Anisotropic black phosphorene nanotube anodes afford ultrafast kinetic rate or extra capacities for Li-ion batteries
Huili Wng, Qin Go, Cheng Liu, Yu Co, Shuo Liu, Boshn Zhng,Zhenpeng Hu,∗, Jie Sun,∗
a Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China
b School of Physics, Nankai University, Tianjin 300071, China
ABSTRACT As an important anode material for fast-charging Li-ion batteries (LIBs), black phosphorus (BP) has attracted extensive attention.Black phosphorene nanotubes (BPNTs) can be theoretically produced by rolling up the black phosphorene nanosheet along armchair (a-BPNTs) and zigzag (z-BPNTs) directions.The effects of curvature, chirality, Li-storage concentrations and strain stress on the Li-storage performance such as Li diffusion barriers and mechanical stabilities of BPNTs are mainly investigated by first principles calculations.The theoretical calculations predict that the a-BPNTs and z-BPNTs have good maximum Li-storage capacities, and the z-BPNTs exhibit better flexibility than a-BPNTs.The mechanical stabilities and Li-migration are all related to the curvature of BPNTs.Additionally, both a-BPNTs and z-BPNTs exhibit fast Li-ion conductivity along the c-axis direction.Moreover, the average Poisson’s ratio of a-BPNTs(0.68) is larger than that of z-BPNTs (0.17), indicating that the strain stress is more difficult to apply on a-BPNTs than z-BPNTs.Our calculations predict that the a-BPNTs can afford ultrafast kinetic rate for fastcharging and high-power LIBs, while the z-BPNTs can provide extra capacity for high-energy LIBs.
Keywords:Black phosphorene nanotubes Lithium-ion battery Anode materials Li-storage performance First principles calculations
Black phosphorus (BP), owning the most thermodynamically stable structure among the allotropes of phosphorus, was rediscovered as a member of two dimensional (2D) layered materials in 2014 [1,2].Due to the anisotropy of BP structure, BP possesses unique anisotropic mechanical, phononic, optical, electronic and ion transport properties [3–8].Studies have also found that black phosphorus has tunable band gap, carrier mobility and magnetic propertiesviachanging the number of layers, in-plane strain, vacancies or adatoms,etc.[9–12].Based on the unique characteristics,BP shows great potential in various applications, such as batteries,supercapacitors, field-effect transistors, diodes and phototransistors[13–19].
As the anode for Li-ion battery, BP possesses high theoretical capacity (2596 mAh/g), good Li-ion conductivity and relatively low,yet safe lithiation potential (0.7 VversusLi+/Li), which can improve the high-rate performance and inhibit the formation of dendrites at overcharging and fast-charge conditions, respectively [8,20-22].Hanet al.predicted that bulk BP can only provide a specific capacity of 164.4 mAh/g (Li0.19P) [23].Kuoet al.suggested the specific capacities of monolayer and bilayer BP were 216.3 mAh/g (Li0.25P)and 432.7 mAh/g (Li0.5P), respectively [24].Alternatively, studies asserted that the specific capacity of monolayer BP was 432.7 mAh/g (Li0.5P) [25,26].However, the BP structures discussed above are all 2D flat configurations, and the layered BP exhibits huge volumetric expansion (∼300%) during lithiation process which leads to the deterioration of its cycling stability, limiting its application.In order to improve the capacity and cycling stability, various strategies have been adopted, such as nanoparticle composites with carbon, p-type doping, heterostructures [27,28].Despite these efforts, these electrodes still suffer from rapid capacity fading during charge-discharge cycling.Nowadays, many nanotubes have been synthesized from 2D layered materials and studies found that the nanotubes offer superior electronic and mechanical performance [29–31].For example, silicon nanotubes show better cycling stability than solid structures in energy storage equipment,as the nanotubes can increase the surface area accessible to the electrolyte and hollow structures can provide buffer space for volume expansion [32,33].Similar to graphite and silicon, BP can be easily exfoliated to single-/few-layer structures through physical or chemical method [34,35].Although no one has reported the synthesis of black phosphorene nanotubes (BPNTs) at present, theoretical calculations predicted that BPNTs can be stable [36–39].Thus,it is necessary to investigate the Li-storage performance of BPNTs to provide theoretical guidance for the experiment.
