Doping transition metal in PdSeO3 atomic layers by aqueous cation exchange: A new doping protocol for a new 2D photocatalyst
Xiuming Zhang, Rongrong Pan, Tailei Hou, Shuping Zhang, Xiaodong Wan, Yuemei Li,Shan Liu, Jia Liu, Jiatao Zhang
Beijing Key Laboratory of Construction-Tailorable Advanced Functional Materials and Green Applications, Experimental Center of Advanced Materials, School of Materials Science & Engineering, Beijing Institute of Technology, Beijing 100081, China
ABSTRACT Elemental doping confined in atomically-thin 2D semiconductors offers a compelling strategy for constructing high performance photocatalysts.Although impressive progress has been achieved based on co-thermolysis method, the choices of dopants as well as semiconductor hosts are still quite limited to yield the elaborate photocatalyst with atomic-layer-confined doping defects, owing to the difficulty in balancing the reaction kinetics of different precursors.This study shows that the cation exchange reaction, which is dictated by the Pearson’s hard and soft acids and bases (HSAB) theory and allowed to proceed at mild temperatures, can be developed into a conceptually new protocol for engineering elemental doping confined in semiconductor atomic layers.To this aim, the two atomic layers of a new type of 2D photocatalyst PdSeO3 (PdSeO3 2ALs, 1.1 nm) are created by liquid exfoliation and exploited as a proof-of-concept prototype.It is demonstrated that the Mn(II) dopants with controlled concentrations can be incorporated into PdSeO3 2ALs via topological Mn2+-for-Pd2+ cation exchange performed in water/isopropanol solution at 30 °C.The resulting Mn-doped PdSeO3 2ALs present enhanced capacity for driving photocatalytic oxidation reactions in comparison with their undoped counterparts.The findings here suggest that the new route mediated by post synthetic cation exchange promises to give access to manifold 2D confined-doping photocatalysts, with little perturbations on the thickness, morphology, and crystal structure of the atomically-thin semiconductor hosts.
Keywords:2D Semiconductor Atomic layers Photocatalysis Doping Cation exchange
Given its significant impact on modulating the electronic structure of semiconductors, elemental doping has been widely demonstrated to be a viable tool for optimizing the functions of semiconductor-based photocatalysts [1–7].This is especially pertinent to the two-dimensional (2D) atomically-thin semiconductors,where the dopants are primarily exposed on the surface and therefore can inhibit the adverse effects associated with doping such as forming recombination centers in the interior of bulk semiconductors [8–12].In the last decade, the Rh-doped Ca-Nb-O [13], Codoped In2S3[14], Ru-doped TiO2[15], Pt or B-doped g-C3N42D layers [16],etc., have well exemplified their great advantages in photocatalytic process with expanded light absorption range, enhanced charge separation and promoted surface redox reaction.Despite much promise, however, the study in this area is hindered by the limited capability to construct such atomic-layerconfined doping systems.This is because currently, dopant incorporation into semiconductor 2D layers is primarily implemented by co-thermolysis of mixed precursors at high temperatures [17,18].The rigorous requirement on balancing the relative chemical reactivity of different precursors causes finite choice of available host semiconductors and the types of dopants.Besides, during the cothermolysis process, the existence of foreign dopants often significantly affects the nucleation and growth mechanism of the host semiconductors, detrimental to keeping the integrity of the atomic-layer structure or leading to unsuccessful doping [19,20].Therefore, there is an urgent need to develop alternative synthetic strategies for engineering doping in 2D semiconductor photocatalysts.
On the other hand, cation exchange has recently emerged as a powerful means to tailor semiconductor nanocrystalsviaversatile composition tuning with morphology retention.In fact, based on cation exchange, a new chemical route has been established for realizing controllable doping in semiconductor nanocrystals and quantum dots in order to regulate their optoelectronic or magnetic properties [21–25].However, these progresses are mainly restricted to chalcogenide (II-VI) and nitride (III-V) semiconductors.Although efforts have been devoted to expanding the applicability of cation exchange, to date such synthetic strategy has achieved limited success in creation of doped oxides and other types of semiconductors (such as doped oxysulfides and oxyselenides).Moreover, cation exchange reactions are generally performed in organic phase involving the use of ligands with long alkyl tails like oleylamine, oleic acid, dodecanethiol,etc.[19,24,26-28].Thus resulting semiconductor nanocrystals confront obstacles to forming uniform dispersion in aqueous solution and to catalyzing chemical reactions because of blocked surface sites [29].As a consequence,cation exchange, though has manifested great potentials in creation of elaborate doped systems toward applications in displays and solid-state lighting, to the best of our knowledge, has seldom been exploited to manipulate elemental doping in photocatalyst materials.
