exhibiting specific capacities of 375 and 186 F/g at the current densities of 1.0 and 100 A/g, respectively.Also, the assembled symmetric capacitor using the NODPC-1.0 as the active material and 2 mol/L acidic Li2SO4(pH 2.5) as the electrolyte shows an outstanding energy density of 74.4 Wh/kg at a high power density of 1.44 kW/kg under a broad voltage window (2.4 V).Relevant comparative experiments indicate that H+ of the acidic aqueous electrolyte plays a crucial part in enhancement the specific capacity, and the abundant pseudocapacitive functional groups on the surface of the NODPC-1.0 sample play the key role in the improvement of electrochemical cycle stability under a broad voltage window.
Keywords:Hierarchically porous carbon Acidic Li2SO4 electrolyte N, O co-doped carbon Supercapacitor High work voltage
Owing to the rapid development of electric vehicles and portable electronic devices, supercapacitors (SCs) have attracted more attention due to their low cost, eco-friendly, high power density and long cycle life [1,2], which gradually become one of the promising candidates for energy storage devices.However, the low energy density (5∼10 Wh/kg) is still the most common obstruction for SCs.To solve the main challenges of the low energy density for SCs, novel carbon-based electrodes for the improvement of specific capacity and electrolytes for the enhancement of working potentials have gradually received extraordinary attention in recent years [3–5].Although it is important to improve the specific capacitance of carbon-based materials, it is very more crucial to increase the working voltage for the improvement of the energy density of SCs, which is due to the energy density is proportional to specific capacity (C) but proportional to the square of the voltage window(V), that is,E= 1/2CV2[6].
Generally, the methods to enhance the specific capacitances of carbon-based materials mainly include increasing the specific surface area (SSA), optimizing the pore structure, introducing the heteroatoms doping,etc.[7–10].For example, Yuanet al.reported an ultra-high specific area (2470 m2/g) carbon material by using edible oil residues as the carbon source and potassium oxalate monohydrate and dicyandiamide as the activation agent, which exhibited a high specific capacitance of 340 and 282 F/g at 0.5 and 50 A/g, respectively [11].Yanget al.optimized the mesopore volume proportion in an ultrahigh surface area meso–/microporous carbon to enable high rate performance, with the specific capacitance of 163 and 129 F/g at 0.5 and 50 A/g, respectively [12].Zhouet al.created anin-situpolymerization and subsequent pyrolysis route to achieve carbon-based material with a large amount of N, O heteroatoms and nanoarchitecture for the SCs, which can exhibit a specific capacitance of 311 F/g at 1 A/g and an energy density of 18.0 Wh/kg at a power density of 350 W/kg [13].It is indicated that the specific capacities of the carbon-based materials can be enhancedviathe optimization of their fine compositions and structures, however, these conventional strategies are still facing some challenges.
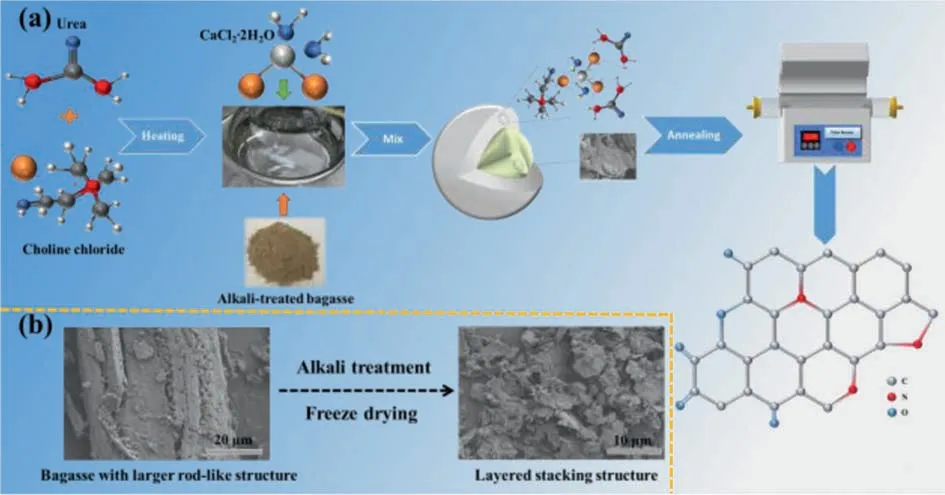
Fig.1.(a) Schematic illustration for the synthesis of NODPC, (b) SEM of primitive bagasse and alkali-treated bagasse (ATB).
