Synchronous mineralization of three aqueous non-steroidal anti-inflammatory drugs in electrochemical advanced oxidation process
Lei Xu, Xinyue Cui, Jianbo Liao, Yufeng Liu, Baoyi Jiang, Junfeng Niu
Research Center for Eco-Environmental Engineering, Dongguan University of Technology, Dongguan 523808, China
ABSTRACT Electrochemical degradation performances of three non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen (ACT), aspirin (ASP) and ibuprofen (IBP), were investigated and compared in their alone and mixture conditions using Ti/SnO2-Sb/La-PbO2.The pseudo-first-order degradation kinetics (k) order was kIBP-A (0.110 min−1) ˃kASP-A (0.092 min−1) ˃kACT-A (0.066 min−1) in their alone condition, while that was kACT-M (0.088 min−1) ˃kASP-M (0.063 min−1) ˃kIBP-M (0.057 min−1) in their mixture condition.The •OH apparent production rate constant of 5.23 mmol L−1 min−1 m−2 and an electrical energy per order (EEO)value of 6.55 Wh/L could ensure the synchronous degradation of the NSAIDs mixture.The mineralization efficiency of NSAIDs mixture was 86.9% at 240 min with a mineralization current efficiency of 1.67%.Acetic acid and oxalic acid were the main products in the mineralization process for the both conditions.In the mixture condition, there were higher k values at lower initial concentrations and higher current density, while the presence of carbonate and humic acid inhibited their degradation.The results indicated electrochemical advanced oxidation process can effectively and synchronously mineralize NSAIDs mixture in wastewater.
Keywords:Electrochemical advanced oxidation Non-steroidal anti-inflammatory drugs Degradation kinetics Synchronous mineralization Wastewater
The presence of non-steroidal anti-inflammatory drugs(NSAIDs), one class of emerging contaminants, in waters has become a topic of increasing concerns [1].NSAIDs have been detected in the effluent of traditional wastewater treatment plants,surface water, ground water and even drinking water, with their concentrations were up to hundred μg/L [2,3].It is proved that NSAIDs can cause considerable threat to the water ecosystem and human health because of their bioactivity and pharmacological activities [4].Based on the risk quotients, NSAIDs could pose a medium risk or high risk to aquatic organisms in the Hai River,Yellow River and Liao River [5].Researchers have found that the adverse effects caused by NSAIDs mixture can be quite toxic at environment concentrations [6].Thus, it is important and urgent to develop effective technology to eliminate NSAIDs mixture from aquatic environment.
Conventional treatment processes,e.g., coagulation, sedimentation and filtration, cannot remove aqueous NSAIDs effectively [7].Advanced oxidation processes (AOPs) are effective methods for decomposition of pharmaceuticals through the generation of reactive oxygen species (ROS), such as•OH, SO4•−and O2•−[8–11].UV/persulfate, photocatalysis and electro-Fenton have been used in the degradation of NSAIDs mixture in water, however, these processes are confronted with the difficulties of low mineralization efficiency and addition of chemical agents [12–15].Electrochemical advanced oxidation appears as an available alternative due to its environmental compatibility, high degradation efficiency,strong oxidation ability and non-addition of chemical agents.Electrochemical advanced oxidation has been widely used in the efficient degradation of aqueous NSAID.For example, the electrochemical mineralization efficiency of ibuprofen (IBP) reached 93.2%at 60 min using Ti/SnO2-Sb/Ce-PbO2at initial concentration of 20 mg/L and current density of 10 mA/cm2[16].Furthermore, trace naproxen was effectively degraded in a flow-through electrochemical advanced oxidation system with an energy consumption of 0.744 Wh/L [17].However, there is still a lack of understanding of the electrochemical mineralization of NSAIDs mixture.
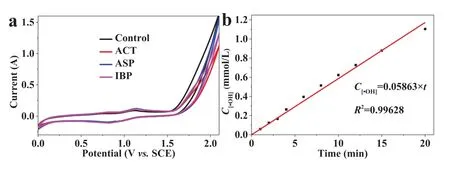
Fig.1.(a) The CV curves of Ti/SnO2-Sb/La-PbO2 electrode in 50 mmol/L Na2SO4 solution with/without 100 mg/L ACT, ASP and IBP at scan rate of 10 mV/s.(b) The production of •OH in the electrochemical advanced oxidation, current density of 10 mA/cm2.
In this study, the electrochemical degradation of NSAIDs mixture, such as acetaminophen (ACT), aspirin (ASP) and IBP, was studied.Ti/SnO2-Sb/La-PbO2was prepared based on titanium plate (effective size of 70 mm×80 mm, thickness of 2 mm) through solgel and electrodeposition methods [18].Electrochemical degradation experiments were conducted in a polymethyl methacrylate cell.Ti/SnO2-Sb/La-PbO2was used as the anode, and two pieces of titanium plates were used as cathodes, with the distances between them were 15 mm.10 mmol/L Na2SO4was used as the electrolyte, and the initial concentrations of the contaminants were 10 mg/L unless otherwise stated.The effective volume of solution was 250 mL with a magnetic stirring rate of 500 rpm in the electrochemical degradation, while 500 mL solution was used in the mineralization process with the current density of 10 mA/cm2.The detailed information of chemicals and materials is presented in Text S1 (Supporting information).The analytical methods of NSAIDs and the produced small molecular carboxylic acids are shown in Texts S2 and S3 (Supporting information), respectively.
