Facilely anchoring Cu2O nanoparticles on mesoporous TiO2 nanorods for enhanced photocatalytic CO2 reduction through efficient charge transfer
Ge Yng, Pei Qiu, Jinyn Xiong, Xueteng Zhu, Gng Cheng,∗
a School of Chemistry and Environmental Engineering, Wuhan Institute of Technology, Donghu New & High Technology Development Zone, Wuhan 430205,China
b College of Chemistry and Chemical Engineering, Wuhan Textile University, Wuhan 430200, China
ABSTRACT Semiconductor-employed photocatalytic CO2 reduction has been regarded as a promising approach for environmental-friendly conversion of CO2 into solar fuels.Herein, TiO2/Cu2O composite nanorods have been successfully fabricated by a facile chemical reduction method and applied for photocatalytic CO2 reduction.The composition and structure characterization indicates that the Cu2O nanoparticles are coupled with TiO2 nanorods with an intimate contact.Under light illumination, all the TiO2/Cu2O composite nanorods enhance the photocatalytic CO2 reduction.In particular, the TiO2/Cu2O-15% sample exhibits the highest CH4 yield (1.35 μmol g-1 h-1) within 4 h irradiation, and it is 3.07 and 15 times higher than that of pristine TiO2 nanorods and Cu2O nanoparticles, respectively.The enhanced photoreduction capability of the TiO2/Cu2O-15% is attributed to the intimate construction of Cu2O nanoparticles on TiO2 nanorods with formed p-n junction to accelerate the separation of photogenerated electron-hole pairs.This work provides a reference for rational design of a p-n heterojunction photocatalyst for CO2 photoreduction.
Keywords:TiO2/Cu2O composite Photocatalytic CO2 reduction Photocatalysis Charge separation p-n Junction
Photocatalytic CO2reduction into solar fuels has attracted increasing attention because it is a compelling approach to tackle the issues of greenhouse gas global warming and energy shortage we are currently facing [1–8].It is of significance to develop stable and highly-active photocatalysts which process the characteristics of good CO2adsorption and solar light harvesting, rapid charge transfer, and strong surface reaction capability [9–16].Among various semiconductor photocatalysts, Cu2O is a typical p-type one with a narrow band gap (∼2.2 eV).It can be excited by the visible light and has more negative conduction band position, and therefore it has great potential in solar-driven CO2photoreduction [17–20].At the same time, n-type semiconductor TiO2has also been widely studied due to its non-toxic, low-cost, and suitable band structure, although it can only absorb the UV light [21–24].However, both of the TiO2and Cu2O suffer from the limitation of rapid recombination of photogenerated electron-hole pairs, resulting in a low photocatalytic performance [25–28].
As a matter of fact, when combining the p-type Cu2O with the n-type TiO2to construct a hybrid with a good contact, a p-n junction would be formed between p-Cu2O and n-TiO2upon light irradiation.In this case, an inner electric field would be built in such formed hybrid, which facilitates the separation of the photoinduced electrons and holes, leading to an efficient photocatalysis[29,30].In recent years, Cu2O/TiO2p-n junction was widely used as an efficient photocatalyst for organic pollutants degradation[31–36] and splitting water to hydrogen [37–39].However,there are few reports relevant to CO2photoreduction upon the TiO2/Cu2O composite.Biet al.[40] and Xuet al.[41] recently reported efficient CO2photoreduction was achieved through employing porous Cu2O/TiO2p-n junction as the photocatalyst.However,it is still a great challenge to develop a facile approach to prepare TiO2/Cu2O heterojunction with a good contact for a high-active photocatalyst.
On the basis of the above background, in this work, the composite of mesoporous TiO2nanorods coupled with Cu2O nanoparticles has been successfully prepared by a facile chemical reduction method.The composition and structure of the as-synthesized TiO2/Cu2O composite were characterized.The enhanced photoreduction capability of the TiO2/Cu2O composite was also studied.
