Insight into the role of iron in platinum-based bimetallic catalysts for selective hydrogenation of cinnamaldehyde
Ying Zhang, Jinfang Su, Junnan Chen, Chengshan Dai, Bingsen Zhang,∗
a Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
b School of Materials Science and Engineering, University of Science and Technology of China, Shenyang 110016, China
c School of Petrochemical Engineering, Liaoning Pertochemical University, Fushun 113001, China
ABSTRACT Selective hydrogenation of cinnamaldehyde (CAL) toward cinnamyl alcohol (COL) is an extremely important and challenging reaction.Herein, a series of PtxFey-Al2O3 bimetallic catalysts with varied Pt to Fe ratios were prepared by incipient wetness impregnation method.The introduction of Fe significantly modifies the electronic and surface properties of Pt, which clearly enhances the C=O hydrogenation selectivity.Among all the catalysts, Pt3Fe-Al2O3 displays the best catalytic performance and the conversion of CAL is 96.6% with 77.2% selectivity of COL within 1 h.In addition, Pt3Fe-Al2O3 had excellent reusability with 76% COL selectivity after five runs of the recycle process.Further characterization of the fresh,used and cycled catalysts revealed that the structure and electronic state of the synthesized PtxFey-Al2O3 are unchanged after hydrogenation reaction.The identical-location transmission electron microscopy (ILTEM) results revealed that the interaction between the nanoparticles and the supports was strong and the catalyst was relatively stable.
Keywords:PtxFey NPs Cinnamaldehyde Hydrogenation Chemoselective IL-TEM
Selective hydrogenation of cinnamaldehyde (CAL), which contains two functional groups of C=C and C=O bond, to cinnamyl alcohol (COL) is an important and challenging process for producing fine chemicals [1].However, COL is more difficult to synthesize because the selective hydrogenation of the C=O bond is thermodynamically unflavored [2,3].In order to increase the selectivity of COL, many efforts and investigations have been made to design and synthesize highly selective and active heterogeneous catalysts.Until now, noble metal catalysts and oxide supports are widely used in catalysis reactions and have excellent activity [4–10].Nevertheless, the selectivity of CAL to COL is generally low due to the intrinsic properties of Pt [11,12].This problem can be solved by adding a second metal into Pt to generate a bimetallic catalyst[13,14].The second metal not only can improve the dispersibility of Pt nanoparticles (NPs), but also can affect the crystallography and electronic structure of Pt NPs, thereby improving the target product selectivity [15–17].For example, Zhaoet al.[18] have designed a catalyst, which used the metal-organic framework MIL-101 to encapsulate Pt, for the hydrogenation ofα,β-unsaturated aldehydes.The Fe3+and Cr3+in the framework of MIL-101 can regulate the active metal Pt and significantly improve the selectivity to unsaturated alcohol.Chenet al.[19] reported that Pt3Sn/CNTs catalyst prepared by lithium naphthalenide-driven reduction method,which presents excellent catalytic performance because large Pt ensembles were diluted by incorporated Sn atoms and the electron density of Pt was increased.Thein-situformed SnOxinterfaces as lewis acid sites facilitate the coordination of C=O bonds, enhancing the selectivity to COL.In our previous work, PtxCoybimetallic NPs supported on oxygen functionalized CNTs were prepared and the electropositive active sites promote the activation of C=O [20].However, due to the agglomeration and detachment of PtCo3NPs,the catalytic activity was decreased during the recycle process.
Despite the widespread investigations, the structural evolution of catalyst under the liquid reaction conditions still remain outstanding challenges.Identical-location transmission electron microscopy (IL-TEM) method is a powerful approach to provide valuable insight into the structural evolution at the same location and the reaction mechanism of catalyst during reaction process [21–23].Meanwhile, it is possible to explain the structure-performance relationship of catalyst combined its structural characteristics and the catalytic activity [24–26].
