Extending aromatic acids on TiO2 for cooperative photocatalysis with triethylamine: Violet light-induced selective aerobic oxidation of sulfides
Hui Li, Xia Li, Jun Zhou, Wenlong Sheng, Xianjun Lang
Sauvage Center for Molecular Sciences, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan 430072, China
ABSTRACT Designing visible light photocatalysts with a metal oxide semiconductor as the starting material could expand a new horizon for the conversion and storage of solar energy.Here, the benchmark photocatalyst TiO2 was used to pursue this goal by anchoring aromatic acids.Extending the aromatic acid was strategically deployed to design TiO2 complexes with violet light-induced selective aerobic oxidation of sulfide as the probe reaction.With benzoic acid (BA) as the initial molecule, horizontally extending one or two benzene rings furnishes 2-naphthoic acid (2-NA) and 2-anthracene acid (2-AA).Moreover, triethylamine(TEA), an electron transfer mediator, was introduced to maintain the integrity of the anchored aromatic acids.Notably, there was a direct correlation between the π-conjugation of aromatic acid ligand and the selective aerobic oxidation of sulfides.Among the three aromatic acids, 2-AA delivered the best result over TiO2 due to the most extensive π-conjugated system.Ultimately, violet light-induced selective aerobic oxidation of sulfides into corresponding sulfoxides was conveniently realized by cooperative photocatalysis of 2-AA-TiO2 with 10 mol% of TEA.This work affords an extending strategy for designing the next-generation ligands for semiconductors to expand visible light-induced selective reactions.
Keywords:Aromatic acid Extending π-conjugation Complex photocatalyst Oxidation of sulfides Cooperative photocatalysis
The development of solar energy conversion and storage is essential for alleviating the looming energy and environmental crisis triggered by the global population explosion and unprecedented economic growth [1–4].However, the intermittent and diffuse nature of solar energy leave ample space to further developments.Increasingly, semiconductor photocatalysis can expand a new horizon for harnessing solar energy [5,6].Whereas visible light utilization rate in the solar spectrum still imposes restrictions on the available scope of transition metal oxides on Earth [7,8].For instance, TiO2can only absorb approximately 5% of natural sunlight owing to its wide bandgaps (3.0 eV for rutile, 3.2 eV for anatase)[9,10].Nevertheless, TiO2turns out to be a stepping stone to new photocatalysts that close the energy gap [11,12].
Particularly, surface-modified TiO2with carboxyl, hydroxyl containing ligands such as ethylenediaminetetraacetic acid [13,14], salicylic acid [15,16], catechol [17,18], glucose [19,20], and cyclodextrin [21,22] have aroused great interest on account of their high absorption of visible light and low recombination of electron-hole to achieve photocatalytic reactions efficiently.Besides, the properties of the TiO2complexes, such as photochemical stability, optical response, and binding mode, are closely associated with the structure of ligands [23–25].Generally, aromatic organic acids containing other functional groups like hydroxyl can form more stable surface complexes than aromatic monocarboxylic acids [26].Nevertheless, theπ-conjugation of aromatic monocarboxylic acid ligands also strongly affects the complex’s stability, surface binding,and charge transfer [27,28].Therefore, one can embrace the concept of molecular design to select redox-active ligand based on a previously disclosed strategy [29].
Hereby, with benzoic acid (BA) as the initial molecule, horizontally extending one or two benzene rings furnish 2-naphthoic acid (2-NA) and 2-anthracene acid (2-AA) which could be anchored onto TiO2for the photocatalytic selective oxidation of sulfides.It is worth noting that the selective oxidation of organic sulfides has recently attracted much interest [30,31] because the resultant sulfoxides are vital intermediates for agrochemicals, pharmaceuticals, and valuable fine chemicals [32–36].Meanwhile, TEA, an electron transfer mediator [37], maintains the integrity of these surface ligands on TiO2.Additionally, TEA also stands out for its low boiling point in consideration of the subsequent separation and purification of sulfoxides [38].Hence, cooperative photocatalysis of 2-AA-TiO2with TEA in solution is establishedviacollisional electron transfer.