Aqueous electrolytes with low cost, eco-friendly, high ionic conductivity, and high safety are usually used for carbon-based SCs,especially for the heteroatom-doped carbon electrodes with intrinsic pseudocapacitance [2].However, the potential window of an aqueous SCs is limited by the low electrochemical stability window (ESWs) of water (<1.23 V), which therefore results in the low energy density of the aqueous SCs [3].It is of great significance to explore wider ESWs for the aqueous electrolytes to achieve high performance SCs [14–16].‘Water-in-salt’(WIS) electrolytes, which were first developed in a 2.3 V lithium ion battery [17], are the commonly used aqueous electrolytes to enhance the operating voltage ranges for the SCs.For example, a superconcentrated aqueous electrolyte consisting of 37 mol/kg potassium bis(fluorosulfonyl)amide (KFSI) was successfully used to assemble a 2.3 V symmetrical carbon-based SC [18], which displayed a specific capacitance of 96.07 F/g at current densities of 1.0 A/g and specific energy of 20.5 Wh/kg at 2.3 kW/kg.Another superconcentrated aqueous electrolyte with 17 mol/L NaClO4and an ESW of 2.3 V can also enable a carbon-based supercapacitor exhibiting high-performance [19], with a specific capacitance of 21.8 F/g at 20 A/g and an energy density of 23.7 Wh/kg at a power density of 1.15 kW/kg.To some extent, this strategy using superconcentrated salts (LiTFSI, NaClO4,etc.) in the aqueous electrolytes should be reduced their cost in practical applications in the future.
Based on the above-mentioned discussion, the combination of the preparation of heteroatoms-doped hierarchically porous carbonviaa new route and the development of a novel aqueous electrolyte with low-cost, eco-friendly, and a wide ESW to synergistically boost the energy density of a SC is urgently needed.Herein, we design a novel route to prepare a N, O co-doped hierarchically porous carbon (NODPC) by utilizing a novel deep eutectic solvent (DES) as the activator and nitrogen source, and also develop a novel aqueous electrolyte (acidic 2 mol/L Li2SO4, pH 2.5) to enable the NODPC-based SC high energy and power densities.It is indicated that the symmetric SC assembled by the asprepared NODPC-1.0 sample, possessing a moderate specific surface area (SSA) and total pore volume (Vt) as well as rich heteroatoms (detail parameters are shown in Table S1 in Supporting information), can exhibit a high energy density of 74.4 Wh/kg at a power density of 1.44 kW/kg and ultra-stable cycle capability under a wide voltage window of 0∼2.4 V.Also, the relative underlying mechanisms for the high performance NODPC-based SC are explored.The acidic 2 mol/L Li2SO4electrolyte used herein will offer some insight for developing other cheap, green, and highvoltage aqueous electrolytes with a low salt concentration for the heteroatoms-doped carbon-based SCs.
The synthetic processes of the NODPC-1.0 sample, using a novel deep eutectic solvent (DES) as the activation agent and nitrogen source as well as the alkali-treated bagasse (ATB) as the carbon precursor, are schematically illustrated in Fig.1a.The main purposes of the use of this novel and cheap DES herein are achieving porous carbons in high yield (Table S1) and simplifying the synthetic procedures meanwhile.Also, the as-obtained ATB (Fig.S1 in Supporting information) as the carbon source can improve the yield of the final carbon materials, specifically, the high yield of 41.3 wt% for the NODPC-1.0 sample can be achieved.For other comparisons, different mass ratios of ATB and DES have been used to prepare other carbons with different structures and compositions.In addition, the raw bagasse as the carbon source for the preparation of another sample (NODPC-n) has been conducted.Specifically, three samples labeled as NODPC-1.5, NODPC-1.0 and NODPC-0.5 were achieved from the different usages of the ATB in the preparation processes.Detail experiments can be found in Supporting information.As shown in Fig.1b, it is found that the morphology of the as-obtained ATB has been changed obviously as compared with the raw bagasse, that is, from a larger rod-like structure into a looser layered stacking structure.In addition, the elemental composition of the ATB has also been changed (Fig.S1).These variations play a positive effect on the fabrication of porous carbons with unique structure and composition, as discussed in the following section.
The morphologies of the as-prepared NODPC-1.5, NODPC-1.0,NODPC-0.5, and NODPC-n samples are shown in Fig.2.Also, the corresponding enlarged views are shown in Fig.S2 (Supporting information).It is indicated that the NODPC-1.5, NODPC-1.0, and NODPC-0.5 samples all display looser porous flake structures and the NODPC-n sample shows a dense stacked structure in a rod shape, which suggests that the ATB as the carbon precursor indeed affect the morphologies of the final carbons.The TEM images(Figs.2e-g) of the NODPC-1.0 sample indicate it exhibits rugged fold morphology with good dispersibility and rich pores, also, the HRTEM image (Fig.2g) indicates this sample is an amorphous structure with partial graphitization in the local region.The elemental mappings (Figs.2i-m) demonstrate the homogeneous distribution of C, N and O elements of the NODPC-1.0, indicating the synthetic route designed herein is a feasible strategy for fabrication of heteroatom-doped carbons with uniform element distribution.