Cyclic voltammetry (CV) curves were performed on an electrochemical workstation (Autolab M204, Metrohm) equipped with a Booster10A module in a conventional three-electrode system(electrolyte of 50 mmol/L Na2SO4), in which Ti/SnO2-Sb/La-PbO2,a piece of titanium plate and a saturated calomel electrode (SCE)were used as work electrode, counter electrode and reference electrode, respectively.As shown in Fig.1a, there was no oxidation current of ACT, ASP and IBP observed in the CV curves.The higher oxidation current of CV curve in control sample than in ACT, ASP and IBP (concentration of 100 mg/L) samples was because of the changes of pH after the addition of the contaminants.That indicated that there is not obvious direct oxidation (oxidation of anode) of ACT, ASP and IBP on Ti/SnO2-Sb/La-PbO2, and the indirect oxidation could be the main degradation mechanism of them.Therefore, salicylic acid (10 mmol/L) was used as the probe of•OH,which is the significant ROS produced in the electrochemical advanced oxidation [19].The analytical method of produced•OH concentration is shown in Text S4 (Supporting information), and the result is shown in Fig.1b.The apparent production rate constant of•OH (k[•OH]) was calculated as 5.23 mmol L−1min−1m−2.
The electrochemical degradation performances of ACT, ASP and IBP in their alone (ACT-A, ASP-A and IBP-A, respectively) and mixture (ACT-M, ASP-M and IBP-M, respectively) conditions were studied, the result of which is shown in Fig.2a.The electrochemical degradation of the NSAIDs followed thepseudo-first-order degradation kinetics.In the alone condition, the kinetics constants order was:kIBP-A> kASP-A> kACT-A, with their values of 0.110 min−1,0.092 min−1and 0.066 min−1, respectively.In the mixture condition, ACT, ASP and IBP could be degraded simultaneously in the electrochemical advanced oxidation process, while their kinetics constants order was:kACT–M(0.088 min−1)> kASP–M(0.063 min−1)> kIBP–M(0.057 min−1).Compared with that in alone condition,the degradation kinetics constant of ACT enhanced 33.3%, while that of ASP and IBP decreased 31.5% and 48.2%, respectively.ACT was preferentially degraded in the mixture condition, which was might because ACT possessed the highest mass transfer rate and the fastest reaction with ROS in the mixture solution.That is an interesting finding, and the deep mechanism would be researched in the further studies.The economic feasibilities of NSAIDs degradation were studied through electrical energy per order (EEO), energy consumption when 90% of contaminant was degraded [19], the detalied calculation method of which is presented in Text S5 (Supporting information).TheEEOvalues of ACT, ASP and IBP in their alone condition were 5.77 Wh/L, 4.06 Wh/L and 3.39 Wh/L, respectively, while the values were 4.24 Wh/L, 5.93 Wh/L and 6.55 Wh/L in their mixture condition, as shown in Fig.2b.The energy consumption of ACT degradation was the lowest in the mixture condition due to its highest kinetics constant.It was worth noting that the three NSAIDs could be degraded simultaneously at the totalEEOvalue of 6.55 Wh/L, indicating that the NSAIDs mixture could be effectively degraded by the generated ROS in the electrochemical advanced oxidation process.
The total organic carbon (TOC) values decreased from 5.59 mg/L,5.36 mg/L, and 6.31 mg/L to 0.68 mg/L, 0.23 mg/L and 0.74 mg/L for ACT, ASP and IBP, respectively, after 240 min in their alone condition.Their mineralization efficiencies were 87.7%, 95.6% and 88.2%, respectively, as shown in Fig.2c.Meanwhile, the mineralization efficiency of NSAIDs mixture was 86.9% (from 17.83 mg/L to 2.34 mg/L), which was slightly lower than the values in the alone condition.ACT, ASP and IBP were synchronously mineralized in the electrochemical advanced oxidation, which was similar to the degradation efficiencies in Fig.2a.The effective mineralization of NASIDs mixture could be achieved by the produced ROS.The mineralization current density (MCE) was studied based on the TOC decrease and electric energy input to evaluate the energy efficiency in the electrochemical advanced oxidation [19], and its calculation method is presented in Text S6 (Supporting information).As shown in Fig.2d, the MCE value of ACT-A decreased from 3.03% at 10 min to 0.54% at 240 min, while the value of ASP-A decreased from 2.02% to 0.50%.The MCE value of IBP-A increased from 2.34% at 10 min to 2.93% at 20 min, and then decreased to 0.66% at 240 min.The MCE value of NSAIDs mixture was estimated by assuming that the mineralization efficiencies of ACT, ASP and IBP were equal with each other in their mixture condition.The MCE value of the mixture increased from 6.88% at 10 min to 6.99%at 20 min, and then decreased to 1.67% at 240 min, which was almost the sum of ACT-A, ASP-A and IBP-A.The above results indicated that the produced ROS in electrochemical advanced oxidation can synchronously mineralize contaminants mixture, which process has high electric energy utilization efficiency.