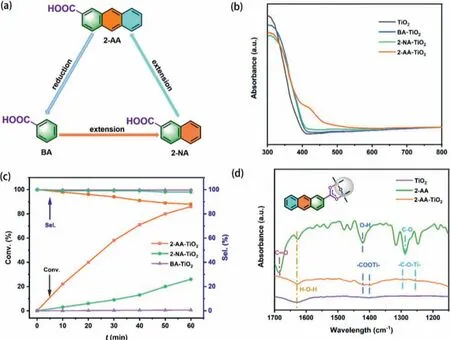
Fig.1.(a) Horizontally extending the benzene ring of BA obtains 2-NA and 2-AA.(b) UV–vis DRS of TiO2, BA-TiO2, 2-NA-TiO2, 2-AA-TiO2.(c) Violet light-induced selective aerobic oxidation of phenyl methyl sulfide over BA-TiO2, 2-NA-TiO2, 2-AA-TiO2.Reaction conditions: CH3OH (1 mL), ligand (1.2 × 10−3 mmol), TiO2 (40 mg), TEA (0.03 mmol),phenyl methyl sulfide (0.35 mmol), aerial O2, violet LEDs (3 W × 4).(d) FTIR spectra of TiO2, 2-AA, and 2-AA-TiO2.
In general, the aromatic monocarboxylic acid-modified TiO2complex is implemented by the acidolysis and exchange of the surface hydroxyl groups of TiO2with carboxylate anions, forming the RCOO-Ti bond [26].However, theπ-conjugated system in aromatic monocarboxylic acids can greatly affect the electrostatic attraction of carboxylate anions to the titanol group, thus affecting the stability and photocatalytic activity of the ligand-modified TiO2.With BA as the initial molecule, horizontally extending theπ-conjugation affords two monocarboxylic acids ligands, namely 2-NA and 2-AA(Fig.1a).In this work, Aeroxide P25 TiO2was adopted as the starting metal oxide to anchor BA, 2-NA, and 2-AA.
Based on the ultraviolet (UV)–visible absorption spectra (Fig.S1a in Supporting information), it can be found that compared with BA, there is no absorption response to visible light completely in 2-NA, while 2-AA can harvest a band of visible light at approximately 400–420 nm.When they were assigned as redox-active ligands for the surface of TiO2, UV–visible diffuse reflectance spectra (UV–vis DRS) (Fig.1b) show that the difference between BATiO2, 2-NA-TiO2, and pristine TiO2is relatively small.Conversely,the difference between 2-AA-TiO2and pristine TiO2is rather obvious.Compared with TiO2,which can only absorb UV light, the response range of 2-AA-TiO2has an obvious redshift.In addition,combining with Fig.S1b (Supporting information), Fig.1b reveals that the light-emitting spectrum of the violet LED overlaps with the absorbance of 2-AA-TiO2.Despite the loading of these ligands,there was no change in the powder X-ray diffraction (PXRD) peaks of rutile and anatase TiO2(Fig.S1c in Supporting information).
Subsequently, these complexes were applied to the violet lightinduced selective oxidation of phenyl methyl sulfide (Fig.1c).It is intriguing to find a correlation between the conversions of phenyl methyl sulfide and the structure of these monocarboxylic acid ligands.With only one benzene ring, the photocatalytic activity of BA-TiO2was almost zero under the standard conditions.With the augment ofπ-conjugation, the violet light-induced conversion of phenyl methyl sulfide increased from 26% over 2-NA-TiO2to 86%over 2-AA-TiO2for 1 h, which was attributed to the more stabilized binding of carboxyl from 2-AA with the surface hydroxyl group of TiO2.Compared with anatase TiO2(ST-01) or rutile TiO2with much higher specific surface areas, Aeroxide P25 TiO2with 2-AA was the best complex photocatalyst (Table S1 in Supporting information).