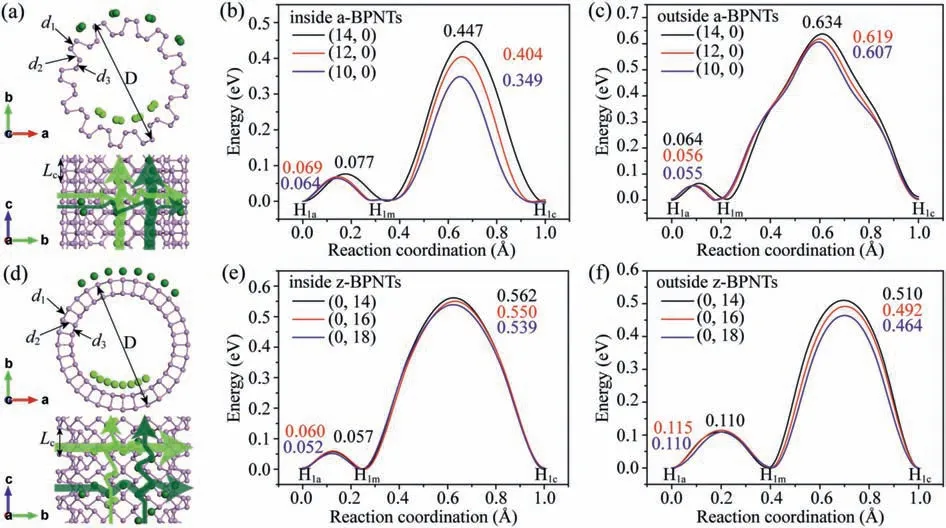
Fig.1.The top view and side view of (a) a-BPNTs and (d) z-BPNTs, and corresponding migration trajectories of some Li atoms on the surface.The energy barriers for Li diffusion on (b, c) a-BPNTs and (e, f) z-BPNTs with different diameters.
BP affords a superior Li-ion conductivity along zigzag direction and a good electronic conductivity along armchair direction due to its structural anisotropy [7,8].Herein, two kinds of BPNTs are taken into account in the present work by rolling up the BP nanosheet along armchair (a-BPNTs) and zigzag (z-BPNTs) directions, respectively.The effects of curvature, Li-storage concentrations and strain stress on the Li-storage performance are mainly investigated by first principles calculations.All the calculations are performed by the VASP code, with the generalized gradient approximation (GGA)of Perdew, Burke, and Ernzerhof (PBE) to describe the exchange correlation potential [40,41].A plane-wave cutoff of 400 eV is used and the energy and Hellmann-Feynman force convergence criteria for electronic and ionic interaction are set at 10−5eV and 0.03 eV/, respectively.The van der Waals (vdW) correction is included using optB86b-vdW functional in our calculations [42].A 3 × 4 × 1 supercell with 20vacuum space is used to simulate the Li adsorption and diffusion on black phosphorene.To avoid the interaction between the nanotubes, the aperiodic length for a-BPNTs and z-BPNTs in thea/b-axial directions is set to 36
The structural characteristics of a-BPNTs and z-BPNTs are firstly discussed.As shown in Fig.S1a (Supporting information), the lattice constants of the optimized black phosphorene area1= 4.50anda2= 3.31in the armchair and zigzag directions, respectively,which are in agreement with the experimental and theoretical prediction [11,26,34].The (n1,n2) BPNTs can be obtained by rolling up black phosphorene along the vectorwheren1andn2are the numbers of the unit cell along the two directions.In this study, we fabricated two kinds of BPNTs by rolling up monolayer BP along armchair (n1, 0) (a-BPNTs) and zigzag (0,n2) direction,respectively, as shown in Figs.1a and d.
In order to investigate the effect of curvature on Li-storage performance, both a-BPNTs and z-BPNTs choose three simulation models with different diameters, (10/12/14, 0) and (0, 14/16/18)for a-BPNTs and z-BPNTs, respectively.The structural parameters of black phosphorene and BPNTs are listed in Table 1.The periodic unit-cell length (Lc) of BPNTs in thec-axial direction is obtained by fitting the energy withLcusing quadratic function as shown in Fig.S2 (Supporting information).It can be seen that the optimized unit-cell lengthLcin the case of a-BPNTs is nearly the same with black phosphorene (3.31, zigzag direction).Differently, theLcof z-BPNTs shrinks, and the smaller radius exhibits the more obvious shrinkage compared with black phosphorene (4.50, armchair direction).In addition, the unit-cell lengthLcof z-BPNTs increases along with increasing diameters.
