Microwave-assisted synthesis of oxygen vacancy associated TiO2 for efficient photocatalytic nitrate reduction
Qin Li, Yunni Liu, Zhe Wn, Hiyn Co, Sho Zhng, Yue Zhou, Xingyu Ye,Xioyn Liu,∗, Dieqing Zhng,∗
a The Education Ministry Key Lab of Resource Chemistry, Joint International Research Laboratory of Resource Chemistry, Ministry of Education, and Shanghai Key Laboratory of Rare Earth Functional Materials, College of Chemistry and Materials Science, Shanghai Normal University, Shanghai 200234, China
b School of Environmental and Geographical Sciences, Shanghai Normal University, Shanghai 200234, China
ABSTRACT The solar-driven photocatalytic technology has shown great potential in nitrate (NO3−) pollutants reduction, however, it has been greatly hindered by the complex preparation and high cost of photocatalysts.Herein, a relatively low-cost photocatalyst, rutile and anatase mixed phase TiO2 was synthesized by a facile microwave-hydrothermal method.Meanwhile, oxygen vacancy is successfully generated, leading to an acidic surface for strong adsorption towards NO3−, which further improved the reduction activity.Compared with the commercial P25, a higher NO3−conversion of ca. 100% and nitrogen (N2) selectivity of 87% were achieved under UV (365 nm) irradiation within 2 h.This research provides a promising strategy for designing efficient noble metal free photocatalyst in the NO3−reduction.
Keywords:Photocatalysis TiO2 Oxygen vacancy Nitrate reduction
Nitrogen (N) is an essential nutrient, but when its concentration accumulated to some threshold value, it could be a source of pollution in water or the atmosphere [1].Nitrates (NO3−) as one of the most common N species contaminants in the world, was mainly resulting from the use of nitrogen fertilizers and the dung from large animal farms [2].However, high intake of nitrate would appear a serious threat to human health, such as an increased risk of natural preterm birth and central nervous system cancers (CNC)in children [2–4].Furthermore, the nitrate can be reduced to dangerous chemicals, including nitrite (NO2−), which caused blue baby syndrome [5].Thus, many strategies such as reverse osmosis, electrodialysis and ion exchange have been widely studied for removing nitrate from ground water [6].However, these works highly concentrated on the nitrate conversion into brines instead of removing it to harmless nitrogen (N2) [7,8].
Photocatalysis as an environment-friendly technology shows great potentials in pollutant removal by directly using solar energy [9–13].As a semiconductor, titanium dioxide (TiO2) has been widely used in photocatalytic nitrogen oxides (NOx) oxidation[10,11,14-17], carbon dioxide (CO2) reduction [18], hydrogen (H2)production [19] and removal of volatile organic pollutants (VOCs),etc.[20], due to its low-cost, nontoxicity and good stability [21,22].However, its application in NO3−conversion has been limited because of the low efficiency of traditional TiO2.Noble metals(e.g., Pd, Au, and Ag) loaded TiO2have been developed for promoted denitrification performance (Table S1 in Supporting information).However, this noble metal involving strategy greatly increased the cost, which is not practical for large-scale application[8,23,24].TiO2with oxygen vacancies has attracted intensive attention in photocatalytic NOxremoval, H2evolution and CO2reduction [25–27] owing to the improved charge separation and reactant molecules adsorption.TiO2materials with oxygen vacancies are traditionally produced by hydrogen reduction or NaBH4reduction, both of which are time and energy consuming [28].
Herein, the mixed anatase and rutile phase TiO2(TiO2-A-R)with oxygen vacancies and proper acid sites was successfully prepared by a simple microwave hydrothermal method.The obtained TiO2-A-R showed outstanding photocatalytic NO3−conversion ofca.100% and high N2selectivity of 89% under ultraviolet irradiation.This study will provide a novel approach for efficient and low-cost nitrate removal from water.