Herein, we demonstrate that aqueous cation exchange can constitute a facile and reliable way for modulating cation doping confined in semiconductor atomic layers to ameliorate their photocatalytic behaviors.As a proof-of-concept prototype, PdSeO3atomic layers, which have been proposed to be a highly promising photocatalyst for overall water splitting based on density functional theory (DFT) calculations [30], were fabricated and employed as the host materials.Our results demonstrated that different amount of Mn(II) dopants can be incorporated into PdSeO3atomic layers(thickness of 1.1 nm, corresponding to two atomic layers)viacation exchange performed in aqueous solution at mild temperature (30°C).The obtained Mn-doped PdSeO3photocatalyst well retained their atomic layer morphology and high crystallinity.With increasing the Mn dopant concentration, the valance band (VB) maximum of PdSeO3atomic layers was gradually down-shifted, indicative of the enhanced oxidation capability for photogenerated holes.Furthermore, we found that the presence of Mn dopants in PdSeO3atomic layers lead to accelerated organics degradation rate under visible light irradiation, contributed by the promoted generation of reactive oxygen species (ROS) such as hydroxyl radicals (•OH).On the basis of these findings, we envision that cation exchange can be developed into an important synthetic approach for elaborate construction and manipulation of doping defects in 2D photocatalysts.
In this work, as illustrated in Fig.1a, we demonstrate a feasible cation exchange strategy for fabricating the 2D PdSeO3atomic layers doped with transition-metal Mn(II) ions, where the morphology and crystal structure of pristine PdSeO3are almost unaffected during the ion exchange process.PdSeO3is a new emerging 2D photocatalyst that has been theoretically predicted to be suitable for splitting water into H2an O2under visible light irradiation.In our prior research, we have conducted the first experimental study on the ultrathin PdSeO3nanosheets that were synthesized through the quaternary ammonium intercalation-assisted electrochemical exfoliation method [31].The resultant PdSeO3nanosheets possessed a thickness of 4–5 nm, corresponding to the stacking of 7 atomic layers of PdSeO3slab.Although such ultrathin 2D photocatalyst showed excellent photocatalytic hydrogen evolution activity, it was failed to drive the water oxidation half reaction, at variance with the DFT predictions for PdSeO3monolayers.Therefore,to optimize the photocatalytic function of PdSeO3photocatalyst, it is desirable to further reduce the layer thickness and exploit the doping strategy to enhance its oxidation ability.
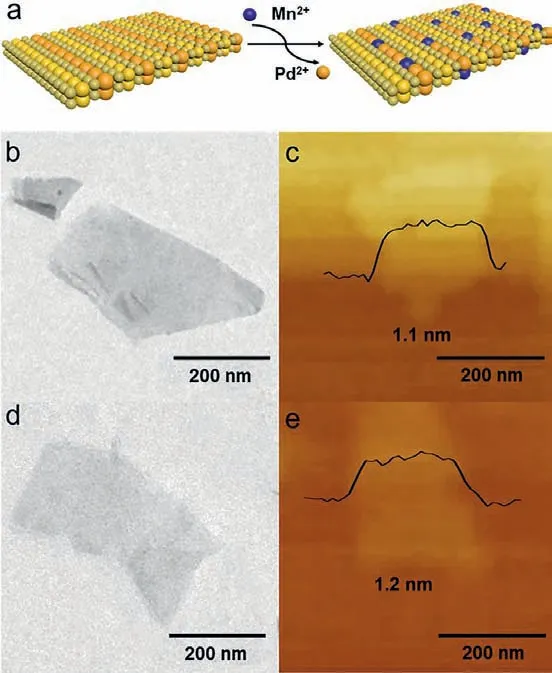
Fig.1.(a) Schematic illustration of doping transition metal ions Mn2+ into PdSeO3 2ALs through cation exchange reaction.(b) TEM image and (c) AFM image of PdSeO3 2ALs; (d) TEM image and (e) AFM image of Mn(3)-PdSeO3 2ALs.
To this end, one of our aims in this work is to develop an alternative synthetic approach for creation of the PdSeO3atomic layers with even reduced thickness.According to previous report,as the surface tension of graphite was well matched with the isopropanol/water (1:1) solution, high quality and yield graphene could be successfully synthesized in isopropanol/water (1:1) solution under ultrasonic treatment [32].In view of the similar cleavage energy of PdSeO3monolayer to that of graphene [30], here sonication-assisted exfoliation in isopropanol/water (1:1) solution was applied to exfoliate the bulk PdSeO3crystals into atomic layers.This generated a light-orange-colored solution, which was highly stable and exhibited a distinct Tyndall effect.No precipitations were observed even after being stored for 2 weeks under ambient conditions, reflecting the well retained colloidal state of the as-exfoliated PdSeO3(Fig.S1 in Supporting information).As shown in Fig.1b, transmission electron microscopic (TEM) image of the as-exfoliated PdSeO3indicates its uniform freestanding and large-area sheet-like morphology with a lateral size ofca.200 nm.The atomic force microscopic (AFM) image in Fig.1c discloses that the resulting PdSeO3layer possesses an average thickness of 1.1 nm, which is consistent with two atomic layers (2ALs)of PdSeO3slab along the z direction.The above results demonstrate that the PdSeO32ALs can be readily accessed by sonicationassisted exfoliation in proper solvent.