Next, the chemical states of Ti, O, and C in 2-AA-TiO2were measured by X-ray photoelectron spectroscopy (XPS) (Fig.S2 in Supporting information).The Ti 2p1/2(464.4 eV) and Ti 2p3/2(458.5 eV) peaks conform to the results of Ti 2p (Fig.S2a), which are consistent with a previous investigation [37].Meanwhile, in Fig.S2b, the XPS data of O 1s can be split into O−Ti at about 529.6 eV,O=C at about 530.9 eV, O−C at about 531.8 eV, which might be due to the presence of excessive 2-AA on the surface of the 2-AATiO2.According to the C 1s XPS (Fig.S2c), the carboxylate group from 2-AA successfully combined with the titanol group of TiO2to generate the 2-AA-TiO2complex.
On the other hand, the formation of 2-AA-TiO2complex was explored by Fourier-transform infrared (FTIR) spectroscopy.In Fig.1d,the two broad peaks at 1423 cm−1and 1403 cm−1were attributed to the stretching vibrations of –COOTi– group.The peak (1423 cm−1) in the FTIR spectrum of 2-AA corresponded to the symmetric stretching of the hydroxy group in a carboxyl group.Therefore, it is reasonable to accept that 2-AA is successfully adsorbed on the surface of TiO2.Nevertheless, a more profound analysis is required to further determine whether the binding mode of 2-AATiO2is through bidentate or monodentateviathe carboxylate ligand.The FTIR spectra of pristine TiO2and 2-AA were also presented in Fig.1d.The primary signal peak of the carboxylic group in 2-AA are analyzed as following: 1683 cm−1is the stretching vibration peak of C=O; the signal peaks at 1582 cm−1, 1481 cm−1,and 1461 cm−1are assigned to C=C skeleton stretching mode;the signal peak at 1423 cm−1is the vibration peak of O−H in carboxylate group; the strong peak at 1290 cm−1is the stretching vibration peak of C−O [39,40].If the 2-AA-TiO2possesses the monodentate mode, the FTIR spectrum of 2-AA-TiO2should show the vibration peak of C=O.However, the actuality that the peak of C=O was vacant in the curve, revealed that 2-AA-TiO2was more tend to the bidentate binding mode.Meanwhile, the peak of−C−O−Ti−presented in the FTIR spectrum of 2-AA-TiO2and the slightly shifted peak position further manifested that the two oxygen atoms in the −COO−connected to the adjacent surface Ti sites respectively and formed the bridging bidentate mode.Furthermore,the FTIR spectra of BA-TiO2and 2-NA-TiO2are also shown in Fig.S3a (Supporting information).
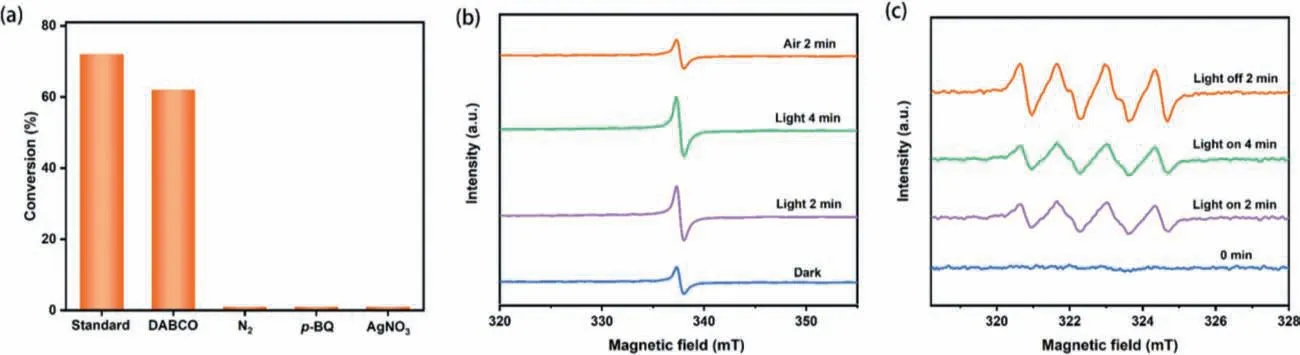
Fig.2.(a) Quenching experiments to identify the ROS for the violet light-induced selective aerobic oxidation of phenyl methyl sulfide.Standard reaction conditions: CH3OH(1 mL), 2-AA (1.2 × 10−3 mmol), TiO2 (40 mg), TEA (0.03 mmol), phenyl methyl sulfide (0.35 mmol), aerial O2, violet LEDs (3 W × 4), 40 min.The EPR signals recoded during the violet light-induced selective aerobic oxidation of phenyl methyl sulfide over the 2-AA-TiO2 photocatalyst, (b) eCB−of 2-AA-TiO2 and (c) O2•– captured by DMPO.