The synthetic procedure of TiO2-A-R was illustrated in Scheme 1.In a typical process, potassium titanium oxide oxalate dihydrate and sodium chloride were dispersed in the mixed solution of ethanol and water, keeping stirring for 15 min.Then the as-prepared solution was transferred to the microwave reaction chamber for further microwave treatment under 200 °C for 30 min.As comparison, pure rutile (TiO2-R) and pure anatase (TiO2-A)were prepared.As shown in Fig.1a, the TiO2-A-R shows the typical X-ray diffraction (XRD) peaks of rutile and anatase phases, which are similar to the commercial P25.Besides, according to the peak intensity, the weight fraction of the rutile in TiO2-A-R,WRcan be calculated from the formula (Eq.1) [29,30].And the weight fraction of the anatase in TiO2-A-R,WAcan be calculated from the formula as follows (Eq.2) [31]:
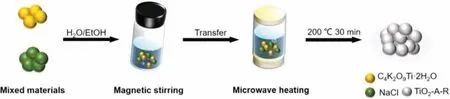
Scheme 1.Schematic illustration of the synthesis processes of TiO2-A-R.
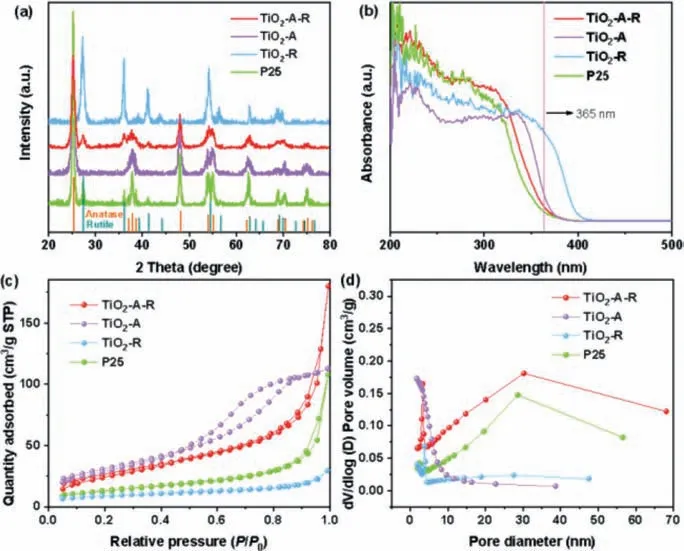
Fig.1.(a) The XRD pattern, (b) UV–vis DRS spectra, (c) N2 adsorption-desorption isotherms and (d) pore size distributions of TiO2-A-R, TiO2-A, TiO2-R and P25 samples.
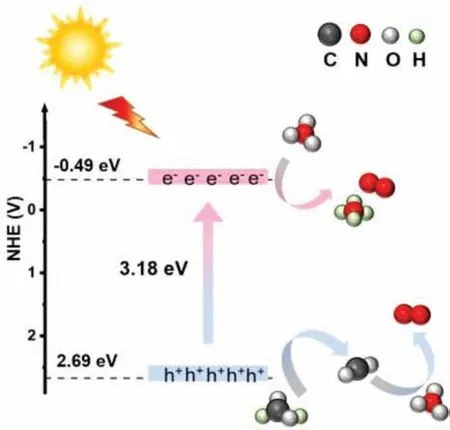
Scheme 2.Schematic illustration of photocatalytic reduction mechanism of NO3−.

In formula,AanaandArutrepresented the diffraction peaks intensity of anatase (101) and rutile (110), respectively.Based on the XRD results, the anatase content of TiO2-A-R was estimated to be 84 wt%.The ultraviolet-visible diffuse reflectance spectra (UV–vis DRS) in Fig.1b demonstrated that all samples exhibited spectral absorption at 365 nm, which ensured the photocatalyst could be effectively excited under 365 nm irradiation.In addition, based on the UV–vis results, the band gap energy (Eg) of the TiO2-A-R was calculated as followed (Eq.3):

whereα,h,v, A andEgrepresented the absorption coefficient, the Planck constant, the light frequency, the constant and band gap,respectively [32].Furthermore,nwas equal to 1/2 or 2 for an indirect or direct band gap semiconductors, respectively.Thus, the estimatedEgof TiO2-A-R is 3.18 eV (Fig.S1 in Supporting information).The calculated flat band potential (EFB) value of TiO2-A-R as shown in Fig.S2 (Supporting information) was –0.95 Vvs.SCE, which is corresponding to −0.29 Vvs.NHE.Besides, TiO2-A-R as an n-type semiconductor, the conduction band (ECB) was 0.2 V belowEFB[33].Thus,ECBlevel of TiO2-A-R was −0.49 eV.And the valence band(VB) was 2.69 eV, which was obtained according to the formula Eq.4 [32,34]:

The nitrogen adsorption-desorption isotherms and pore size distributions were displayed in Figs.1c and d.All the samples showed typical IV isotherms and a pore size distribution ranging from 2 nm to 50 nm, indicating the mesoporous structures.The detailed surfaces areas, pore size and pore volume were listed in Table 1.Compared with other photocatalysts [35–37], TiO2-A-R had the distinguishing features of larger Brunauer-Emmett-Teller (BET)surface area (97.6 m2/g), the greater pore volume (0.3 cm3/g) and the pore diameter (11.4 nm), which might significantly enhance its adsorption ability of reactants and therefore facilitate the targeted reaction [38].