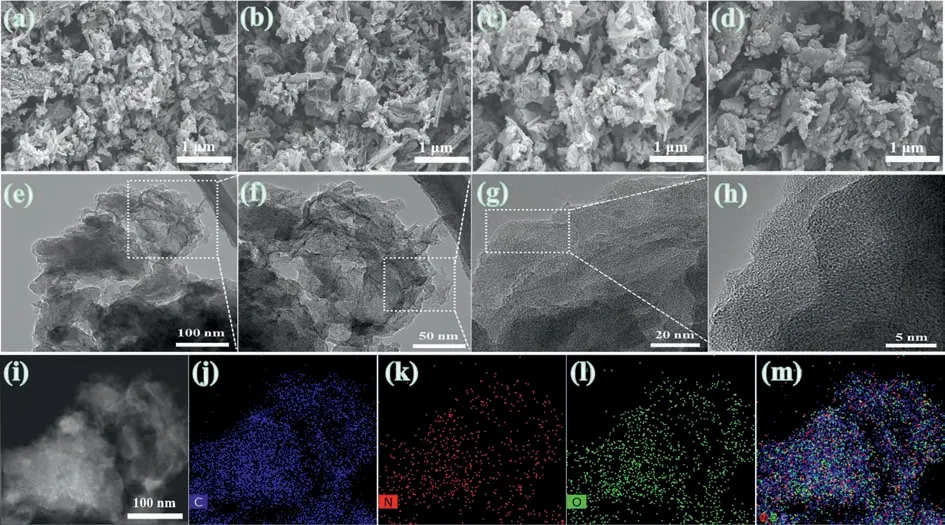
Fig.2.SEM images of (a) NODPC-1.5, (b) NODPC-1.0, (c) NODPC-0.5 and (d) NODPC-n.(e-g) The TEM and (h) HRTEM for the NODPC-1.0.(i-m) TEM image and the element mapping patterns for C, N and O elements of NODPC-1.0..
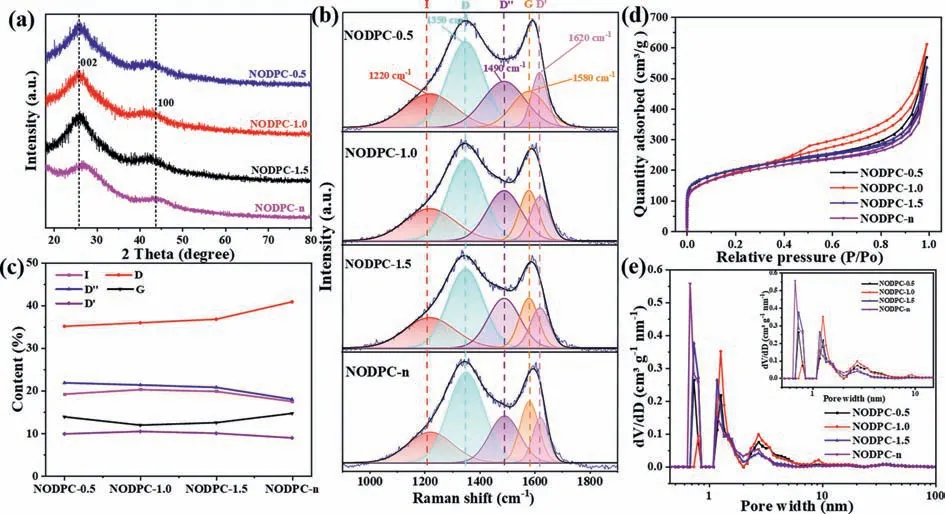
Fig.3.(a) XRD patterns, (b) the Raman fitting spectra and (c) contents of integrated peaks of the four materials.(d) N2 adsorption isotherms and (e) pore size distribution of the four materials.
XRD patterns, Raman spectra, and N2-adsorption/desorption isotherms of the as-prepared carbons are shown in Fig 3.As shown in Fig.3a, the four samples all exhibit two broad peaks located at 25.6° and 43.5° corresponded to the (002) and (100) planes of graphitic-type carbon, respectively, indicating their typical amorphous structures.After comparison their XRD patterns carefully, it is noted that the carbons derived from the ATB precursor show stronger diffraction peaks than that of the NODPC-n sample.The Raman spectra fitted by five peaks corresponded to the Gaussianshaped band (D′′ 1490 cm−1) and the Lorentzian-shaped bands(I 1220 cm−1, D 1350 cm−1, G 1580 cm−1, and D′ 1620 cm−1)[20] and their specific contents are illustrated in Figs.3b and c,respectively.Compared with the NODPC-n sample, the content of D band was deceased in the other three samples, which suggests that the carbons derived from the ATB precursor possess some lower disorder degree.This result is also consistent with the higher reflection intensity of the (002) crystal plane for the NODPC-1.5,NODPC-1.0, and NODPC-0.5 samples.However, these three samples display higher content of D′′ band, as a comparison to that of the NODPC-n sample, indicating the presence of multitudinous defects in the graphene layers, which may contribute to increasing some active sites to absorb ions.The N2-adsorption/desorption isotherms of the four NODPC samples, as shown in Fig.3d, demonstrate similar shape, in a combination of I and II type curves and obvious H4 hysteresis loops in theP/P0range of 0.45–0.9, which suggests that these four samples exhibit hierarchically porous structure.However, after careful examination of the isotherms, it is observed that the NODPC-1.0 sample displays the largest H4 hysteresis loop and the NODPC-n sample shows the smallest H4 hysteresis loop, indicating the richest and minimal mesopores of these two samples, respectively.Based on the detailed parameters shown in Table S1, it is found that the ratios of the mesopore volume(Vmeso) and micropore volume (Vmicro) of NODPC-1.5, NODPC-1.0,and NODPC-0.5 samples display the upshift trend, which agrees well with the increasing mass ratio of EDS and ATB.The pore size distribution plots shown in Fig.3e indicate the pore size distributions of the four samples exhibit their own characteristics, although with similarities.Compared with the other three samples exhibiting micropore size concentrated at ∼0.7 nm, the NODPC-1.0 sample shows the micropore size is concentrated at∼1.5 nm.In addition, the NODPC-1.0 sample displays much richer mesopores with a wide pore size distribution from 2 nm to 10 nm.Specifically, the NODPC-1.5, NODPC-1.0, NODPC-0.5, and NODPC-n samples exhibit the SSAs of 713.6, 775.4, 743.9 and 683.4 m2/g,withVtof 0.59, 0.73, 0.54 and 0.40 cm3/g, respectively, as shown in Table S1.It is worth pointing out that the external SSAs of the carbons derived from the ATB carbon source are much larger than that of the NODPC-n sample.Generally, the carbon-based material with higher external SSA can provide more active sites for ion adsorption, and thus improving its specific capacitance as an active material for a SC.In addition, a largeVtand suitable ratio ofVmicroandVmesoof carbon-based electrode materials will offer large numbers of pores for ion accumulation and transport [21,22].