The TiO2/Cu2O composite was fabricated according to the schemed process displayed in Scheme 1, in which TiO2nanorods are firstly prepared and subsequently anchoring Cu2O nanoparticles on its surface.XRD pattern obtained for titanium glycolate precursor is shown in Fig.S1a (Supporting information), which displays amorphous characteristics [42].As shown in Fig.S1b (Supporting information), the as-prepared titanium glycolate precursor is composed of rod-like nanostructures with a length of 2–6μm and diameter of 0.7–2 μm.As can be seen in Fig.S1c (Supporting information), the inside of the titanium glycolate precursor is solid.Fig.S2a (Supporting information) shows the XRD pattern of the TiO2prepared from titanium glycolate precursor, and it can be seen that the diffraction peaks belong to the standard pattern(JCPDS No.4–477) of anatase TiO2.As shown in Figs.S2b and c (Supporting information), after refluxing 95 °C for 1 h, the asprepared TiO2still keeps a rod-like structure, and the nanorod is comprised of nanoparticles, and accordingly forms a mesoporous structure.Fig.S3a (Supporting information) shows the XRD pattern of the as-synthesized Cu2O, all the peaks match well with cuprite Cu2O (JCPDS No.5–667).As can be seen in Figs.S3b and c (Supporting information), the as-synthesized Cu2O are nanoparticles with a size of 20–50 nm.
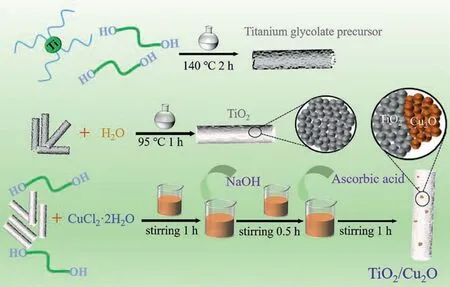
Scheme 1.Illustration for fabrication of TiO2/Cu2O composite, where titanium glycolate precursor and TiO2 nanorods preparation was involved.
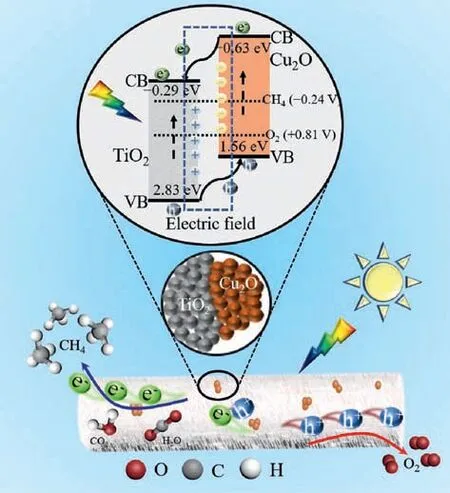
Scheme 2.Schematic illustration of photocatalytic CO2 reduction upon the TiO2/Cu2O composite.
Fig.1a shows the XRD patterns of the TiO2/Cu2O composites.It can be seen that all the diffraction peaks can be indexed to anatase TiO2(JCPDS No.4–477) and Cuprite Cu2O (JCPDS No.5–667).With increasing Cu2O anchoring, the TiO2/Cu2O composite obviously exhibits the characteristic peaks of Cu2O in XRD pattern.Fig.1b shows the UV-DRS spectrum of the as-prepared products.TiO2displays the characteristic absorption edge at about 389 nm.With increasing Cu2O anchoring, all the composites show the enhanced absorption intensity in the visible light region from 400 nm to 800 nm.
The composition and states of elements for TiO2, Cu2O, and the TiO2/Cu2O composite are analyzed by the X-ray photoelectron spectroscopy (XPS).As depicted in Fig.1c, Ti and O elements exist in the TiO2sample, and Cu and O elements exist in the Cu2O sample.For the TiO2/Cu2O-15% sample, Ti, Cu and O elements can be observed.Fig.1d shows the high-resolution XPS spectrum of Cu 2p.The binding energy of 932.3 and 952.2 eV is attributed to Cu 2p3/2and Cu 2p1/3of Cu2O, respectively [43,44].The peaks at binding energy of 934.2, 942.0, and 953.9 eV show the appearance of CuO in the sample [45–47].Fig.1e shows the high-resolution XPS spectrum of Ti 2p.The two typical binding energies at ∼458.2 and ∼464.0 eV can be attributed to the Ti 2p3/2and Ti 2p1/2, respectively, indicating the existence of Ti4+in the TiO2and the TiO2/Cu2O-15% samples [25,48].Fig.1f displays the high-resolution XPS spectrum of O 1s.For the TiO2and the TiO2/Cu2O-15% samples, the peaks located at 530.0 and 531.2 eV correspond to the lattice oxygen and the surface hydroxyl groups [49,50].For the Cu2O sample, the main peak is located at 530.7 eV, which is a signal of surface absorbed oxygen molecule [46].It can be observed that the Ti 2p and Cu 2p bind energy of the TiO2/Cu2O-15% sample shift to the higher one, compared with pristine TiO2and Cu2O.This result indicates an interaction exists between the Cu2O and TiO2.In other words, when the p-type Cu2O and n-type TiO2have an intimate contact, a heterojunction could be formed, and an electron transfer could occur from the p-type Cu2O to n-type TiO2until the system keeps equilibration [51].