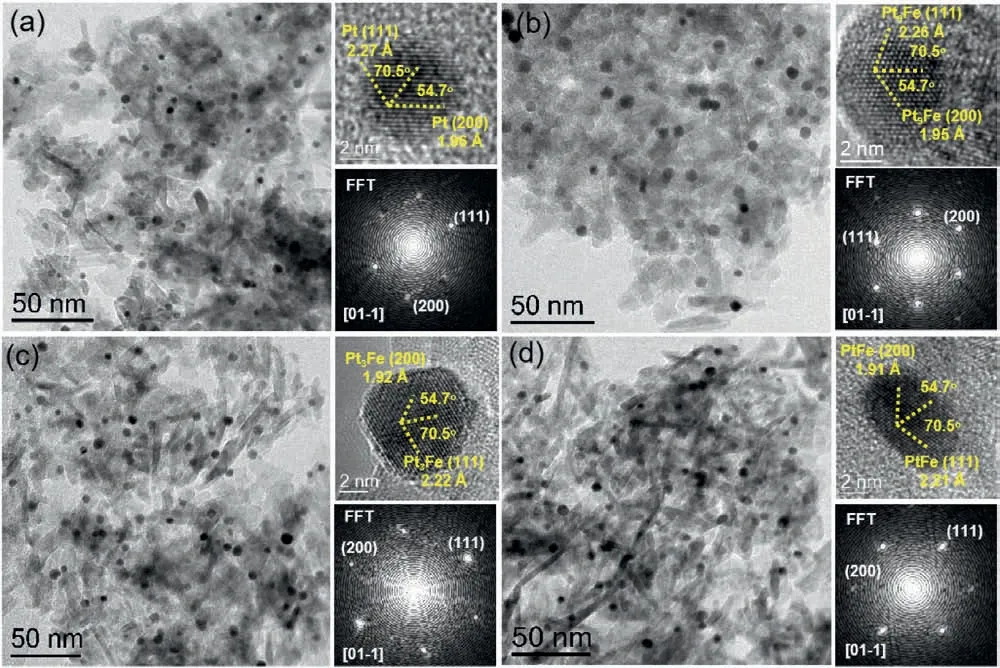
Fig.1.TEM, HRTEM images (top right) and corresponding FFT (bottom right) of Pt-Al2O3 (a), Pt9Fe-Al2O3 (b), Pt3Fe-Al2O3 (c) and PtFe-Al2O3 (d), respectively.
In order to synthesize a stable and efficient catalyst, a series of PtxFeybimetallic catalyst with varied Pt to Fe ratios supported onγ-Al2O3were prepared in this work.The chemoselective hydrogenation of CAL was chosen as probe reaction to explore the influence of second metal Fe in Pt-based catalyst.IL-TEM method was employed to explore the structural evolution during the liquid phase reaction, and the corresponding structure-activity relationship was proposed.
The morphology and structure of the synthesized PtxFey-Al2O3catalysts were characterized by TEM (Fig.1 and Fig.S1 in Supporting information).Highly dispersed PtxFeyNPs on Al2O3support can be observed from the low-magnification high-angle annular dark-field scanning TEM (HAADF-STEM) images.The particle size distribution (PSD) histograms of the corresponding NPs (insets of Fig.S1) display that the average particle diameters of PtxFeyNPs are 3.5, 5.3, 5.2 and 4.7 nm regarding Pt-Al2O3, Pt9Fe-Al2O3, Pt3Fe-Al2O3and PtFe-Al2O3, respectively.HRTEM images show that the PtxFeyNPs have two crystal planes (200) and (111) with a characteristic acute angle of 54.7°, which belongs to face centered cubic (FCC)structure of Pt.The corresponding Fast Fourier Transform (FFT) patterns also display the structure of PtxFeyNPs and their good crystallinity.With the increase of Fe content, the d-spacing of (200)and (111) gradually decreased, which further confirms the formation of PtxFeyNPs.