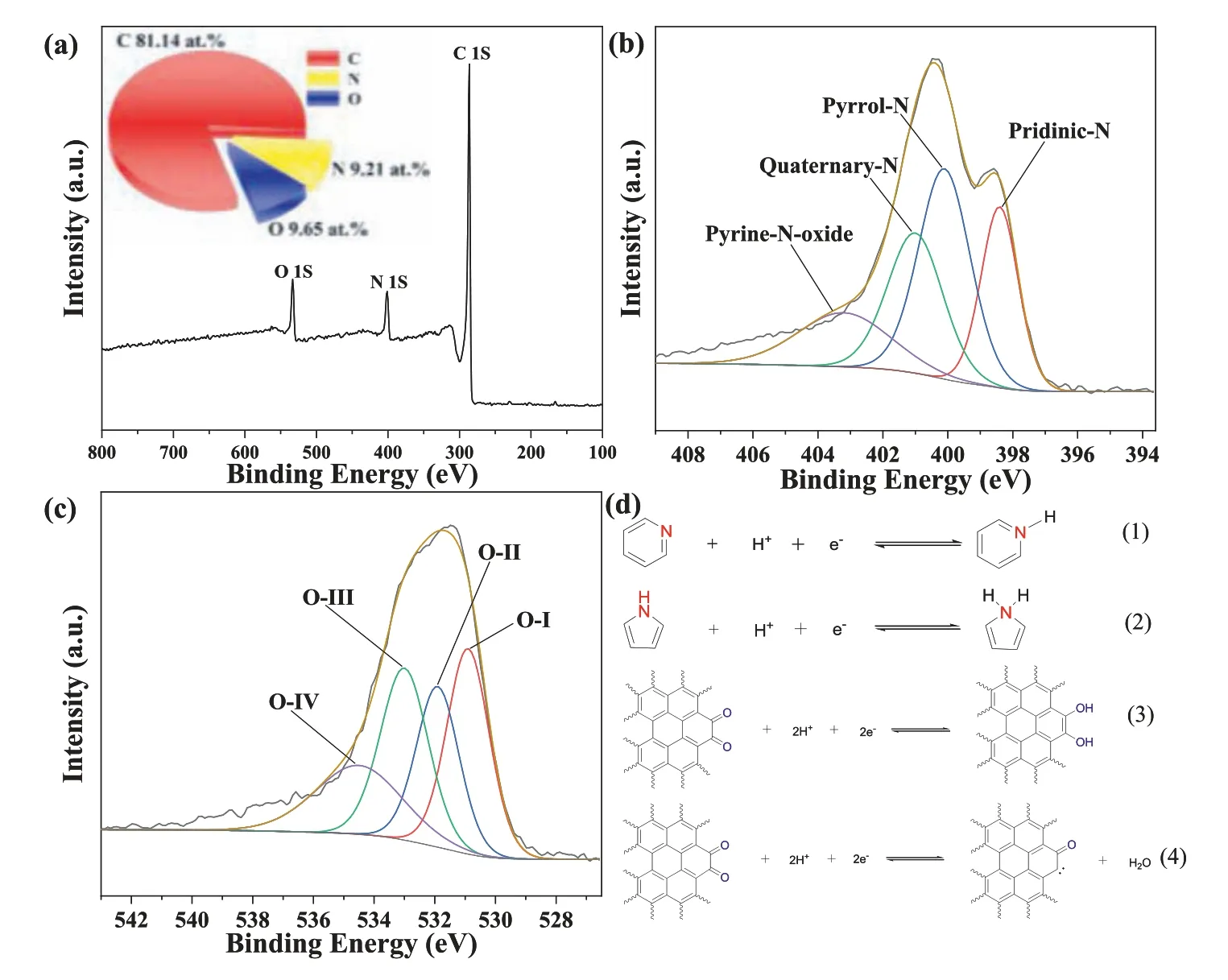
Fig.4.XPS spectrum and valence state of NODPC-1.0: (a) The full scan spectrum of NODPC-1.0 with the corresponding 3D-pie chart high-resolution spectra of (b) N 1s, (c)O 1s, (d) the possible reactions.