After confirming 2-AA-TiO2complex, various control experiments were conducted for the selective aerobic oxidation of sulfide into sulfoxide.It can be found that photocatalyst synthesizedin situis more effective than the pre-prepared 2-AA-TiO2for the selective aerobic oxidation of phenyl methyl sulfide to sulfoxide (Table S2 in Supporting information).Meanwhile, kinetic studies have shown that there is no induction time when aromatic acids modified TiO2wasin situformed (Fig.S3b in Supporting information).Hereafter, the exploration of the effect of different wavelengths of LEDs is stated in Fig.S4a (Supporting information), proposing that the corresponding conversion is commensurate to the UV–vis DRS of 2-AA-TiO2.In Fig.S4b (Supporting information), it was found that very few sulfide were transformed into the corresponding sulfoxide with 2-AA.When 2-AA was loaded onto the surface of TiO2to form a complex photocatalyst, the conversion of phenyl methyl sulfide was significantly improved (Fig.S4b).Synchronously, the recycling tests were implemented to testify the durability of the 2-AA-TiO2photocatalyst (Fig.S4c in Supporting information).The recovered 2-AA-TiO2was also reused twice with the temperate refurbishing of 2-AA, implying its superior endurance.
Importantly, all the components of the photocatalytic system were indispensable based on the thorough control experiments.Further, the effect of the amounts of aromatic acids was explored(Fig.S5a in Supporting information).The conversion of phenyl methyl sulfide sharply increased once 2-AA was added.However,with the further increase of the dosage of 2-AA, the conversion of phenyl methyl sulfide increased slightly.With the amount of TiO2enhanced, the yield of phenyl methyl sulfoxide advanced gradually and the selectivity of the product declined slightly (Fig.S5b in Supporting information).TEA, the electron transfer mediator, exhibited a considerable effect on the violet light-induced selective aerobic oxidation of sulfide over the 2-AA-TiO2photocatalyst (Fig.S5c in Supporting information).Furthermore, when TEA was replaced by trimethylamine, the conversion of phenyl methyl sulfoxide decreased slightly (Table S3 in Supporting information).CH3OH, as a redox-active solvent, plays an important role in the formation of phenyl methyl sulfoxide (Table S4 in Supporting information).With the increasing power of violet LEDs, the conversion of phenyl methyl sulfide presented an increasing trend (Fig.S5d in Supporting information).
Next, different scavengers were introduced into the system to determine the reactive oxygen species (ROS) (Fig.2a).First, a singlet oxygen (1O2) scavenger, 1,4-diazabicyclo[2.2.2]octane (DABCO)was added to the reaction system.The slightly reduced conversion indicated that1O2did not produce a decisive impact on the reaction.In contrast, whenp-benzoquinone (p-BQ) was introduced to capture O2•–, the selective oxidation of sulfide was almost completely restrained, suggesting that O2•–was the pivotal ROS.Nevertheless, no reaction occurred in an atmosphere of N2, suggesting O2is the terminal oxidant.Besides, AgNO3completely restrained the reaction by capturing eCB−.Furthermore, according to Fig.S6a(Supporting information), the flat band potential that is closely related to the conduction band (CB) potential could be linearly fitted to be −0.85 Vvs.Ag/AgCl from the intercept of the x-axis, which is negative than the potential of O2/O2•–(−0.48 Vvs.Ag/AgCl).According to the value of the bandgap and the CB potential, the valence band (VB) potential was calculated as +1.9 Vvs.Ag/AgCl (Fig.S6b in Supporting information).