Based on the Pearson’s hard and soft acids and bases (HSAB)theory, the coordination ability of tributylphosphine (TBP, soft base) with Pd2+(soft acid, hardness of 6.75) is stronger than that of Mn2+(hard acid, hardness of 9.02) [33–35].Therefore, when TBP ligands and Mn2+cations are both introduced into the colloidal suspension of PdSeO32ALs, a certain amount of Pd2+would be dragged out from the lattice of PdSeO3by the TBP ligand and replaced with the guest Mn2+ions.In view of this, we performed the Mn2+-for-Pd2+cation exchange reaction on PdSeO32ALs in isopropanol/water (1:1) mixture at 30 °C.The resultant samples are denoted as Mn(X)-PdSeO32ALs, where X (X% = 0.75%, 1.5%, 3%and 6%) represents the theoretical percentage of Mn/Pd molar ratio during synthesis.As shown in Fig.S2 (Supporting information), the concentration of Mn dopants in Mn(X)-PdSeO32ALs was measured at 0.6%, 0.75%, 1.4% and 1.5% when increasing the Mn/Pd molar ratio from 0.75% to 1.5%, 3% and 6%, correspondingly.
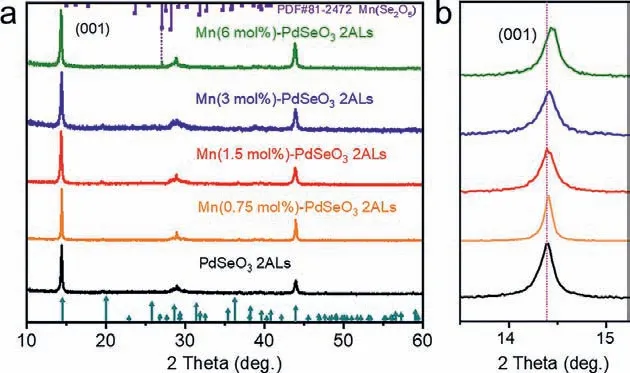
Fig.2.(a) XRD patterns of pristine PdSeO3 2ALs and Mn(X)-PdSeO3 2ALs with different dopant concentrations.(b) The detailed analysis of XRD patterns shows gradual shift of the (001) diffraction peak after cation exchange reaction with increased concentration of Mn2+ dopants.
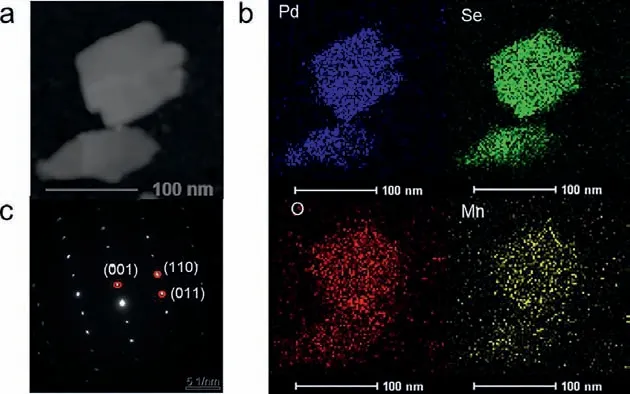
Fig.3.(a) HAADF-STEM image of an individual sheet of Mn(3)-PdSeO3 2ALs, (b) the corresponding EDS elemental maps for Pd, Se, O, and Mn, and (c) the corresponding SAED pattern.