The elemental compositions in the macroscopic state and the surface of the four samples are checked by elemental analysis (EA)and X-ray photoelectron spectroscopy (XPS), respectively.The relative data are shown in Fig.4, Fig.S3 and Table S2 (Supporting information), and.Based on the EA results in Table S2, it is found that the NODPC-1.5, NODPC-1.0, and NODPC-0.5 samples possess higher N-doped content than that of the NODPC-n sample, with respective values of 9.28, 9.95, 8.51 and 7.75 wt%.XPS results (Fig.4,Fig.S3 and Table S3 in Supporting information) indicate that the NODPC-1.5 sample shows lower N content (7.83 at%) than that of the NODPC-0.5 sample (9.18 at%), although the EA results indicate the higher N content of the NODPC-0.5 sample.This inconsistent result may be attributed to the lowert-plot external surface area of the NODPC-1.5 sample (Table S1).In this regard, the NODPC-1.0 sample displays the highest N content among the four samples, resulting from the synergetic effect of its high external specific surface area and intrinsic rich N content.In addition, the four samples exhibit different surface oxygen contents, with the respective value of 8.28, 9.65, 6.39, and 11.80 at% in the NODPC-1.5, NODPC-1.0, NODPC-0.5 and NODPC-n samples.Previous studies indicated that the unique N- and O-functional groups on the surface of carbon materials play an important effect on the capacitive performanceviathe pseudocapacitive behavior in acid electrolytes [23–26], as schematic by the equations 1∼4 shown in Fig.4d.Herein,the detail surface N-(pyridinic and pyrrolic N) and O-functionalities(C=O quinone group) of the four samples are identified by the deconvolution of their high resolution N 1s and O 1s core level peaks,respectively.Specifically, the deconvoluted N1s spectra were fitted by four peaks corresponding to the pyridinic N (N-6 at 398.5 eV),pyrrolic N (N-5 at 400.2 eV), quaternary N (N-Q at 401.1 eV) and oxidized N (N-X at 403.2 eV) [7,11], and the deconvoluted O-1s spectra were fitted by four peaks corresponding the quinonetype (O-I, 530.9 eV), phenol-type (O-II, 531.9 eV), ether-type (OIII, 533.1 eV) and carboxyl-type (O-IV, 534.5 eV) [7,8], respectively.Based on the detailed results shown in Tables S3 and S4 (Supporting information), the NODPC-1.0 sample exhibits the highest pyridinic and pyrrolic N contents and C=O quinone group content.All the above-discussed results indicate that the ratio of the DES and ATB as well as the carbon source both affect the SSA,Vt, pore size distribution and chemical compositions of the as-prepared carbons.These unique structural characteristics and chemical compositions also affect their electrochemical performances, as in the subsequent discussions.
The CV curves of the four samples were achieved from the three-electrode system in 1 mol/L H2SO4electrolyte, as shown in Fig.5a and Fig.S4 (Supporting information).It is found all samples show nearly rectangular shapes even at the high scan rate of 500 mV/s, indicating the excellent capacitive behavior, with predominant electric double layer capacitance (EDLC) contribution and some pseudocapacitance.As for thepseudo-capacitance phenomenon in the CV curve in Fig.5a, obvious pseudocapacitance peaks in the discharging process are observed, while only blunt peaks are in the charging process.These various observations may be due to their different reaction kinetics.Moreover, benefitting from the synergistic effect originated from the largest SSA, suitable pore size distribution, unique local crystal structure, and the highest surface pyridinic N, pyrrolic N, and quinone (C=O) contents, the NODPC-1.0 sample exhibits the highest specific capacitance and superior rate capability among these samples (Fig.5b), with specific capacitances of ∼380 and 251 F/g at 0.5 and 100 A/g, respectively.Comparisons of the specific capacitances of the NODPC-1.0 sample and the previously reported carbons obtained from various activation methods are listed in Table S5 (Supporting information).The galvanostatic charge/discharge curve (GCD) profiles (Fig.5c)and the cycle performance (Fig.5d) of the NODPC-1.0 sample indicate its high reversibility of charge/discharge processes in 1 mol/L H2SO4electrolyte, and its specific capacitance shows a slight increase by slow activation of carbon material during the long cycle processes.