Fig.S2 (Supporting information) shows the X-ray diffraction(XRD) patterns of Al2O3support and PtxFey-Al2O3catalysts.Fig.S2b is the local enlarged drawing curve in the red rectangle of Fig.S2a.The diffraction peaks of Al2O3support match well with the standard XRD pattern (JCPDS No.46-1131).For Pt-Al2O3, the diffraction peaks related to Pt were detected at 2θ= 39.7°, 46.2°and 67.4°, which corresponding to the (111), (200) and (220) crystal planes of Pt with FCC structure (JCPDS No.01-1311), respectively.The broaden diffraction peak at 39.7° indicates that the size of Pt NPs is small.The particle size calculated based on the Scherrer formula in XRD are 8.7 nm, 9.0 nm, 10.4 nm, and 9.2 nm, respectively, which is slightly difference with the result from TEM.The detailed analysis is discussed in Supporting information.Furthermore, the position of the diffraction peak at 2θ= 39.7°
The real Pt and Fe contents of Pt-Al2O3, Pt9Fe-Al2O3, Pt3Fe-Al2O3and PtFe-Al2O3were determined by inductively coupled plasma mass spectrometry (ICP-MS) and the results are listed in Table S1.Pt contents were examined as 3.85, 4.00, 2.95 and 2.20 wt%, and Fe contents were 0, 0.20, 0.32 and 0.65 wt%, respectively.These measurements indicate that Pt/Fe atomic ratios are approximately close to the desired ratios.
X-ray photoelectron spectroscopy (XPS) was used to further analyze the surface chemical states of Pt.Since Al 2p peak overlaps with Pt 4f peak, which is the most prominent platinum electron line, the Pt 4d5/2was analyzed instead (Fig.2a).It can be seen that the introduction of Fe has a direct impact on the binding energy of Pt 4d, which shifts to higher values slightly with Fe content increased.It indicates that there is electron transfer from Pt to Fe in these bimetallic catalysts.The surface atomic ratio of Pt and Fe on the basis of XPS analysis are listed in Table S1 (Supporting information).
Furthermore,in-situdiffuse reflection IR Fourier transform spectroscopy (DRIFTS) of CO as probe molecule was conducted to study the surface properties of PtxFey-Al2O3catalysts.As shown in Fig.2b, there are two vibration regions of CO adsorbed on Pt-Al2O3and Pt9Fe-Al2O3catalysts.The band at about 2095 cm−1is assigned to CO adsorbed on PtOxsites [27], and the band at around 2065 cm−1is assigned CO linearly adsorbed on the Pt (100) facets[28,29].For Pt3Fe-Al2O3and PtFe-Al2O3catalysts, a main CO band at 2084 cm−1and a shoulder band at around 2054 cm−1were detected.The main one could be attributed to the CO linearly absorbed on the Pt(111) planes [29,30], and the shoulder band is ascribed to CO adsorbed on Pt sites at the step edges, corners, and defects [28].The DRIFTS results indicate that the relativity strong interaction between Pt and Fe occurred in the synthetic process.Moreover, Pt(100) facets are less selective for COL formation than Pt(111) facets [31], so the Pt-Al2O3and Pt9Fe-Al2O3catalysts may be less selective for COL formation when compared with the other two catalysts.
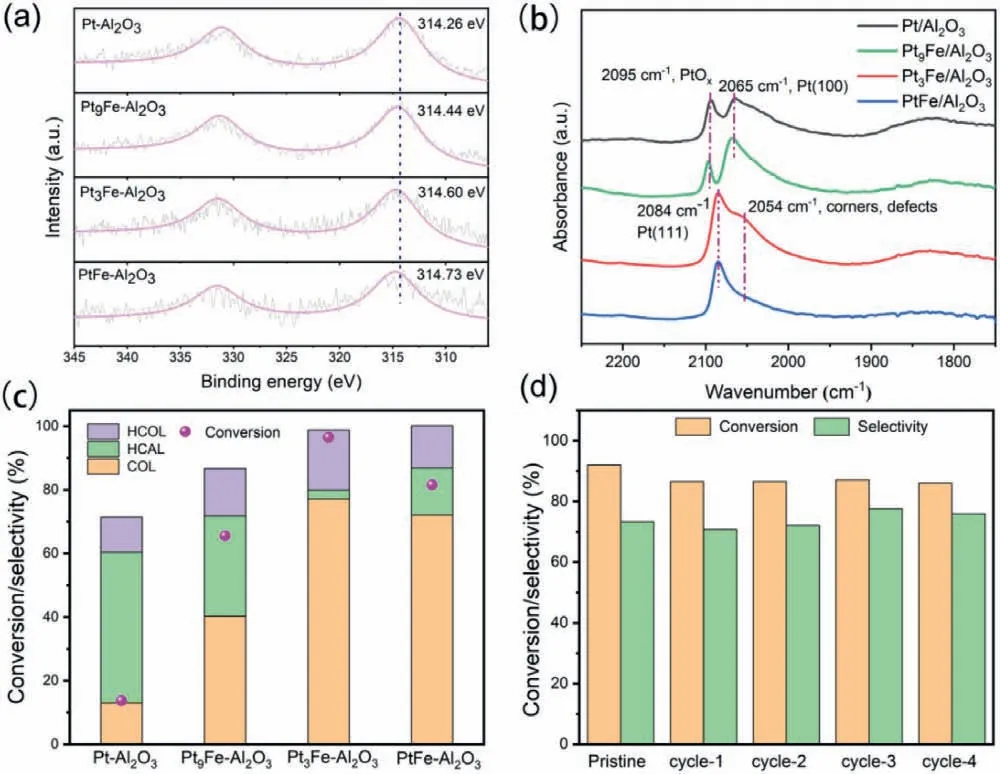
Fig.2.Pt 4d XPS spectra (a), in-situ CO-DRIFTS spectra (b) and catalytic performance of PtxFey-Al2O3 (c) and cycling test of Pt3Fe-Al2O3 for CAL hydrogenation to COL (d).Reaction conditions: T = 80 °C, P = 1.5 MPa, 5 mmol CAL, 30 mg catalysts, 10 mL diethylene dioxide was used as solvent, reaction time: 1 h.gradually shifts to a higher angle with the increase of Fe content (Fig.S2b), which proves that Fe enters Pt lattice and they form platinum iron structure.These results are consistent with the structural features reflected by HRTEM analysis in Fig.1.