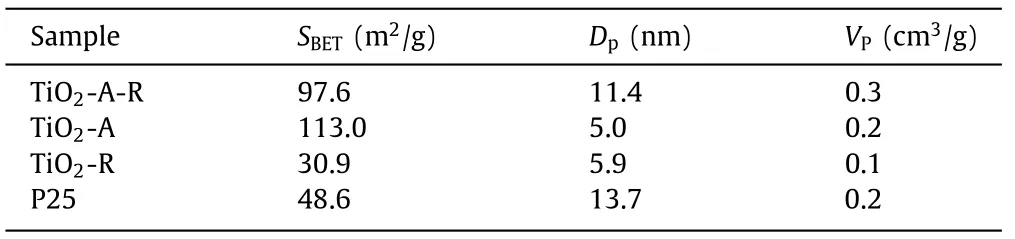
Table 1 BET properties of TiO2-A-R, TiO2-A, TiO2-R and P25 photocatalysts.
TEM images in Figs.2a and b suggested that TiO2-A-R was composed of flaky petal-like structure.Moreover, obvious diffraction rings could be observed in Fig.2c (the selected area electron diffraction, SAED), indicating the TiO2-A-R had good crystallinity[39].Meanwhile, the lattice spacing of 0.351 nm and 0.325 nm were also clearly detected in Fig.2d, which corresponded to (101)and (110) plane of anatase and rutile, respectively [40].The element mappings demonstrated the homogeneous distribution of Ti and O in TiO2-A-R (Fig.2e).These results further confirmed the successful synthesis of mixed-phase titanium dioxide.
In order to probe the surface chemical compositions and the binding configuration of all the samples, X-ray photoelectron spectroscopy (XPS) measurement was performed.P25 and TiO2-R show the peaks located at 458.9 and 464.6 eV corresponding to Ti 2p3/2and Ti 2p1/2(Fig.3a).These peaks of TiO2-A slightly shifted to lower binding energies.Notably, a clearly negative shift was also observed in the TiO2-A-R, indicating the existence of Ti3+[41].Meanwhile, the O 1s XPS spectra of TiO2-A-R presented two peaks centered at ∼529.8 and ∼531.6 eV (Fig.3b), representing for the lattice oxygen and oxygen vacancy, respectively [7].And the area ratio of oxygen vacancy peak (named O2) to the sum area of the O1 and O2 peaks (named Os) is shown in Table S2 (Supporting information).The O2/Os of TiO2-A-R had the largest percentage(22.7%), which demonstrated that oxygen vacancy rooted more in the TiO2-A-R sample [28].