As indicated by the TEM image of Mn(3)-PdSeO32ALs in Fig.1d, the cation exchange reaction dose not obviously affect the morphology of the sample.The corresponding AFM image in Fig.1e reveals that the ultrathin feature of the exfoliated PdSeO32ALs is well kept after the cation exchange process (thickness of 1.2 nm for Mn(3)-PdSeO32ALs).It was found that the suspension of Mn(X)-PdSeO32ALs was highly stable over several days, inferring the colloidal state of the sample was little perturbed under the exchange reaction condition.The X-ray diffraction (XRD) patterns of PdSeO32ALs before and after cation exchange are presented in Fig.2a.One can see that all the samples are highly crystallined showing diffraction peaks coincided well with the crystallographic data reported for PdSeO3, expect for Mn(6)-PdSeO32ALs which additionally exhibits the characteristic peak of MnSe2O5(JCPDS card No.81–2472, thus in the following studies this sample will not be further discussed due to the presence of impurity).Careful examination of the XRD patterns further uncovers that the (001) peaks of Mn(X)-PdSeO32ALs are monotonically shifted to higher angles with increasing the dopant concentration (Fig.2b).This is consistent with lattice contraction caused by the substitution of Pd2+by Mn2+with smaller ionic radius.The high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and the associated energy-dispersive Xray spectroscopy (EDX) elemental analyses demonstrate that the elements of Pd, Se, O, and Mn are uniformly distributed within the Mn(3)-PdSeO32ALs atomic layers (Figs.3a and b).From Fig.3c,the selected area electron diffraction (SAED) pattern collected from an individual nanosheet of Mn(3)-PdSeO32ALs clearly reveals its single crystallined nature, in good agreement with the XRD result.
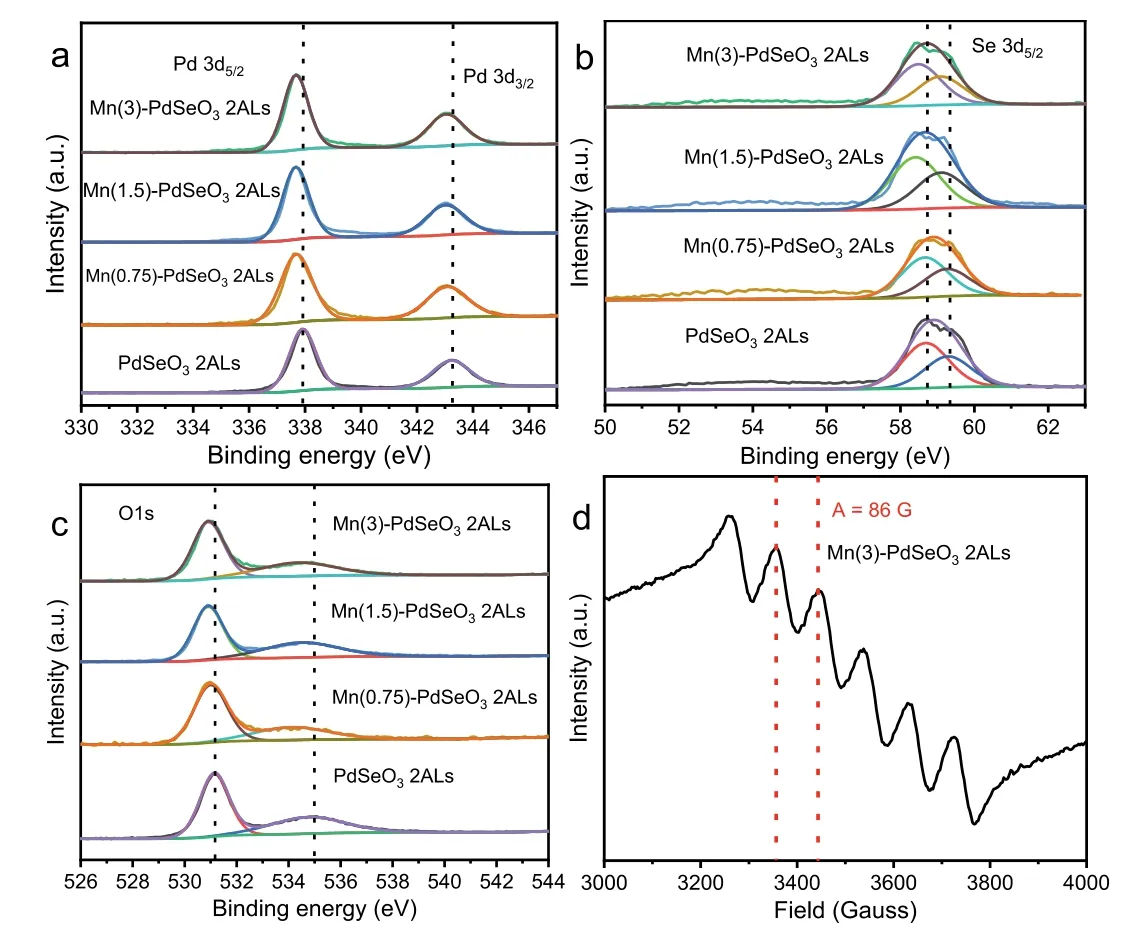
Fig.4.High-resolution (a) Pd 3d, (b) Se 3d, and (c) O 1s XPS spectra for PdSeO3 2ALs and Mn(X)-PdSeO3 2ALs.(d) X-band EPR spectrum of Mn(3)-PdSeO3 2ALs at room temperature.