The strain energy, defined as the energy difference of per phosphorus atom between BPNTs and black phosphorene, is used to evaluate relative stabilities of BPNTs.The smaller strain energy predicts more stable structure.As shown in Table 1, the strain energy for both a-BPNTs and z-BPNTs decreases with the increase of diameters, indicating that BPNTs become more stable as the diameters increase.In addition, the stability of a-BPNTs is better than z-BPNTs when their diameters are similar, due to that the former possess lower strain energy which is in line with the results of Zenget al.[37].Bond length is another criterion to evaluate structural stability.As shown in Figs.1a and d,d1,d2andd3represent three types of P-P bonds and their lengths are listed in Table 1.In the case of black phosphorene, the longest bond isd2(∼2.26).In the case of a-BPNTs, the bond lengths ofd1,d2andd3are about 2.25, 2.27and 2.20, respectively.Similar to black phosphorene,d2bond is the most unstable one for a-BPNTs, and is a little larger than that of black phosphorene.The averaged1(∼2.45in z-BPNTs is much larger than the averaged2(∼2.21),d3(∼2.18),and the longest bonds in both a-BPNTs (d2∼2.27) and black phosphorene (d2∼2.26), respectively.In addition, thed1in z-BPNTs decreases with the increasing diameters, from 2.48for (0,14) to 2.42for (0, 18), meaning an increasing stability.Considering the strain energy and the longest P-P bond, the a-BPNTs are more stable than z-BPNTs with the similar diameter, and the stability of both a-BPNTs and z-BPNTs increases with diameter.
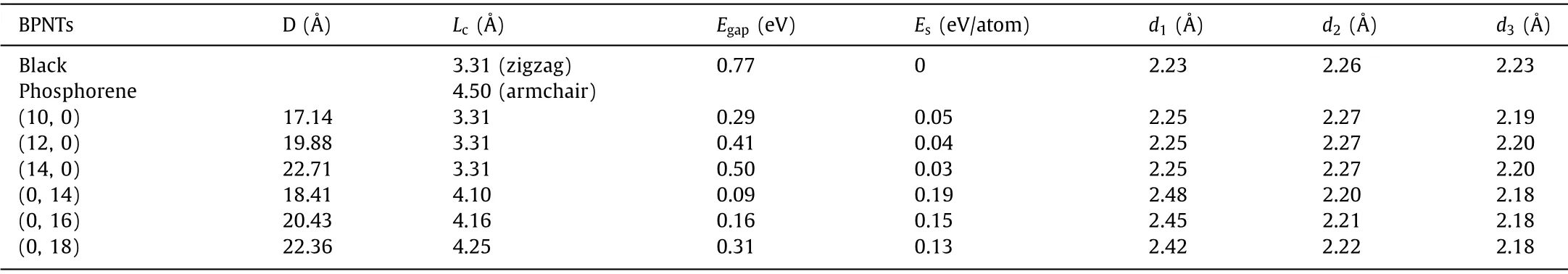
Table 1 The diameter D, unit-cell length Lc in the axial direction, bandgap Egap, the strain energy Es, and P-P bond length d1, d2 and d3.
The charge-discharge rate is a crucial indicator for rechargeable batteries which mainly depends on the migration speed of the intercalated metal-ions.To quantify this, it is essential to investigate the diffusion properties of Li atoms on black phosphorene and BPNTs [43].The climbing image nudged elastic band method was performed to estimate the Li diffusion barrier [44].The adsorption and diffusion of Li atoms on black phosphorene were firstly examined as shown and discussed in Fig.S1 (Supporting information).The results indicate that the Li atom will transfer to the nearest equivalent H1msite no matter which direction it migrates along armchair (H1a-H1b) or zigzag (H1a-H1c) directions.The rate-limiting steps that Li diffusion along zigzag direction and armchair direction on black phosphorene are H1a-H1mand H1m-H1c, respectively,and the corresponding barriers are 0.074 eV and 0.603 eV, respectively.