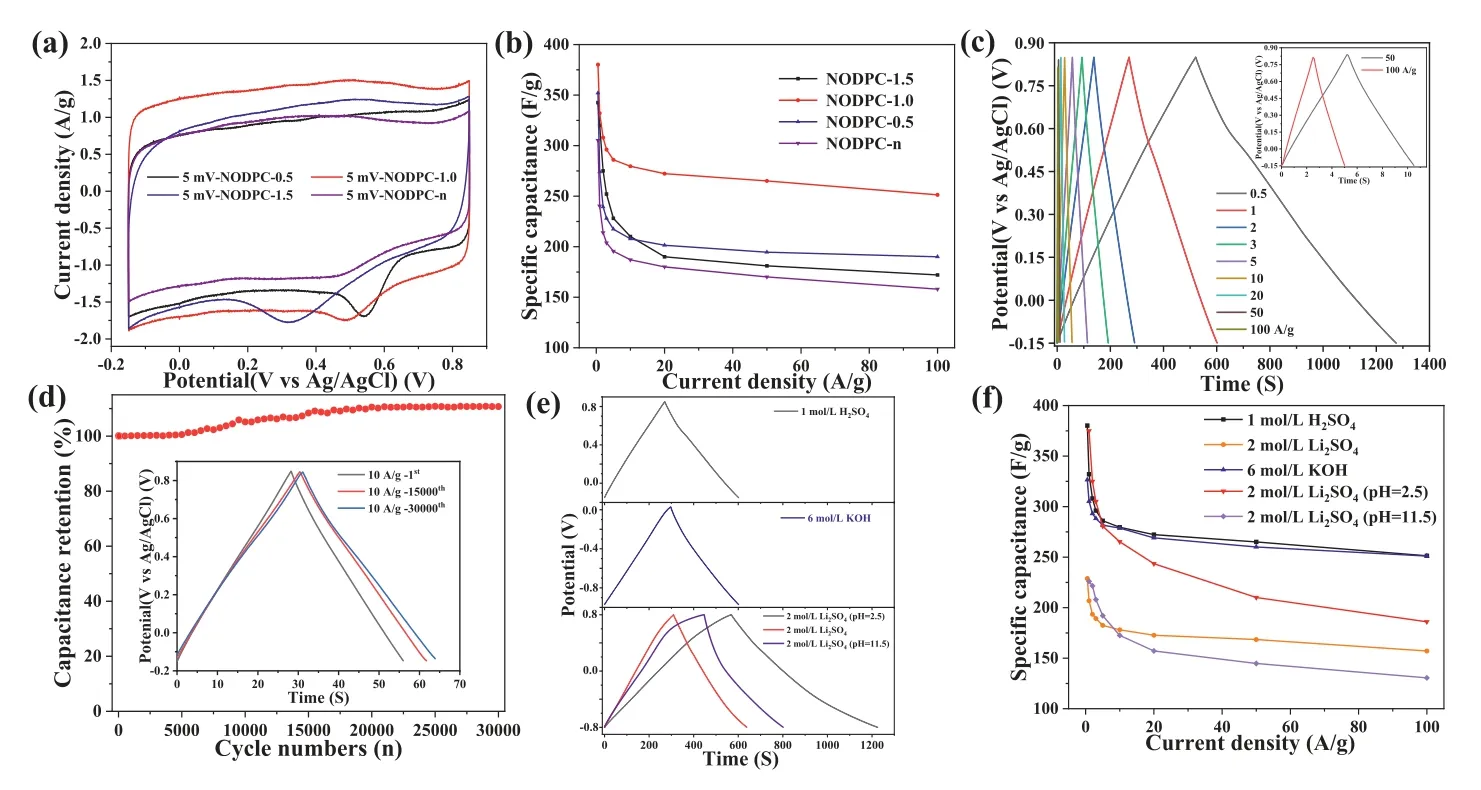
Fig.5.Electrochemical performance in three-electrode systems.(a) CV curves and (b) specific capacitances of NODPC series in 1 mol/L H2SO4.(c) GCD curves at various current densities of NODPC-1.0 in 1 mol/L H2SO4.(d) Long-term cycling test at 10 A/g with the GCD curves for the 1st, 15000th and 30000th cycle in 1 mol/L H2SO4 electrolyte.(e) GCD curves at 1 A/g and (f) Specific capacitances of NODPC-1.0 in different electrolytes.