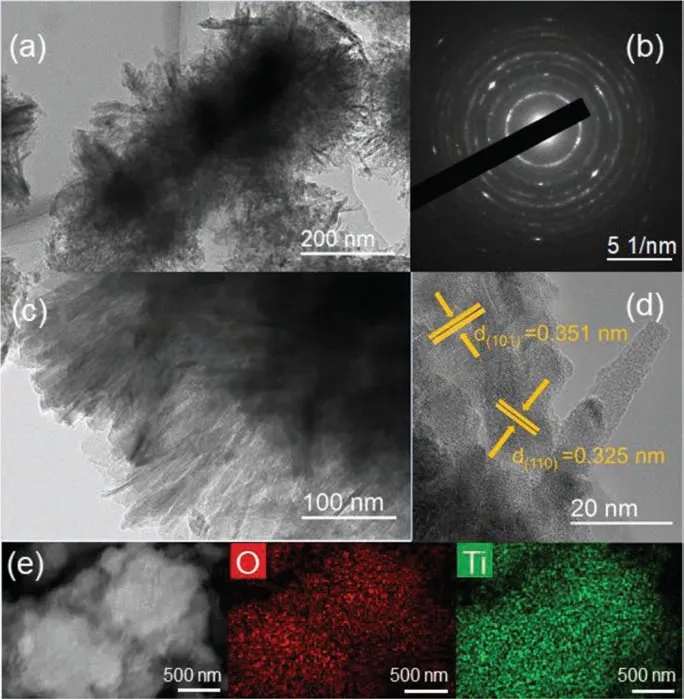
Fig.2.(a, b) TEM images, (c) SAED pattern, (d) HRTEM image, (e) SEM image and corresponding elemental mappings of TiO2-A-R sample.
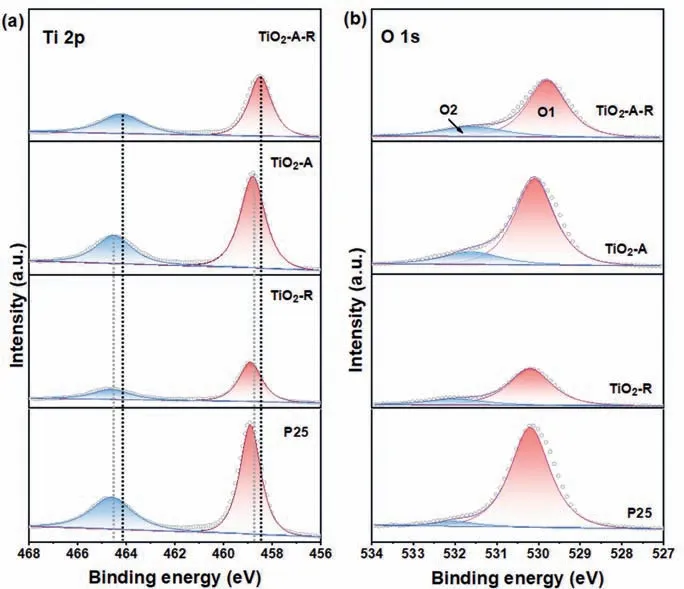
Fig.3.XPS spectra of (a) Ti 2p and (b) O 1s over TiO2-A-R, TiO2-A, TiO2-R and P25 samples.
Moreover, the strong electron paramagnetic resonance (EPR)signal (Fig.4a) in TiO2-A-R with a g-value of 2.001 further verified the existence of oxygen vacancy [42], which might play a vital role in promoting the rapid conversion of nitrate as previously reported [28].Furthermore, the catalysts’activities are closely related to their surface properties such as alkaline and acidity properties [43].Moreover, the NO3−presents Lewis base due to its electronegativity, which implies that it is easier to combine with the Lewis acid catalyst surface [44,45].Thus temperature-programmed desorption of ammonia (NH3-TPD) of P25 and TiO2-A-R were performed from 50 °C to 800 °C to find out their surface properties,and the curves were illustrated in Fig.4b [46,47].The desorption peaks of NH3located below 200 °C, 200–400 °C and above 400 °C are considered as indicators of the weak, medium and strong acid sites, respectively [48].P25 exhibited three NH3desorption peaks at 200, 487 and 611 °C, respectively.The former one is assigned to the weak acid sites and the other two peaks are indexed to the strong acid sites.As comparison, there are only medium (suggested by the peaks at 277 and 384 °C) and strong acid sites (suggested by the peaks at 540, 606 and 706 °C) observed, demonstrating its more acidic surface.As displayed in Table S3 (Supporting information), TiO2-A-R shows a larger peak area than that of P25, confirming there are more active sites to possibly absorb and reduce the NO3−[44,49].