To better comprehend the reaction process, electron paramagnetic resonance (EPR) experiments were then carried out.The eCB−signal of 2-AA-TiO2gradually increased with the prolongation of exposure time in the absence of O2(Fig.2b).Once O2was poured,the eCB−signal reduced to the initial state, indicating the relevance between O2and eCB−.The EPR signal of O2•–was then tested with 5,5-dimethyl-1-pyrrolineN-oxide (DMPO) as the capturing agent(Fig.2c).The EPR signal of O2•–dramatically increased under violet light irradiation from 0 min to 2 min and continued to slightly increase at 4 min, suggesting that O2•–captured by DMPO was accumulated and maintained after switching off the light.
Tentatively, a mechanism of violet light-induced selective aerobic oxidation of sulfide over 2-AA-TiO2photocatalyst with TEA is proposed in Scheme 1.Firstly, the carboxyl group of 2-AA is in combination with the surface hydroxyl group of TiO2, constituting the 2-AA-TiO2photocatalyst, which enables the reaction to take place at the violet light range.Next, the charge separation is generated over the surface complex by the stimulation of violet light.2-AA would straightly infuse electrons into the CB of TiO2to form 2-AA•+, without running into an excited state of 2-AA∗.Meanwhile, O2•–is generated by the reduction of O2at the CB of TiO2.Thirdly, the transition between TEA and TEA•+is driven by collisional electron transfer with 2-AA•+, and the resulted TEA•+interacts with phenyl methyl sulfide to deliver a sulfur-centered radical cation, which is pivotal to connect the TEA redox cycle and photocatalytic cycle.This connection can effectively prevent the 2-AA-TiO2photocatalyst from being undermined by ROS.Fourthly,the sulfur-centered radical cation combines with O2•–to generate phenyl methyl persulfoxide.Ultimately, protons from CH3OH terminate phenyl methyl persulfoxide to afford phenyl methyl sulfoxide.
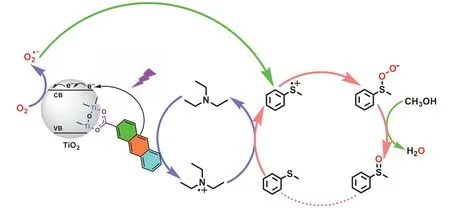
Scheme 1.A proposed mechanism of violet light-induced selective aerobic oxidation of phenyl methyl sulfide by cooperative photocatalysis of 2-AA-TiO2 with TEA.
Then, the range of organic sulfides subjected to the photocatalytic protocol was investigated (Table 1).Phenyl methyl sulfide was oxidized to corresponding phenyl methyl sulfoxide in 88% selectivity and 85% conversion for 50 min (Table 1, entry 1).With a comparable reaction time, the sulfides containing electrondonating groups generally afforded higher conversions than that of phenyl methyl sulfide (Table 1, entries 2–4).On the contrary, conversions of sulfides containing electron-withdrawing groups were lower than that of phenyl methyl sulfide (Table 1, entries 7–12).The sulfides with anm–methoxy group or ano–methoxy group holding the fairly strong steric hindrance effect desired an extended reaction time (Table 1, entries 5 and 6).A similar trend was observed in sulfides containing electron-withdrawing groups(Table 1, entries 11 and 12).Therefore, affected by the steric effect,the required reaction time of the regioisomer augmented in the order ofpara < meta < orthoisomer.In addition, when the methyl group of phenyl methyl sulfide was replaced by an ethyl group or a phenyl group, longer reaction time was acquired (Table 1, entries 13 and 14).The selective oxidation of 2-naphthyl methyl sulfide took a longer time to reach a similar conversion (Table 1, entry 15).Moreover, the selective oxidation of aliphatic sulfides required less reaction time to achieve the considerable conversion (Table 1,entry 16) or achieved a higher conversion and selectivity with the standard reaction time (Table 1, entry 17).