The chemical state of the elements in PdSeO3and Mn(X)-PdSeO32ALs was analyzed by X-ray photoelectron spectroscopy(XPS).For pristine PdSeO32ALs, the high-resolution XPS spectrum of Pd 3d (Fig.4a) shows two bands at 343.2 and 337.8 eV,corresponding to Pd2+3d3/2and Pd2+3d5/2, respectively [28].It can be seen obviously that after the cation exchange reaction,the binding energies of Pd 3d are decreased with the increment of Mn dopant concentration, which can be well rationalized by the changed electronic environment as a result of the incorporation of Mn into PdSeO3[36–39].Similar phenomena are discernible for the high-resolution XPS spectra of Se 3d and O 1s(Figs.4b and c), where the characteristic peaks assigned to Se4+and O2−in Mn(X)-PdSeO32ALs are shifted to lower binding energies in comparison with pristine PdSeO32ALs.These observations strongly suggest the occurrence of cations substitution in PdSeO32ALs after the exchange reaction.More importantly, we exploited the electron paramagnetic resonance (EPR) measurement,which is a vital tool for detecting the valence state and distribution of transition metals in nanomaterials, to provide direct evidence for the existence of Mn2+dopants in Mn(X)-PdSeO32ALs.As shown in Fig.4d, the EPR signal for Mn(3)-PdSeO32ALs manifests six equally spaced lines showing an equal intensity (with hyperfine coupling constant, atA= 86 G).Such observation is exactly the same as those reported for Mn-doped nanocrystals,which unambiguously verifies the residence of Mn2+dopants inside the atomically-thin PdSeO3host with a homogeneous distribution (in other words, the Mn2+species are not adsorbed on the surface of PdSeO32ALs, and are not clustered together)[40,41].
It is well-known that doping would affect the band structure of semiconductors and consequently influences their photocatalytic activities.UV–vis absorption spectra in Fig.5a demonstrate that the band gap of the Mn(X)-PdSeO32ALs samples are all around 2.59 eV, which is slightly smaller than that of pristine PdSeO32ALs(2.71 eV), indicative of the enhanced capacity for harvesting visible light [42].Besides, the valence band (VB) position of the samples was determined by the XPS valence band spectra as shown in Fig.5b.The pristine PdSeO32ALs display a VB maximum edge at 1.32 V, while for the Mn(X)-PdSeO32ALs samples, their VB maximum edges are gradually down-shifted with increasing the concentration of Mn2+dopants.These results suggest that the introduction of Mn2+into PdSeO32ALs is beneficial for improving the oxidation ability of the photogenerated holes [43].The band structures for each sample are depicted in Fig.5c.Especially, for Mn(3)-PdSeO32ALs, its VB and conduction band (CB) edges are at 1.64 and −0.95 V, respectively, which is thermodynamically capable of driving overall water splitting reaction.
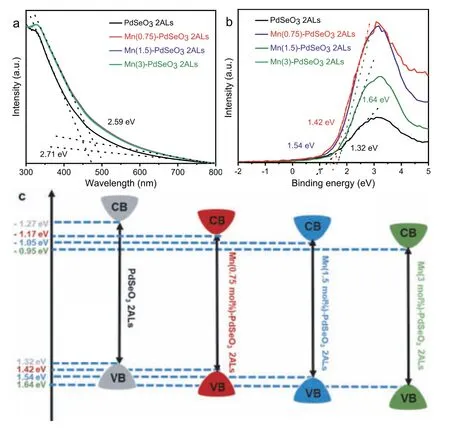
Fig.5.(a) UV–vis absorption spectra of PdSeO3 2ALs and Mn(X)-PdSeO3 2ALs.(b)Valence-band XPS spectra of PdSeO3 2ALs and Mn(X)-PdSeO3 2AL.(c) Schematic illustration of the band structures determined for PdSeO3 2ALs and Mn(X)-PdSeO3 2ALs.
Encouraged by the theoretical studies predicting that PdSeO3layers can function as a promising water splitting photocatalyst[30], we first conducted photocatalytic overall water splitting reaction in pure water under visible light irradiation.However, it was found that no H2or O2gasses were evolved from the reaction system for both PdSeO3and Mn(3)-PdSeO32ALs (Fig.S3 in Supporting information).Even with the assistance of electron sacrificial reagents like AgNO3and NaIO3, these two samples still cannot generate O2viaphotocatalytic water oxidation reaction (Fig.S4 in Supporting information).We further carried out electrocatalytic oxygen evolution reaction (OER) in 1 mol/L KOH electrolyte to evaluate the intrinsic water oxidation ability of the samples.As indicated by the polarization curves in Fig.S5 (Supporting information), the pristine PdSeO32ALs require an overpotential of 1.38 V (vs.RHE) at the current density of 10 mA/cm2, while Mn(3)-PdSeO32ALs can reach the current density of 10 mA/cm2with an overpotential of 1.27 V (vs.RHE).Such large overpotential values are close to that reported for g-C3N4of 1.56 V [44],unfavorable for the proceeding of water oxidation half reaction.However, the reduced overpotential (110 mV) observed for Mn(3)-PdSeO32ALs relative to PdSeO32ALs evidences that doping of Mn2+into PdSeO3indeed can improve the oxidation ability of the catalyst.