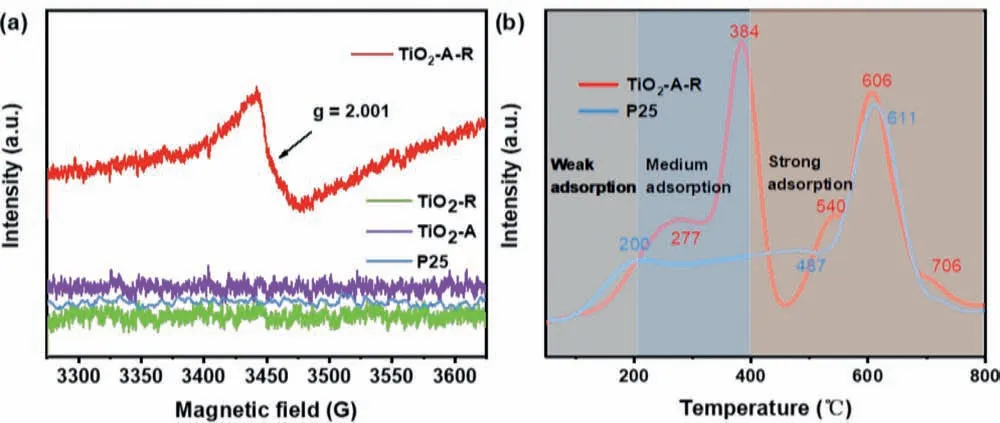
Fig.4.(a) EPR spectra of TiO2-A-R, TiO2-R, TiO2-A and P25 samples.(b) NH3-TPD analysis over TiO2-A-R and P25 sample.
Normally, NO3−could be reduced to N2, NO2−and ammonium(NH4+), but both NO2−and NH4+are hazardous to the environment.An ideal photocatalyst should have high NO3−conversion and good N2selectivity [24,50].In order to inhibit the rapid recombination of electron-hole pairs, formic acid (FA) was selected as a hole scavenger in this reaction [51].To exclude the catalytic effect of FA on NO3−reduction, we conducted a control experiment (in FA without photocatalyst added) and the results were shown in Fig.S3a (Supporting information).No catalytic activity was observed in the absence of the photocatalyst, and thus we can conclude that FA itself will not react with NO3−and promote NO3−reduction.Then, TiO2-A-R (0.060 g) and various amounts of FA were dispersed into 60 mL nitrate solution (50 mg/L) to evaluate the optimal photocatalytic performance.As shown in Figs.5a-c, the activity sequence involving different amounts of FA were summarized as 5 mL FA ≈4 mL FA>3 mL FA.Meanwhile, almost no NO2−was detected in the reaction process of the three controlled trials.When the reaction progressed to 120 min, the average conversion of NO3−involving 3 mL FA was 98%, the average selectivity of N2and NH4+were 88% and 12%, respectively.The experiments with 4 mL or 5 mL FA showed similar nitrate conversion (almost 100%),N2selectivity (89%) and NH4+selectivity (11%).Therefore, we determined adding 4 mL FA as the optimal hole scavenger amount for this reaction.
According to the literature, different hole scavengers such as oxalic acid (OC) and methanol may also be favorable for the photocatalytic nitrate reduction [50].Thus, the 4 mL 0.1 mol/L methanol and OC solution was introduced for photocatalytic NO3−(50 mg/L)reduction experiment as shown in Fig.5d.The OC involving system demonstrated the NO3−conversion of 11% and the 78% N2selectivity, which were much lower than that of FA.No NO3−conversions were observed in methanol involving system or hole scavengers absence system, indicating that FA significantly improved the photocatalytic activity [24].Based on the previous study [51],we confirm that carbon dioxide anion radical (CO2•−) generated by reacting FA with photogenerated holes of photocatalyst has strong reductive ability for NO3−conversion to N2.Then, EPR test was carried out to probe the production of CO2•−in the TiO2-A-R system.As shown in Fig.6, no signals were detected under dark conditions.While under light irradiation, a six-line DMPOCO2•−spin adduct signal was formed with hyperfine parameter of magnetic factorg=2.0059 (Fig.S3b in Supporting information), which can be assigned to reductive CO2•−species for further promoting NO3−degradation [52–54].These results clearly indicated the important promoted effect of FA in the photocatalytic NO3−reduction reaction using noble-metal free TiO2as photocatalysts.