Table 1 Violet light-induced selective aerobic oxidation of sulfides into sulfoxides with air by cooperative photocatalysis of 2-AA-TiO2 with TEA.a
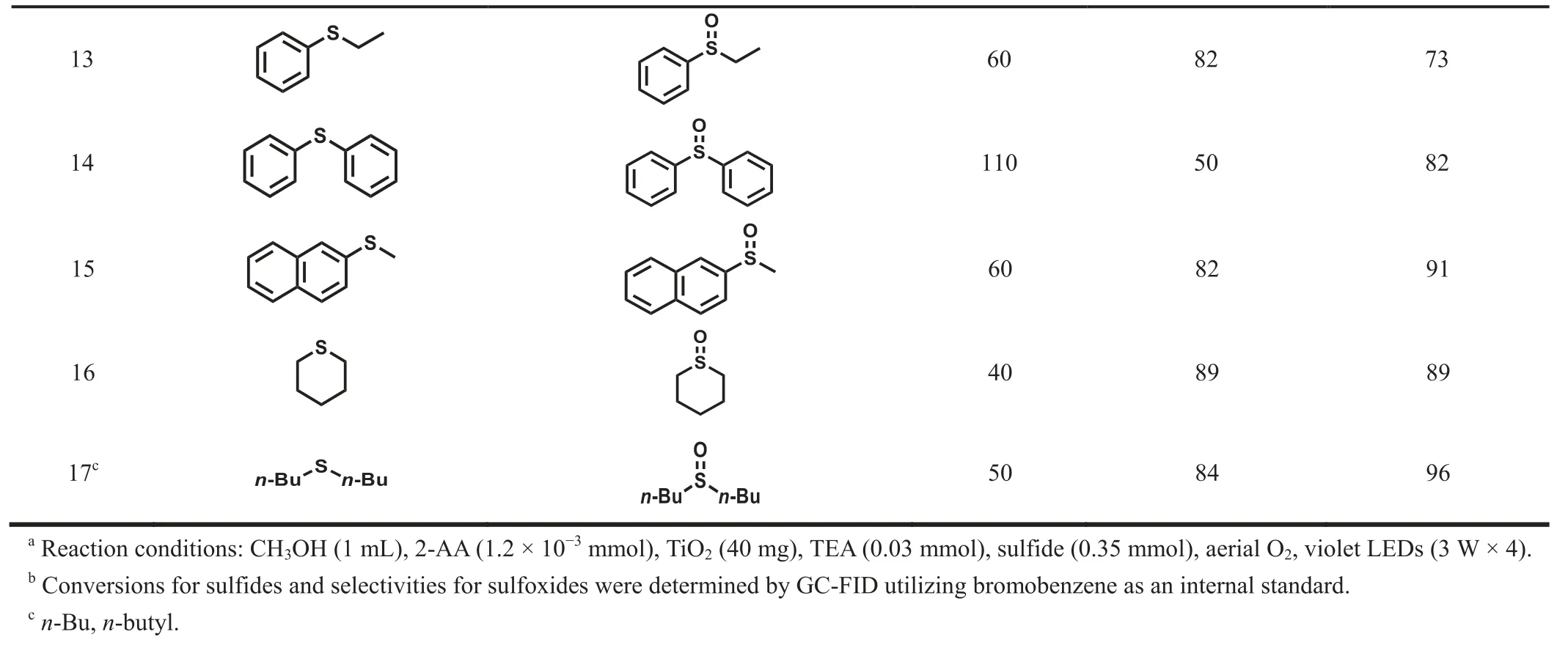
Table 1 (continued)
(continued on next page)
In summary, extending theπ-conjugation of aromatic acids has been adopted to construct TiO2complex visible light photocatalysts.Intriguingly, with BA as the initial molecule, horizontally extending one or two benzene rings acquires 2-NA and 2-AA.Among three aromatic acids, 2-AA has delivered the best result over TiO2due to the most extendedπ-conjugation.Importantly, TEA maintains the integrity of anchored aromatic acids and accelerates electron transfer.Conveniently, violet light-induced selective aerobic oxidation of sulfides to sulfoxides has been realized by cooperative photocatalysis of 2-AA-TiO2with 10 mol% of TEA.This work extends the library of next-generation ligands for semiconductors to expand visible light-induced selective organic reactions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was funded by the National Natural Science Foundation of China (Nos.22072108 and 21773173).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.10.068.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