As the diffusion of alkali metal passing through the BP layer is very difficult [23], we first discussed the migration of Li adsorbed on the most stable adsorption H1site on the surface of a-BPNTs and z-BPNTs.To investigate the effect of curvature on Li diffusion, the diffusion barriers of Li on both a-BPNTs and z-BPNTs with three different diameters are calculated.According to the results discussed (Fig.S1), the periodic Li diffusion pathways on a-BPNTs alongc-axis (zigzag) direction and perpendicularly to thec-axis (armchair) direction are H1a-H1m-H1band H1a-H1m-H1c, respectively.The Li diffusion pathways on z-BPNTs alongc-axis (armchair) direction and perpendicularly to thec-axis (zigzag) direction are H1a-H1m-H1cand H1a-H1m-H1brespectively.The Li diffusion alongc-axis direction of a-BPNTs and z-BPNTs is first discussed which plays a key role in Li-ion conductivity of the nanotubes, and the corresponding rate-limiting steps are H1a-H1mand H1m-H1c, respectively.As shown in Figs.1b and c, when Li migrates on a-BPNTs along thec-axis direction, the average barriers of Li migrating inside and outside the nanotubes are about 0.070 eV and 0.058 eV, respectively.The average barriers of Li migrating inside and outside z-BPNTs along thec-axis direction are 0.55 eV and 0.49 eV, respectively (Figs.1e and f).Accordingly, Li migrates a little slower inside than outside the nanotubes alongc-axis direction.As the barriers alongc-axis directions of a-BPNTs and z-BPNTs decrease with decreasing diameters, the larger curvature of BPNTs, the faster diffusion of Li.For the first-layer Li, the a-BPNTs show better Li-ion conductivity along thec-axis direction than z-BPNTs.Moreover, the transmission of Li atoms perpendicularly to thec-axis of BPNTs is also considered.The average diffusion barriers of Li transports perpendicularly to thec-axis inside and outside a-BPNTs from H1mto H1care 0.40 eV and 0.62 eV, respectively, indicating that the transport is difficult.Differently, the z-BPNTs show fast Li-ion conductivity perpendicularly to thec-axis direction as the average barriers of Li migrates inside and outside the nanotubes from H1ato H1mare only 0.06 eV and 0.11 eV, respectively.
According to the above analysis of the strain energy, the mechanical stability of a-BPNTs and z-BPNTs is strongly dependent on their curvature.In order to investigate the effect of curvature on the mechanical stability of lithiated BPNTs, the Li intercalation on a-BPNTs and z-BPNTs with different curvature is investigated.The Li-storage concentrations are another important factor affecting the structural stability and are crucial for practical applications in LIBs.Thus, the single-layer Li insertion at different concentrations are investigated by gradually increasing the number of Li atoms at the most energetically stable H1sites.The notation of LixP is used to represent the lithiated nanotubes withxrepresenting the Li-intercalation concentration, which is equal to the ratio of Li atoms to P atoms.Two main Li-intercalation patterns, Li laying inside or outside the BPNTs, are also taken into account.The effect of strain energy on the structural stability of lithiated BPNTs is also studied by the change of unit-cell lengthLcin thec-axis direction.Thec-axis strain is defined asεc-axis= (Lc–Lc0)/Lc0, whereLcandLc0are the strained and relaxed unit-cell length of pristine a-BPNTs and z-BPNTs, respectively.The positive value (εc-axi>0)means a tensile strain, and negative value (εc-axi<0) corresponds to a compressive strain.
In the case of a-BPNT (10, 0) as shown in Fig.2a, the structural stabilities of nanotubes with internal Li-intercalation are poor as the lithiated nanotubes can maintain stable structures with suitable unit-cell lengthLc, but the structures are easy to broken into fragments under the action ofc-axis strain stress.The lithiated a-BPNT (10, 0) can remain stable under the strain stress when the Li atoms are embedded outside nanotubes with low Li concentrationsx= 0.05 and 0.125, while the nanotubes with high Li-concentrationx= 0.25 are unstable coupled with the breaking of P-P bonds as shown in Fig.2b.Whether Li atoms insert inside or outside the nanotubes, the lithiated a-BPNT (12, 0) can maintain stable structures under the strain stress, shown in Figs.2c and d.Similarly,the lithiated a-BPNT (14, 0) also exhibit good structural stabilities under the strain stress (Figs.S3a and b in Supporting information).Therefore, it can be drawn that the a-BPNTs exhibit better Li-storage performance when their diameters are larger than that of a-BPNT (12, 0).