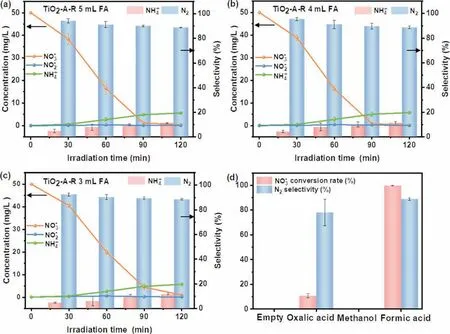
Fig.5.Photocatalytic nitrate reduction activity of TiO2-A-R (a-c) with 3–5 mL formic acid and (d) with different hole scavengers.
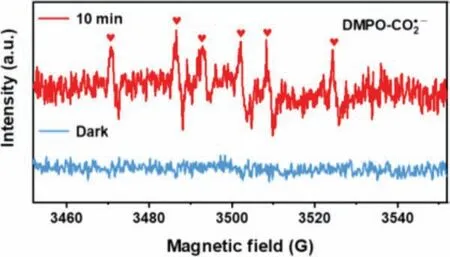
Fig.6.DMPO spin-trapping EPR spectra of TiO2-A-R.
In order to compare the contribution of TiO2-A-R, the performance of TiO2-A, TiO2-R and commercial P25 were evaluated in 4 mL FA and NO3−(50 mg/L) mixture solution system (Figs.7a-c.).The photocatalytic removal rate of these samples followed the order of TiO2-A-R (100%)>TiO2-A (82%)>P25 (61%)>TiO2-R (36%).And the selectivity of N2presented the trend of TiO2-A-R (89%)>TiO2-A (88%)>P25 (87%)>TiO2-R (80%).Obviously, TiO2-A-R showed the enhanced ability of NO3−reduction, which may be attributed to the existence of Ov and acid sites [28].In addition, the cycling durability of TiO2-A-R was conducted and displayed in Fig.7d.After five cycles, the photocatalyst still had 98% NO3−conversion, demonstrating its great stability and big potential for practical application.Moreover, when the concentration of the initial NO3−solution was diluted to 30 mg/L (Fig.8a), the NO3−conversion was achieved 100% after 90 min reaction and the selectivity of N2and NH4+reached 86% and 14%, respectively.Even when the NO3−concentration was increased to 100 mg/L, a high NO3−removal of 77% and N2selectivity of 91% were achieved after 120 min reaction (Fig.8b), implying the excellent activity of TiO2-A-R in a wide NO3−concentrations range.