The morphology of the TiO2/Cu2O-15% composite is further observed by SEM images.As displayed in Figs.2a and b, the composite still keeps the same rod-like structure as the TiO2supporter,while the Cu2O nanoparticles are deposited on the surface of the rods (Fig.2c).EDX mapping was performed to get more information to confirm the composition of the TiO2/Cu2O composite.As shown in Figs.2d-h, the red, green, and blue colors represent the existence and distributions of O, Ti and Cu, respectively.It can be seen that Cu2O is uniformly coated on the TiO2nanorods.This result further confirms the TiO2/Cu2O composite has been successfully prepared by such a facile chemical reduction method.
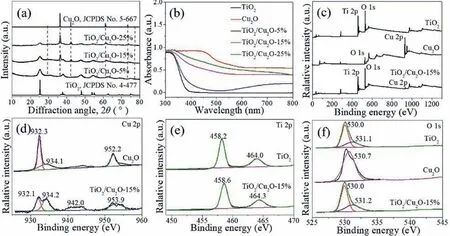
Fig.1.(a) XRD patterns of the TiO2/Cu2O composites.(b) UV-DRS spectra of the TiO2, Cu2O and TiO2/Cu2O composites.(c) XPS survey spectra of TiO2, Cu2O and TiO2/Cu2O-15% samples; high-resolution XPS spectra of (d) Cu 2p, (e) Ti 2p and (f) O 1s for different samples.
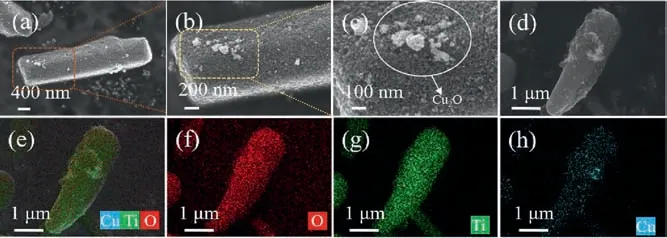
Fig.2.(a-c) SEM images of the TiO2/Cu2O-15% sample; (d-h) SEM image of the TiO2/Cu2O-15% sample and its EDX mapping images of O, Ti, and Cu elements.
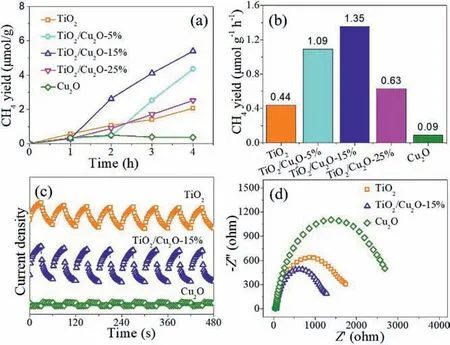
Fig.3.Photocatalytic CO2 reduction activity (a) and production rates (b) of TiO2,TiO2/Cu2O-5%, TiO2/Cu2O-15%, TiO2/Cu2O-25%, and Cu2O under light irradiation;(c) transient photocurrent responses and (d) electrochemical impedance spectra of TiO2, TiO2/Cu2O-15%, and Cu2O samples.