The effects of curvature, Li-storage concentrations and strain stress on the structural stability of z-BPNTs are also investigated.As shown in Fig.3, when the Li concentration is low (x ∼0.05), the shape of all lithiated z-BPNTs changes accompanied by the breaking of P-P bonds, but the nanotubes do not break into fragments under the strain stress.When the unit-cell lengthLcis smaller than 4.55, the z-BPNT (0, 14) with internal Li intercalationx= 0.125 can maintain the stable structures and the total energies decrease with the increasing unit-cell lengthLc, while the nanotubes are destroyed coupled with the breaking of P-P bonds when the unitcell lengthLcis larger than 4.55When the unit-cell lengthLcis in the range of 3.80to 4.80, all the z-BPNTs (0, 14) with external Li intercalationx= 0.125 are destroyed.In comparison, the z-BPNTs (0, 16) with Li concentrationx= 0.125 show good structural stabilities no matter Li atoms insert inside or outside the nanotubes.When the Li concentration is up tox= 0.25, the (0, 14) and(0, 16) with internal Li intercalation show good structural stabilities, while the nanotubes with external Li intercalation can remain the stable structures only at small tensile strain.Good structural stabilities are also observed in z-BPNT (0, 18) after Li intercalation as shown in Figs.S3c and d (Supporting information).Similarly, it can be also drawn that the z-BPNTs exhibit better Li-storage performance when their diameters are larger than that of z-BPNT (0,16).As the diameters of a-BPNT (12, 0) and z-BPNT (0, 16) are all about 20, we can get that the a-BPNTs and z-BPNTs show better Li-storage performance when their diameters are larger than 20,that is, the curvature is less than 0.1.

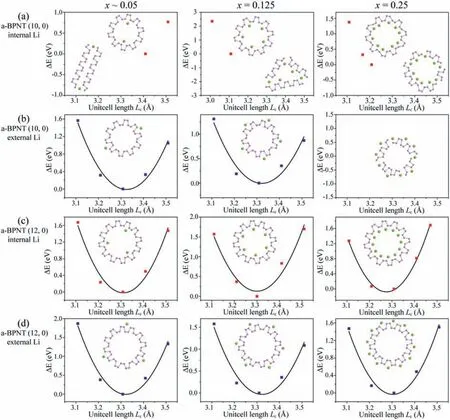
Fig.2.Energy profiles of linear scans about the unit-cell length Lc along the c-axis for (a, b) a-BPNTs (10, 0) and (c, d) a-BPNTs (12, 0) with different internal or external Li-intercalation concentrations.
The Li adsorption ability of LixP can be evaluated from their binding energy whereE(LixP) andE(P) are total energies of lithiated and pristine BPNTs, respectively,E(Li) is the average energy of per Li atom in the body-centered cubic (bcc) Li metal, andxis the Li concentration.The effect of curvature on the Li adsorption ability is discussed by comparing the binding energies with Li concentrationx= 0.125 (Fig.S4 in Supporting information).The binding energies of a-BPNTs with external Li decrease slightly with the increasing diameters, indicating the gradually enhanced Li adsorption ability with decreasing curvature.However, the binding energies of a-BPNTs with internal Li intercalation and z-BPNTs with internal or external Li intercalation increase a little with increasing diameters,indicating the gradually weakened Li adsorption ability with decreasing curvature.In addition, theEb(x) of Li inserting to z-BPNTs is about 0.7 eV less than the correspondingEb(x) of Li inserting to a-BPNTs with similar diameters, indicating that Li atoms are more inclined to intercalate to z-BPNTs than a-BPNTs.
The unit-cell lengthLcand diameters of pristine and lithiated a-BPNTs (12, 0) and z-BPNTs (0, 16) with the single-layer Li intercalation concentration less thanx= 0.25 are summarized in Tables S1 and S2 (Supporting information), respectively.When the Li atoms insert inside the a-BPNT (12, 0), theLcdecreases a little while the diameter increases a little with the increasing Li concentrations.However, when the Li atoms insert outside the a-BPNT(12, 0), both theLcand the diameter decrease a little with the increasing Li concentrations.In the case of z-BPNT (0, 16), theLcand the diameters increases obviously after lithiation and can be affected evidently by the Li concentrations.