Photocatalytic degradation of organic dyes or toxic pollutants is of great significance in environmental pollutant treatment [45,46].Metronidazole (MTZ) is a commonly used antibiotic drug, however its entry into water can cause great damage to environment and ultimately endanger human health [47,48].In this study MTZ was employed as the probe molecule to evaluate the performance of PdSeO3and Mn(3)-PdSeO32ALs in photocatalytic degradation reaction under visible light irradiation (λ >420 nm).As shown in Figs.6a and b, during the reaction the characteristic absorption peak of MTZ at 318 nm was gradually disappeared and blueshifted, indicating the degradation of MTZ into new substances with the presence of catalysts under visible light.Fig.6c shows the comparison of the photocatalytic degradation curves for the two samples (the blank measurement with absence of catalyst is also exhibited as a reference).After 120 min of irradiation, the removal ratio of MTZ was only 45% for PdSeO32ALs.However,Mn(3)-PdSeO32ALs exhibited significantly enhanced photocatalytic activity with removal ratio of MTZ up to 85% in the same time period.The photocatalytic decomposition kinetics of MTZ are further plotted as presented in Fig.6d, which corroborate that the photocatalytic processes are all well fitted with the first-order reaction dynamic model (Eq.1):

whereC0is the MTZ concentration after 30 min of stirring in dark,whileCandkare the temporal concentration of MTZ at reaction timetand the degradation rate constant, respectively.For Mn(3)-PdSeO32ALs, its kinetic constant k was up to 0.016 min−1, which was about 2 times higher than that of PdSeO32ALs (0.007 min−1),clearly substantiating the beneficial effect brought about by Mn2+doping into PdSeO32ALs.
The hydroxyl radicals (•OH) and superoxide radicals (•O2−) as highly reactive oxygen species (ROS) play an important role in photocatalytic degradation reactions.In order to investigate the photocatalytic degradation mechanism, the spin-trapping electron spin resonance (ESR) measurements were conducted to probe the generation of•OH and•O2−species during the photocatalytic reaction.As displayed in Fig.7a, no signals for•OH were detected under dark conditions.However, when the sample was irradiated with visible light, the four characteristic peaks for DMPO-•OH were detected immediately for both PdSeO3and Mn(3)-PdSeO32ALs.The signal intensities of Mn(3)-PdSeO32ALs were obviously much stronger than those of PdSeO32ALs, suggesting that a higher amount of•OH radicals were produced on the surface of Mn(3)-PdSeO32ALs.This is in good accordance with the conclusion that Mn2+doping can markedly enhance the oxidation ability of PdSeO32ALs.With regard to the•O2−radicals, similar observations were found indicative of the presence of•O2−radicals during photocatalytic reaction and their higher production efficiency on Mn(3)-PdSeO32ALs (Fig.7b).The promoted generation of both•OH and•O2−species on Mn(3)-PdSeO32ALs can largely contribute to boosting the degradation process of MTZ under visible light.Besides, the photoelectrochemical (PEC) currents and electrochemical impedance spectroscopy (EIS) Nyquist plots were also compared for PdSeO3and Mn(3)-PdSeO32ALs (Figs.7c and d).The results demonstrate that doping of Mn2+can improve separation of photogenerated charge carriers and facilitate their transfer through the solid/liquid interface, additionally conducive to photocatalytic degradation of MTZ.