The catalytic performance was evaluated in the selective hydrogenation of CAL over Pt-Al2O3, Pt9Fe-Al2O3, Pt3Fe-Al2O3, and PtFe-Al2O3catalysts, as shown in Fig.2c.The CAL conversion and the COL selectivity gradually increase with the increasing of Fe content.The Pt3Fe-Al2O3catalyst exhibits the best catalytic performance with 77.2% selectivity to COL at a 96.6% CAL conversion after 1 h of reaction.In contrast, Pt-Al2O3catalyst showed the highest HCAL selectivity and the lowest COL selectivity (13.04%), indicating that pure Pt preferentially hydrogenates the C=C double bond.With the introduction of Fe, the selectivity of COL over PtxFey-Al2O3catalysts is significantly improved, indicating that the addition of Fe can inhibit the hydrogenation of C=C bonds.It is because the addition of Fe changes the electronic state of Pt, and the electrondeficient Pt is more conducive to the adsorption and hydrogenation of C=O bonds [20].The catalytic performance decreased with the further increasing of Pt content due to the excess Fe covers the active species of Pt on the surface.That is, the introduction of an appropriate amount of Fe atoms is beneficial to increase the conversion rate of CAL and the selectivity to COL.A comparison of the catalytic performance with some representative reported catalysts for the CAL hydrogenation is listed in Table S2 (Supporting information).Herein, the Pt3Fe-Al2O3catalyst showed significant advantages in conversion.
The reuse performance is an important factor in evaluating heterogeneous catalysts quality.So the catalyst stability of Pt3Fe-Al2O3was further investigated based on its excellent catalytic performance.As shown in Fig.2d, the high selectivity to COL can be basically remained at 76% with a slight decrease (only 6%) of the CAL conversion after five cycles of reaction.It shown that Pt3Fe-Al2O3catalyst has good stability and can be reused.
The fresh, used and cycled Pt3Fe-Al2O3catalysts were collected and further characterized by TEM.The analysis on HRTEM images(Fig.3) of the fresh, used and cycled Pt3Fe-Al2O3catalysts indicates that the crystal structure of the catalysts remains unchanged.The FFT patterns also prove that the crystal structure is stable.The HAADF-STEM images and the corresponding particle size statistics(Figs.3d, h, i) exhibit that Pt3Fe NPs are uniformly dispersed on the Al2O3supports and no particle growth or agglomeration was detected after reusing.
The structure of the fresh, used and cycled Pt3Fe-Al2O3catalysts were also investigated by XRD (Fig.S3 in Supporting information).The main diffraction peak position of all the Pt3Fe-Al2O3catalysts are almost same, indicating that the structure of the catalysts did not change.XPS are used to characterize the electronic properties of the catalysts, as shown in Fig.S4 (Supporting information).The Pt 4d binding energy of the used and cycled Pt3Fe-Al2O3catalysts(314.6 eV) is consistent with that of the fresh catalyst (314.6 eV),indicating that Pt electronic state of the reused (up to five runs)Pt3Fe-Al2O3catalyst remains unchanged.It proves that the electronic properties of the catalysts are stable after hydrogenation reaction.