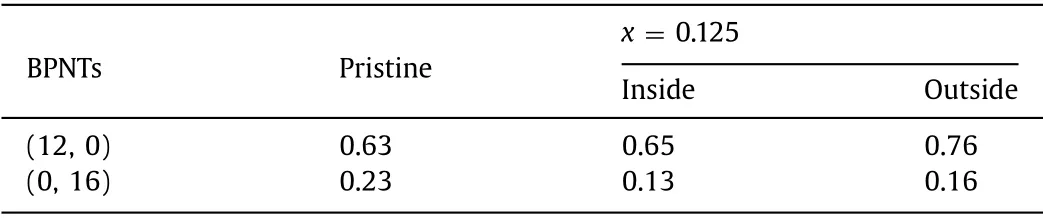
Table 2 The Poisson ratio of pristine and lithiated a-BPNTs (12, 0) and z-BPNTs (0, 16).
Electronic conductivity is another significant indicator of the electrode materials for rechargeable batteries.Fig.S5 (Supporting information) shows the total density of states of the lithiated a-BPNTs (12, 0) and z-BPNTs (0, 16) with different Li-intercalationconcentrations.Similar to 2D BP [8], the lithiated a-BPNTs and z-BPNTs are converted from semiconductor to conductor with some electronic states distributed near the Fermi level, indicating an enhanced electronic conductivity.
Generally, a compression (or stretch) applied in an axial direction leads to an expansion (or compression) in the transverse direction.In the above, we define thec-axis strainsεc-axis, and the corresponding transverse strain can be defined asεtrans= (D–D0)/D0, whereDandD0are the diameters of strained and relaxed BPNTs, respectively.Poisson’s ratio (ν) is defined as the ratio between thec-axis strainsεc-axisand the corresponding transverse strainεtransasν=-dεtrans/dεc-axis.Fig.S6 (Supporting information) displays the appliedc-axis strain and their corresponding transverse strain of pristine and lithiated a-BPNT (12, 0) and z-BPNT (0, 16), and the Poisson’s ratio is listed in Table 2.It can be seen that all the Poisson’s ratios are positive and the Poisson’s ratio of lithiated a-BPNT (12, 0) and z-BPNT (0, 16) with Li concentrationx= 0.125 is nearly equal to that of corresponding pristine nanotubes.In addition, the Poisson’s ratio of a-BPNT (12, 0) is larger than that of z-BPNT (0, 16) which means the strain stress is more difficult to apply on a-BPNT (12, 0) than z-BPNT (0, 16).
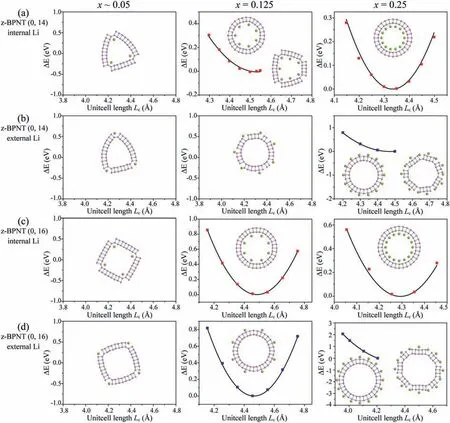
Fig.3.Energy profiles of linear scans about the unit-cell length Lc along the c-axis for (a, b) z-BPNTs (0, 14) and (c, d) z-BPNTs (0, 16) with different internal or external Li-intercalation concentrations.