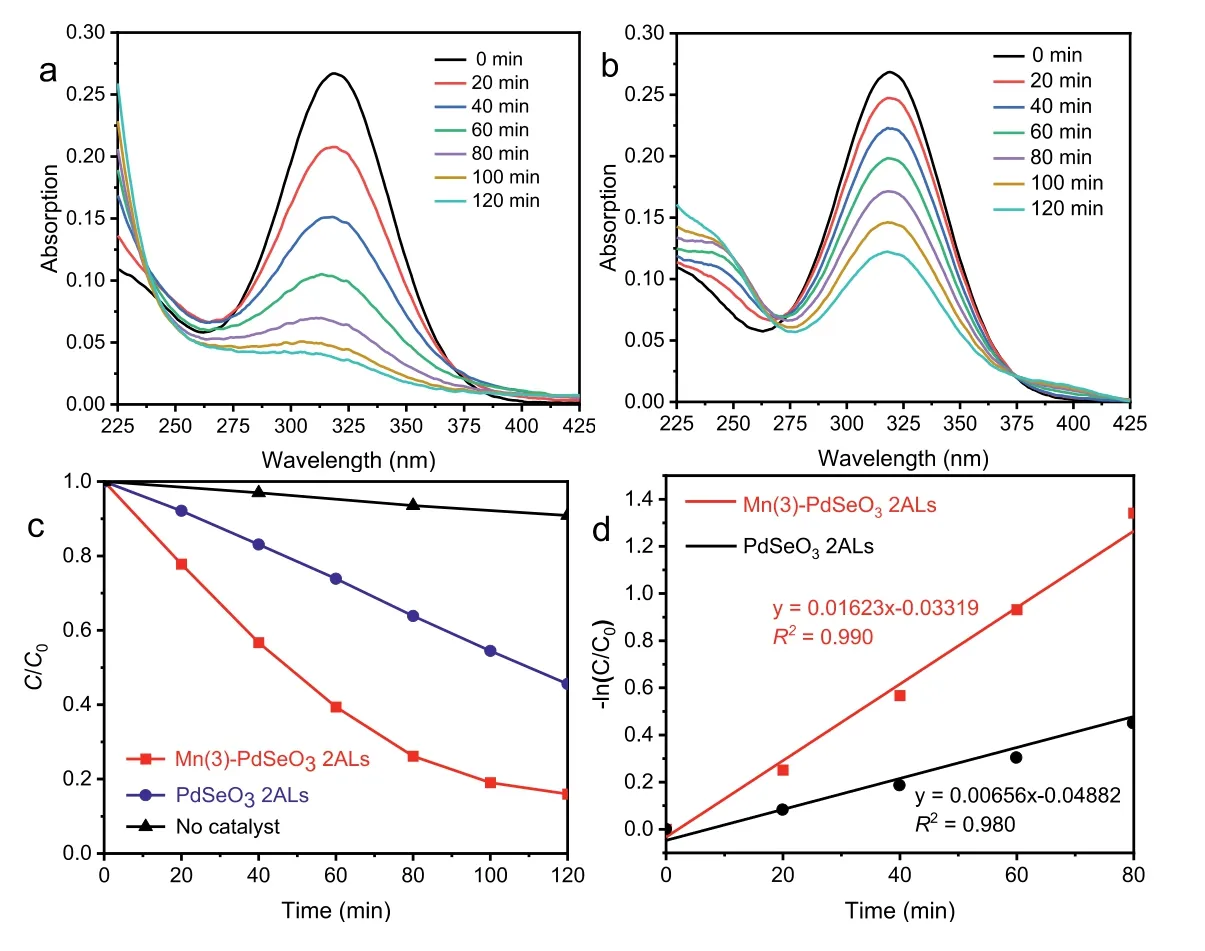
Fig.6.The evolution of UV–vis absorption spectra of MTZ solution under visible light irradiation in presence of (a) Mn(3)-PdSeO3 2ALs and (b) PdSeO3 2ALs.(c) Photocatalytic degradation curves of MTZ with (blue line for PdSeO3 2ALs; red line for Mn(3)-PdSeO3 2ALs) or without catalysts (black line) under visible light irradiation.(d) The kinetics of MTZ photocatalytic degradation over PdSeO3 2ALs and Mn(3)-PdSeO3 2ALs.