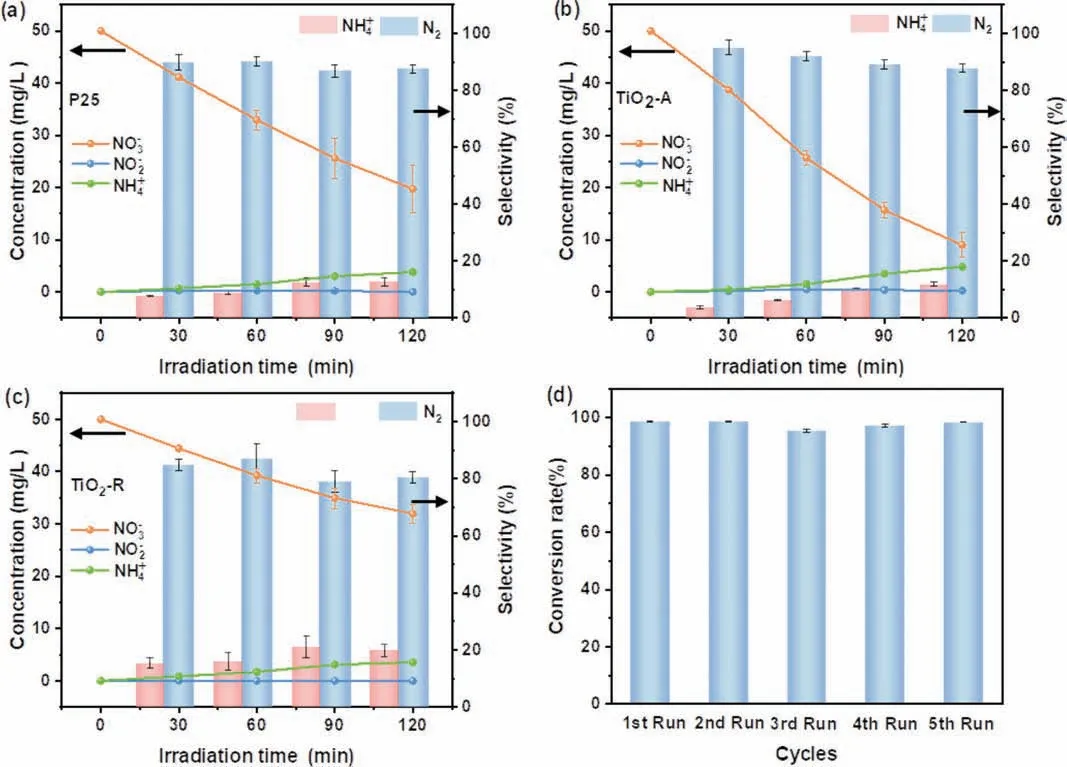
Fig.7.Photocatalytic nitrate reduction activity of (a) P25, (b) TiO2-A, and (c) TiO2-R samples.(d) Cycling stability test of TiO2-A-R.
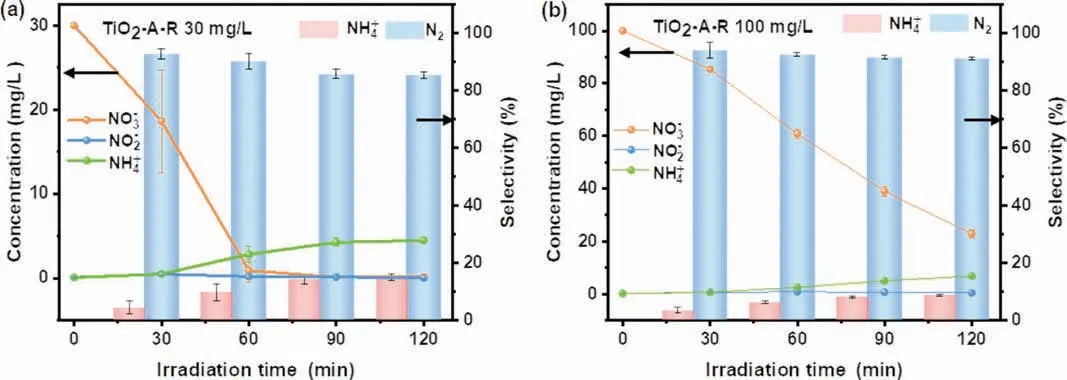
Fig.8.Photocatalytic nitrate reduction activity over the TiO2-A-R sample involving different NO3−initial concentrations of (a) 30 and (b) 100 mg/L.
The photocurrent response (Fig.9a) was carried out to evaluate the charge transport properties [10].Compared with commercial P25, TiO2-A-R has a higher photocurrent density, which demonstrates the improved light source usage rate and effective separation of e−and h+excited by photons [55].In addition, the steadystate photoluminescence (PL) spectrum was measured to investigate the electrons and holes recombination (Fig.9b).Notably, a lower emission peak of TiO2-A-R can be obtained, demonstrating the improved charge carrier separation efficiency [56].And TiO2-AR showed the smaller radius under dark and 365 nm UV-LED irradiation (Figs.9c and d), indicating a better conductivity [9].These factors co-contributed the excellent NO3−conversion and good N2selectivity.