The above works have discussed the cases that only single-layer Li atoms are adsorbed on the internal or external wall of BPNTs.In practice, Li may exist inside and outside the BPNTs at the same time.The adsorption of Li with both internal and external intercalation for the stable a-BPNT (12, 0) and z-BPNT (0, 16) is investigated.As shown in Figs.S7a and b (Supporting information), the maximum Li concentration in a-BPNT (12, 0) is 0.375 with internal Li concentrationx= 0.25 and external Li concentrationx= 0.125,and the maximum Li concentration in z-BPNT (0, 16) is 0.625 with internal Li concentrationx= 0.375 and external Li concentrationx= 0.25.As shown in Fig.S7c (Supporting information), the maximum Li concentration of Li inserting outside the a-BPNT (12, 0)is 0.5, which is equal to that of Li inserting inside the nanotube.Therefore, compared with the single Li-intercalation method, the adsorption of Li in a-BPNTs with both internal and external intercalation is unfavorable for Li-storage.Interestingly, it shows different effects in z-BPNTs.The maximum Li concentrations of Li inserting inside or outside z-BPNTs using a single Li-intercalation method before the structural distortion are 0.375 and 0.25, respectively.Thus, the adsorption of Li in z-BPNTs with both internal and external intercalation can serve higher Li-storage capacity (x= 0.625)than using a single Li-intercalation method.
To verify whether the hollow structures of BPNTs are conducive to Li-storage and can inhibit the volume expansion, the properties of multilayer Li intercalation are discussed.As shown in Fig.4a,when the Li-intercalation concentration of a-BPNT (12, 0) is up tox= 0.5, the Li atoms are uniformly distributed in the cavity, and the shape of the nanotube collapses a little with no broken P-P bonds.When the Li-intercalation concentration of a-BPNT (12, 0)is larger thanx= 0.5, the tubular structures break into fragments with some overflowed Li atoms.In the case of z-BPNTs (0, 16)shown in Fig.4b, when the Li-intercalation concentrations is up tox= 0.75, the tubular structures can keep well even if some PP bonds break, while the nanotube was destroyed when the Liconcentration is up tox= 1.
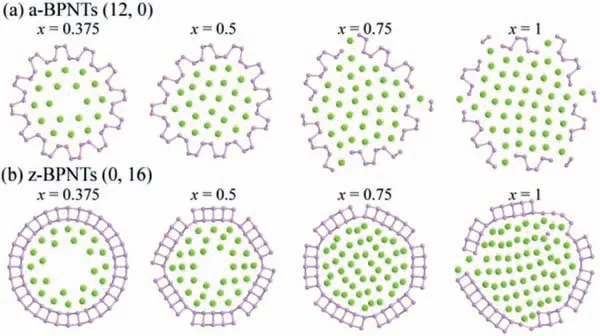
Fig.4.Optimized structures for (a) a-BPNTs (12, 0) and (b) z-BPNTs (0, 16) with different internal Li-intercalation concentrations.
The Li-storage capacityC(mAh/g) can be computed as follows:

wherexis the Li concentration,zis the valence number (z= 1 for Li),Fis Faraday constant (96,485 C/mol), andMPis the atomic mass of P (30.97 g/mol).According to the definition, the theoretical capacities of a-BPNT (12, 0) and z-BPNT (0, 16) are 432.7 mAh/g(Li0.5P) and 649.0 mAh/g (Li0.75P), respectively.Compared with the Li-storage capacity of bulk BP (164.4 mAh/g), it can be seen that the Li-storage performance can also be enhanced by rolling the BP nanosheet into nanotubes.
The Li intercalation voltage is also calculated to explore the electrochemical performance of BPNTs.Usually the effects of entropy and volume are negligible during the reaction.The average voltage (Vavg) of lithiated BPNTs defined as LixP with Li concentration in the range ofx1< x≤x2can be determined by:
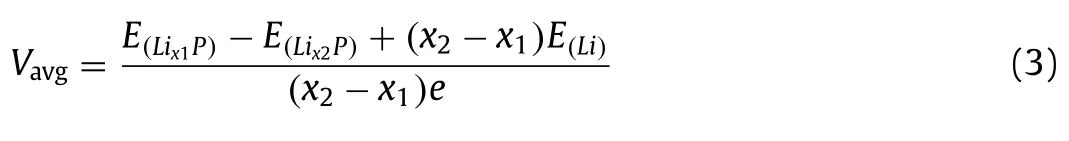
whereE(Lix1P),E(Lix2P)andE(Li)are the energy of Lix1P, Lix2P and a single Li atom, respectively.According to the definition, the average voltages of a-BPNT (12, 0) and z-BPNT (0, 16) with external Li intercalation (0< x≤0.25) are 1.94 V and 2.58 V, respectively.The Li storage inside the BPNTs is divided into two steps: single-layer Li intercalation and multilayer Li intercalation.Based on this, the average voltages of a-BPNT (12, 0) with the Li concentration in the range of 0< x≤0.25 and 0.25< x≤0.5 are 2.15 V and 2.04 V, respectively.In addition, the average voltages of z-BPNT (0, 16) with the Li concentration in the range of 0< x≤0.25 and 0.25< x≤0.75 are 2.31 V and 1.93 V, respectively.Studies found that the average voltage of monolayer BP is 2.9 V (0 ≤x≤0.25) [8], which is much higher than that of BPNTs.Hence, we can draw the conclusion that the BPNTs possess lower average voltage than monolayer BP.