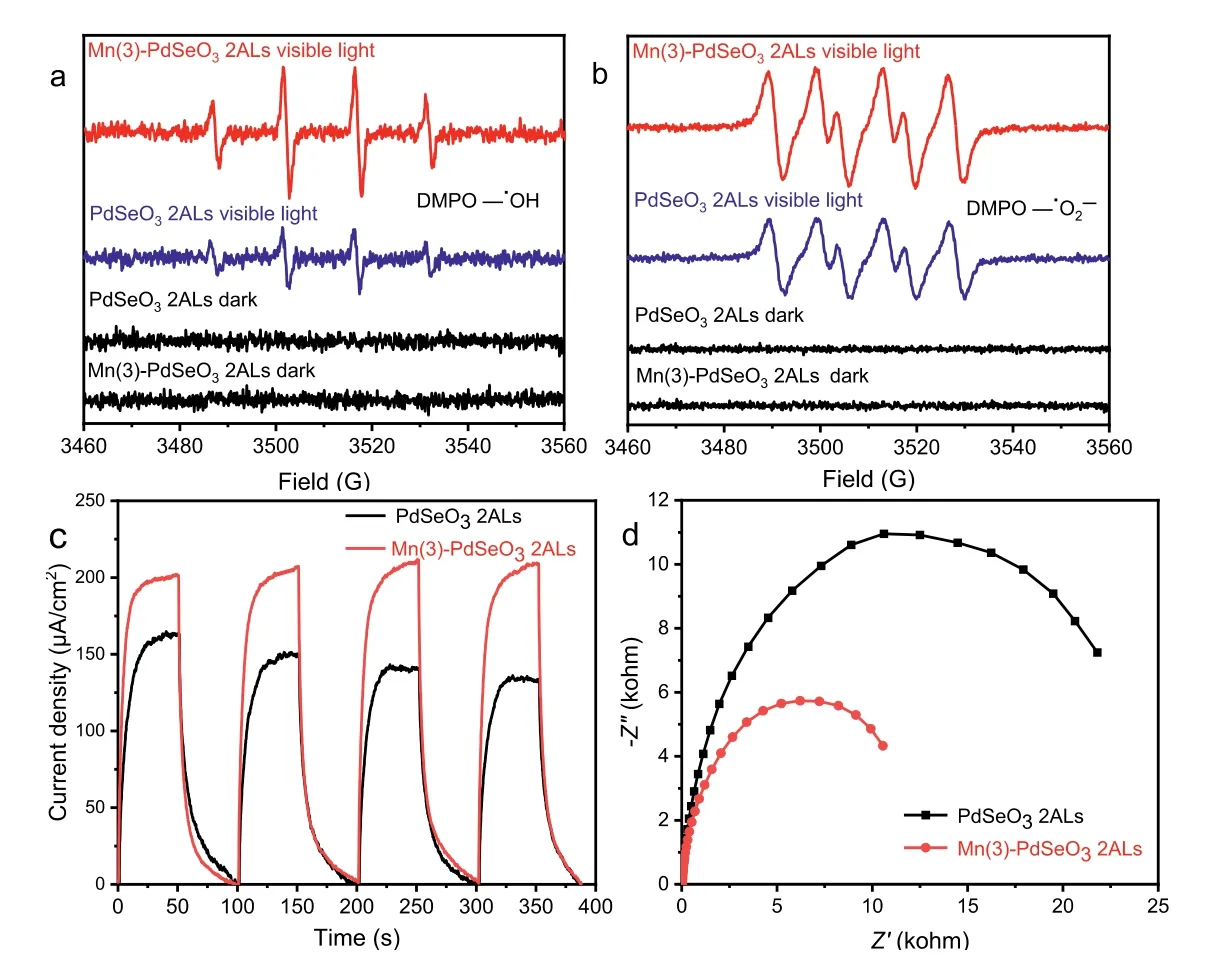
Fig.7.DMPO spin-trapping ESR spectra for detecting the in situ formed (a) •OH and (b) •O2−radicals over PdSeO3 2ALs and Mn(3)-PdSeO3 2ALs before and after irradiation by visible light for 5 min.(c) Photocurrent density-time curves and (d) EIS Nyquist plots for PdSeO3 2ALs and Mn(3)-PdSeO3 2ALs.
In summary, two atomic layers of PdSeO3are firstly created through a facile exfoliation method by matching the surface tension components between material and solvent.The resultant PdSeO32ALs afford a new prototype for promising 2D photocatalyst and based on which, we demonstrate that aqueous cation exchange at ambient temperature can be utilized to realize confined doping of Mn(II) into the atomically-thin 2D photocatalysts with well-preserved thickness, morphology and crystal structure.We further show that programmable enhancement in the oxidation ability of Mn-doped PdSeO32ALs is achievable by raising the Mn(II) dopant concentration, in view of the downward-shifted VB edges, the reduced OER overpotentials and the promoted generation of•OH radicals under visible light irradiation.These advantages make the Mn-doped PdSeO32ALs display a much higher rate for photocatalytic degradation of metronidazole in comparison with the undoped PdSeO32ALs.Our results strongly indicate that distinct from the co-thermolysis processes, the HSAB theorydirected cation exchange can offer a conceptually new protocol for manipulating elemental doping confined in atomic-layered 2D photocatalysts.This may open up new possibilities for creating a large variety of atomic-layer-confined doping photocatalyst systems and help to deepen the insights into the role of atomic structure of doping defects on photocatalytic properties.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.52072035, 51631001, 21801015, 51702016,51902023, 51872030), Joint R&D Plan of Hongkong, Macao, Taiwan and Beijing (No.Z191100001619002), the Fundamental Research Funds for the Central Universities (No.2017CX01003), and the Beijing Institute of Technology Research Fund Program for Young Scholars.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.10.088.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