The excellent capacitive performances of the NODPC-1.0 sample in 1 mol/L H2SO4electrolyte inspire us to investigate its capacitive behavior in different aqueous electrolytes to simultaneously achieve high specific capacitance and a wide work potential range and thus acquire high energy and power densities finally.Herein, we investigated the capacitive performance of the NODPC-1.0 sample in 6 mol/L KOH, 2 mol/L Li2SO4(pH 5.8), acidic 2 mol/L Li2SO4(pH 2.5) and alkaline 2 mol/L Li2SO4(pH 11.5) electrolytes.As shown in Figs.5e and f, the NODPC-1.0 sample displays unique capacitive behaviors in these electrolytes: (1) It shows high specific capacitances in 1 mol/L H2SO4and acidic 2 mol/L Li2SO4(pH 2.5)electrolytes, moderate specific capacitance in 6 mol/L KOH, and small specific capacitance in 2 mol/L Li2SO4and alkaline 2 mol/L Li2SO4(pH 11.5) at the current density of 1.0 A/g.The high specific capacitances are mainly attributed to the typical increase of pseudocapacitance caused by the functional groups in acid electrolytes [7,11,24-27], as illustrated in Fig.4d; (2) It can work well under a wide potential range (−0.8∼0.8 Vvs.Ag/AgCl) in the acidic 2 mol/L Li2SO4(pH 2.5) electrolyte (Fig.S5 in Supporting information), which is originated from the enhancing of the onset overpotential for hydrogen evolution reaction (HER), induced by the fast faradic redox reactions on the surface functional groups and H+[26]; 3) the sample shows the best rate performance in the 6 mol/L KOH electrolyte, which is mainly due to the high ionic conductivity (low solution resistance value shown in Fig.S6 in Supporting information) [28] and the little pseudocapacitance contribution(Fig.5e).In addition, the different ion concentrations and activities of those electrolytes, demonstrated in the EIS and Bode plots(Fig.S6), resulted in various overpotentials and different shapes of the CV curves shown in Fig.S6.The high specific capacitance and its high voltage resistance of the NODPC-1.0 sample in acidic 2 mol/L Li2SO4(pH 2.5) electrolyte will enable it to exhibit potential high energy density in a symmetric SC, and therefore, the electrochemical performances of a coin-type symmetric SC assembled by the NODPC-1.0-based electrodes with a mass load of 4 mg/cm2and acidic 2 mol/L Li2SO4(pH 2.5) electrolyte have been measured and investigated.As shown in Figs.6a and b, the symmetric SC can stably work in a potential range of 0∼2.4 V, without significant HER and OER phenomena.Furthermore, the NODPC-1.0 electrode exhibits a high capacitance of ∼93 F/g at 0.5 A/g in the assembled SC (Fig.6c).This value is much higher than that(75 F/g) of this electrode in the 2 mol/L Li2SO4electrolyte in the same voltage window (Fig.S7 in Supporting information).The decreased capacitance could be attributed to not enough H+in the origin 2 mol/L Li2SO4electrolyte (pH 5.8) for providing the extra pseudocapacitance [23,25].Previous results suggested that the ESW of the Li2SO4electrolyte can be expanded to 2.2 V, due to the forceful solvation properties of Li+and SO42−and strong hydration enthalpies [29,30].However, in most assemble symmetric SCs using the carbon-based electrodes, the practical stable work potential windows in 2 mol/L Li2SO4electrolyte are 1.6 or 1.8 V[22,24,27,28,31].Herein, the as-prepared hierarchically porous N, O co-doped carbon can exhibit stable cycle up to tens of thousands of cycles (92.4% capacity retention after 30,000 cycles) over a voltage range of 0 ∼2.4 V, with high specific capacitance and superior rate performance (Figs.6c and d), which indicated that the unique surface chemical composition and pore structure of the asprepared porous carbon may play key roles on the high voltage resistance when it was used to an active material for an aqueous SC.Although detailed mechanism on the explanation the real deepseated reasons of this interesting fact are not very clear at present,as we suggested, the rich surface groups (pyridinic N, pyrrolic N and quinone (C=O) groups) in the NODPC-1.0 sample offering enough interaction sites for absorbing the H+and thus increasing the HER overpotential are one of the most crucial potential reasons [32,33].In addition, the assembled SC using the NODPC-1.0-based electrodes exhibits inferior specific capacitance and rate performance as well as a lower ESW (<2.0 V) in acidic 1 mol/L Li2SO4(pH 2.5) electrolyte (Fig.S7), indicating the optimization of the electrolyte compositions is also very key for achieving excellent electrochemical performances of a specific carbon material [34,35].