In the case of Li-intercalation concentrationx= 0.375 as shown in Fig.4, the Li atoms are dispersed into two layers.The structural parameters of a-BPNT (12, 0) and z-BPNT (0, 16) with Liintercalation concentrationx= 0.25 and 0.375 are shown in Table S3 (Supoprting information).Interestingly, the tubular structures of both a-BPNT (12, 0) and z-BPNT (0, 16) with Li-intercalation concentrationx= 0.375 not only remain well but also have decreased diameters compared with corresponding that with Li-intercalation concentrationx= 0.25.The migration of the second-layer Li is also considered.The migration pathways along thec-axis direction are selected and the optimized pathways are shown in Fig.5.The calculated diffusion barriers for the second-layer Li along thec-axis direction of a-BPNT (12, 0) and z-BPNT (0, 16) are 0.10 eV and 0.12 eV, respectively.These results indicate that the intercalation of the Li atoms in the second layer not only is beneficial to maintain the structural stability but also provides new Li transport channels.Compared with other anode materials, such as graphene (0.28 eV)[45], silicon (0.23 eV) [46] and MoS2(0.21 eV) [47], the a-BPNTs(0.07 eV) and z-BPNTs (0.12 eV) exhibit super high Li-ion conductivity along thec-axis direction.
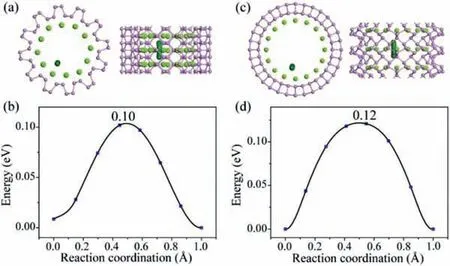
Fig.5.The diffusion pathways and energy barriers for Li diffusion on (a, b) a-BPNT(12, 0) and (c, d) z-BPNT (0, 16) along c-axis direction.
In summary, the effect of curvature, chirality and strain stress on the Li-storage performance and mechanical stability of a-BPNTs and z-BPNTs were investigated by first principles calculations.Our calculations show that the cavity structures of both a-BPNTs and z-BPNTs are conducive to Li-storage, and the maximum Li-storage capacities of a-BPNTs and z-BPNTs are 432.7 mAh/g and 649.0 mAh/g, respectively.The curvature plays a key role in mechanical stabilities and Li-ion conductivity for both a-BPNTs and z-BPNTs.The lithiated nanotubes can maintain tubular structures well under the condition ofc-axis strain tress when their curvature is less than ∼0.1.Furthermore, the larger curvature of BPNTs, the more conducive to the migration of lithium ions along thec-axis direction, especially the transmission of Li laying outside the a-BPNTs and z-BPNTs.The diffusion barrier of first-layer Li alongc-axis direction on a-BPNTs (0.06 eV) is about one-tenth of that on z-BPNTs(0.52 eV), while the difference between the diffusion barriers of the second-layer Li on a-BPNTs (0.10 eV) and z-BPNTs (0.12 eV)alongc-axis direction is very small.Moreover, the pristine and lithiated a-BPNTs and z-BPNTs all have positive Poisson’s ratios,and the average Poisson’s ratio of a-BPNTs (0.68) is larger than that of z-BPNTs (0.17).Our results predict that both the a-BPNTs and z-BPNTs can serve as good anode materials for high-power LIBs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No.2019YFE0118800), National Natural Science Foundation of China (Nos.22005215, 21773124) Tianjin Science and Technology Project (No.19YFSLQY00070) and Hebei Province Innovation Ability Promotion Project (Nos.20544401D,20312201D).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.030.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