The photocatalytic CO2reduction performance of the asprepared samples are evaluated under 300 W Xe lamp irradiation,and the gas products were detected by gas chromatography.As displayed in Fig.3a, the pristine Cu2O nanoparticles almost have no activity towards photocatalytic CO2reduction under light irradiation within 4 h.Compared with pristine TiO2, the TiO2/Cu2O composites can enhance the photocatalytic performance for CO2reduction.Among the different composites, the TiO2/Cu2O-15% shows the highest activity.As shown in Fig.3b and Fig.S4 (Supporting information), within 4 h light illumination, the TiO2/Cu2O-15%sample exhibits the CH4yield with a rate of 1.35 μmol g−1h−1,which is 3.1 and 15.0 folds higher than that of pure TiO2and pure Cu2O samples, respectively.As shown in Fig.S5 (Supporting information), the XRD pattern of the TiO2/Cu2O-15% after reaction correspond well to the standard Cu2O (JCPDS No.5-667) and TiO2(JCPDS No.5-667), indicating that the TiO2/Cu2O-15% sample keeps the same composition before and after reaction.Recently Xu and co-workers [52] found the formation of Cu(I)/Cu(0) from the initial atomically dispersed Cu(II) was proposed to be more effective for CH4formation.In present work, as shown in Fig.S6a (Supporting information), with increasing of recycling test times, the yield of CH4decreases, and it is 0.59 μmol g−1h−1after four cycles.The XRD pattern of the as-cycled TiO2/Cu2O-15% sample is shown in Fig.S6b (Supporting information), it is found that the diffraction peaks of the Cu2O disappear.It might result in the decrease of the catalytic performance.Further study is underway.
Photo/electrochemical measurements are performed to study the interfacial charge transfer of the above photocatalysts, which has a significant impact on the photocatalytic performance [52–56].As shown in Fig.3c, the TiO2/Cu2O-15% sample exhibits superior photocurrent intensity than the pristine TiO2and Cu2O, and it indicates the composite has enhanced capability of electron-hole pairs separation.Fig.3d reveals the electrochemical impedance spectra (EIS) of the TiO2, Cu2O, and TiO2/Cu2O-15% samples.It can be obviously observed that the TiO2/Cu2O-15% sample has the smallest semicircle, suggesting the smallest resistance existence and rapid charge transfer at the interface of the TiO2/Cu2O-15%sample.
Taking into the band structure of the photocatalyst is related to the thermodynamics of the CO2photoreduction.UV–vis diffuse reflectance spectroscopy and valence-band XPS spectrum were employed to determine the band gap energy and the valence band position of the samples.As shown in Fig.S7 (Supporting information),the band gap of TiO2and Cu2O calculated from the Kubelka-Munk function is 3.12 and 2.19 eV, respectively.As displayed in Fig.S8(Supporting information), according to the valence-band XPS spectrum, the valence band position for TiO2and Cu2O is 2.83 and 1.56 eV, respectively.
Based on the above results, the conduction band of TiO2and Cu2O is calculated to be −0.29 and −0.63 eVvs.NHE (pH 0), respectively.As mentioned in the XPS result and reported previously [34,36,39], the p-n heterojunction would be formed when the TiO2and Cu2O have an intimate contact.In this case, as shown in Scheme 2, the diffusion of electrons from Cu2O to TiO2could occur, while the holes diffuse from TiO2to Cu2O.Accordingly, an internal electric field from n-type TiO2to p-type Cu2O would be established.Under light illumination, photogenerated electron-hole pairs would be produced due to the excitation of TiO2and Cu2O,and the formed internal electric field would facilitate the migration of electrons to the TiO2and the transfer of holes to Cu2O.In this regard, efficient charge separation would be achieved.Finally,more photoinduced electrons would participate in the photocatalysis process, and the photocatalytic CO2reduction to CH4is improved.
In summary, a facile chemical reduction method has been used to fabricate the TiO2/Cu2O composite, in which the Cu2O nanoparticles couple with the TiO2nanorods.Under light illumination, the TiO2/Cu2O composites show superior performance than TiO2and Cu2O towards photocatalytic CO2reduction to CH4.The TiO2/Cu2O-15% composite exhibits the highest CH4yield rate of 1.35 μmol g−1h−1within 4 h.Based on the XPS, band structure,and photo/electrochemical measurements, the TiO2/Cu2O composite with an intimate contact would allow the establishment of internal electric field, which greatly improves the separation and migration of photoinduced charge carriers, and therefore promotes photoreduction of CO2into CH4.It is expected that this work could offer an efficient approach to design p-n junction for CO2photoreduction.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No.21501137).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.10.047.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