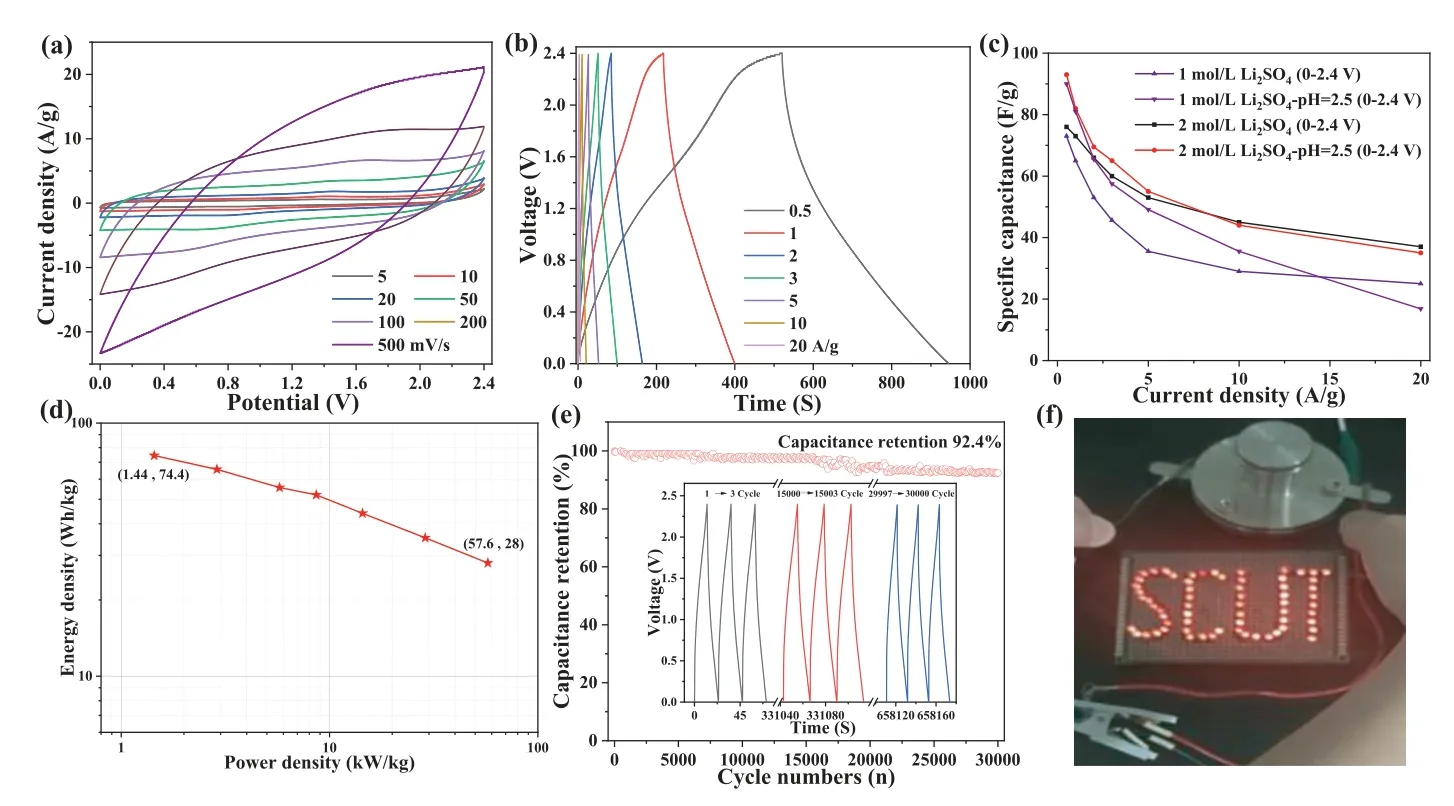
Fig.6.Electrochemical performance of the NODPC-1.0//NODPC-1.0 symmetric SC.(a) CV curves at various scan rates from 5 mV/s to 500 mV/s and (b) GCD curves at various current densities in 2 mol/L Li2SO4 (pH 2.5) at 0∼2.4 V potential window.(c) Rate performance of the NODPC-1.0-based SCs in different electrolytes.(d) Ragone plots.(e)Cycling performance at 10 A/g in acidic 2 mol/L Li2SO4 (pH 2.5).(f) A photo of 64 LED powered by the assembled SC.
Combining the wide ESW with the high specific capacitance and excellent rate capability of the symmetric SC assembled by the NODPC-1.0-based electrodes and acidic 2 mol/L Li2SO4(pH 2.5),it can display high energy and power density simultaneously.As shown in Fig.6e, the symmetric SC presents a high energy density of 74.4 Wh/kg at 1.44 kW/kg and retains 28 Wh/kg at 57.6 kW/kg.These values are superior to most of the carbon-based SCs reported in the literatures [18,19,36-41], and more comparisons of the electrochemical performances for the recently reported symmetric SCs in aqueous electrolytes are listed in Table S6 (Supporting information).After being charged, the NODPC-1.0 based assembled SC was tested to power a group of LED lights in Fig.6f, to further testify its practical application.It is also found that 64 LED lights can be lighted up and continued for more than 2 min (Fig.S8 in Supporting information).The comparisons of the SEM images (Fig.S9 in Supporting information) of the fresh and the cycled electrode indicated that the active material is still well adhered onto the collector, although more obvious pores were observed.The pores generated in the repeated cycles are conducive to the transmission of ions, and thus result in the enhancement of the specific capacitance.
In summary, a hierarchically porous N, O co-doped carbon has been successfully prepared by a novel and low-cost DES as the activator and heteroatom source meanwhile.The resultant NODPC-1.0 sample exhibits an outstanding specific capacitance of 375 and 186 F/g at 1.0 and 100 A/g in 2 mol/L Li2SO4(pH 2.5) electrolyte,respectively.Also, a symmetric SC based on this active material and an acidic 2 mol/L Li2SO4(pH 2.5) electrolyte can stably work for more than 30,000 cycles in a wide potential window (0∼2.4 V),displaying a high energy density of 74.4 Wh/kg at a power density of 1.44 kW/kg and capacitance retention of 92.4%.Taking into account the excellent energy density and superior cycle stability in a wide voltage window, our research will provide some new insight to explore new carbon-based materials with unique compositions and structures for expanding the energy and power densities in low cost and environmentally friendly aqueous SCs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21875071 and 22178125) and the Guangdong key R&D Program of China (No.2019B090908001).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.057.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