IL-TEM method was employed to investigate the structural evolution and stability of the catalysts.The acquired TEM images of fresh, used and cycled Pt3Fe-Al2O3at identical location are shown in Fig.4 and Fig.S5 (Supporting information).The morphologies of Al2O3supports are almost same after used and cycled, and most of Pt3Fe NPs are stable on the Al2O3support after cycling process(marked by red circle).Only very few NPs are detached from the support (marked by yellow circle).The high stability of catalyst is attributed to the strong interaction between Pt3Fe NPs and support.
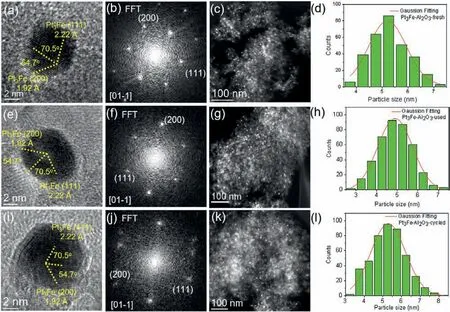
Fig.3.HRTEM (a, e, i) and corresponding FFT (b, f, j), HAADF-STEM images (c, g, k) and PSD histograms (d, h, l) of fresh (a–d), used (e–h) and cycled (i–l) Pt3Fe-Al2O3 catalyst, respectively.
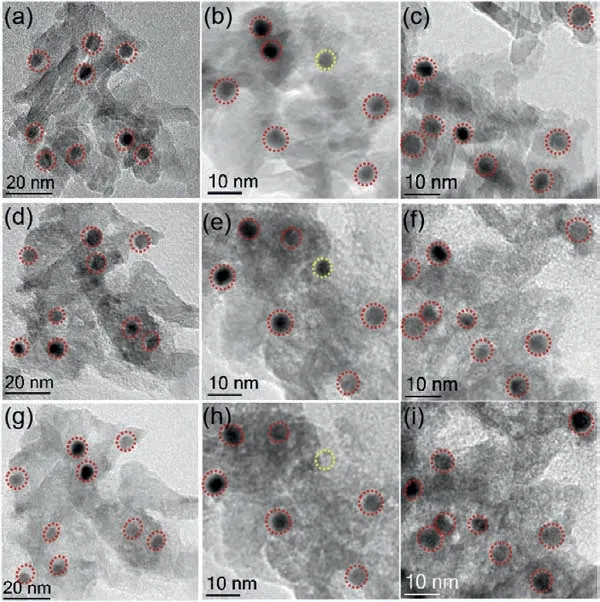
Fig.4.TEM images at identical location of fresh (a–c), used (d–f) and cycled (g–i)Pt3Fe-Al2O3 catalysts.
In summary, a series of PtxFey-Al2O3catalysts with varied Pt to Fe ratios were prepared by the incipient wetness impregnation method and tested in the chemoselective hydrogenation of CAL to evaluated their catalytic performance.Due to the electronic and surface properties of Pt can be adjusted by the introduction of Fe,the COL selectivity and CAL conversion increased dramatically with the decrease of Pt to Fe ratio.The Pt3Fe-Al2O3catalyst performs the highest catalytic activity with 77.2% COL selectivity at 96.6%CAL conversion.However, a further increase of the amount of Fe leads to the active sites on the surface are covered by excess Fe,which resulting in decrease in activity.Further characterization of the fresh, used and cycled catalysts revealed that the structure and electronic state of Pt3Fe NPs were stable.IL-TEM method was conducted to investigate the structural evolution of the catalyst at the identical location under real reaction conditions.The results show that the interaction between the Pt3Fe NPs and Al2O3support is strong.The stable structure can be maintained after reused,indicating that the prepared PtxFey-Al2O3catalysts are relatively stable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (No.21773269, 22072164, 21761132025, 51932005) and LiaoNing Revitalization Talents Program (No.XLYC1807175).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.077.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