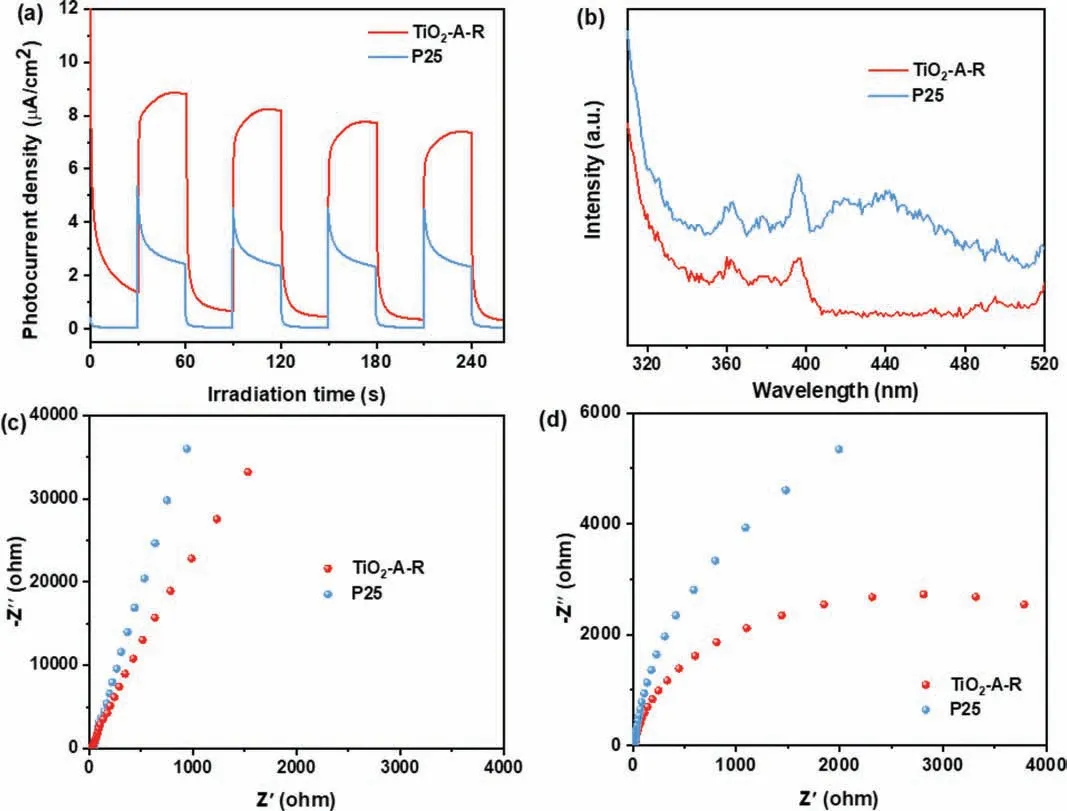
Fig.9.(a) Photocurrent density, (b) PL spectra (ex=290 nm), electrochemical impedance spectra (c) in dark and (d) light irradiation of TiO2-A-R and P25 samples.
Based on the above discussion, the possible NO3−degradation mechanism is proposed as shown in Scheme 2.Firstly, TiO2-A-R is excited to produce photo-generated electron-hole pairs under the UV-LED irradiation (Eq.5).Then the electrons are consumed by NO3−to generate N2or NH4+(Eqs.6 and 7) [24].Meanwhile,the photo-generated holes are scavenged by FA to produce CO2•−species, which further reduces NO3−to N2(Eqs.8 and 9) [8,44,57-59].
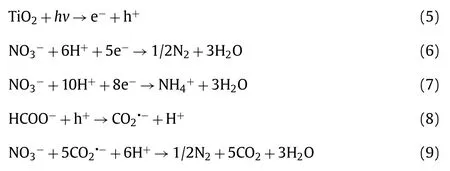
In conclusion, the mixed TiO2photocatalyst with oxygen vacancy was successfully synthesizedviaa facile microwave-assisted method.It has a NO3−conversion up toca.100% under 2 h ultraviolet radiation, which is much high than that of commercial P25(61%).Moreover, the N2selectivity is as high as 89%.This work provides a novel strategy to design noble metal free photocatalysts for cheap, safe and efficient nitrate removal.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No.2020YFA0211004), and National Natural Science Foundation of China (Nos.21876112, 21876113,22022608, 92034301), “111” Innovation and Talent Recruitment Base on Photochemical and Energy Materials (No.D18020),Ministry of Education, and Shanghai Key Laboratory of Rare Earth Functional Materials, Shanghai Engineering Research Center of Green Energy Chemical Engineering (Nos.18DZ2254200)and Shanghai government (Nos.18SG41, 309-AC9103–21–413002,19YF1436600).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.025.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