The carboxylic acids with small molecular were analyzed during the mineralization process, the results of which are shown in Fig.3.Formic acid, glyoxylic acid, oxalic acid and acetic acid were all detected in the ACT-A, ASP-A and IBP-A degradation processes,while there was hardly any detection of pyruvic acid in the ACTA and ASP-A degradation processes.In the three electrochemical degradation processes, the concentrations of acetic acid and oxalic acid were found higher than those of the other three small molecular carboxylic acids.The concentrations of produced acetic acid were all increased and then decreased along with the reaction, its highest concentrations were 3.24 mg/L, 5.66 mg/L and 5.29 mg/L at reaction time of 40 min, 20 min and 90 min for ACT-A, ASP-A and IBP-A, respectively.The concentrations of oxalic acid reached their highest values at 60 min for all the three NASIDs, the values of which ranged from 2.10 mg/L to 2.43 mg/L.However, the concentrations of oxalic acid were not decreased in the following processes, which might be due to its gradual production in the processes and/or its high resistance to the electrochemical advanced oxidation.Formic acid, glyoxylic acid and pyruvic acid (only for IBP-A) were all produced and then decreased in the electrochemical degradation of the three NASIDs, the highest values of which were all lower than 1.00 mg/L.In the electrochemical degradation process of NASIDs mixture, the concentration change tendencies of all the five carboxylic acids (Fig.3d) were similar to those in Figs.3a-c.The concentrations of formic acid, pyruvic acid, glyoxylic acid and acetic acid in the mixture system were all higher than in the alone system, for example, the highest concentration of acetic acid was 11.38 mg/L at 90 min.However, the highest concentration of oxalic acid in the mixture system was 2.36 mg/L, which was similar to that in ACT-A, ASP-A and IBP-A system.The acetic acid and oxalic acid at 240 min were the main constituent of the residual TOC.It is worth noting that the combined process of electrochemical advanced oxidation and other AOPs could be more effective in the mineralization of organic pollutants mixture, due to the higher ROS production and the reaction kinetics between organic pollutants and various ROS [20].Meanwhile, there could be higher degradation performance using reactive electrochemical membrane in the flow-through electrochemical advanced oxidation system, in which the mass transfer of organic pollutants and utilization ratio of ROS could be enhanced as proved in the previous studies [17,21].

Fig.2.(a) The electrochemical degradation performance, (b) EEO values, (c) mineralization efficiency and (d) MCE values of the NSAIDs in their alone and mixture conditions.
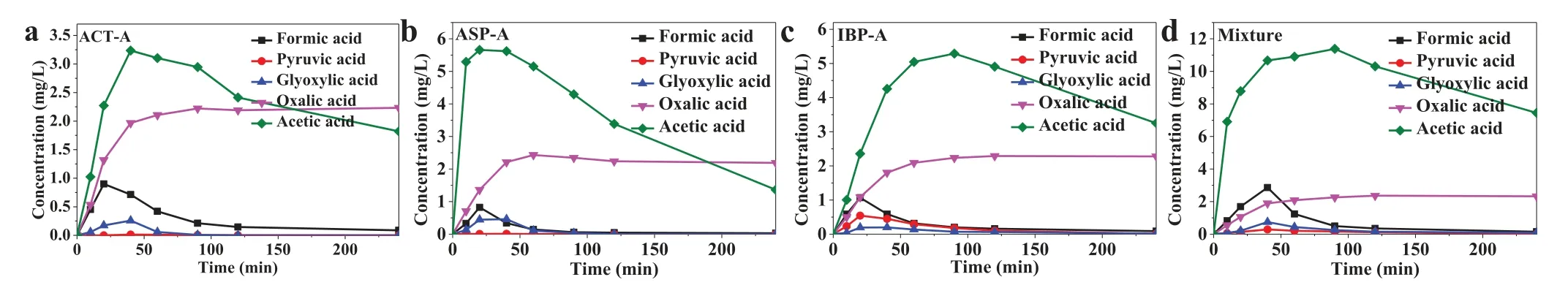
Fig.3.The production of carboxylic acids with low molecular in the electrochemical advanced oxidation of ACT (a), ASP (b), IBP (c) and their mixture (d).

Fig.4.The effects of (a) initial concentration, (b) current density, (c) carbonate and (d) humic acid on the pseudo-first-order degradation kinetics of the NSAIDs in their mixture condition.
The influencing factors,e.g., initial concentrations, current densities, carbonate concentrations and dissolved organic matter concentrations, on the degradation performance of NASIDs mixture were thoroughly studied.As shown in Figs.S1–S4 (Supporting information), the electrochemical degradation performances of the three NASIDs were all obviously effected by the studied influencing factors.Thekvalues of ACT-M, ASP-M and IBP-M at their concentrations of 5 mg/L were 0.142 min−1, 0.085 min−1and 0.095 min−1, respectively.These values were decreased to 0.066 min−1,0.035 min−1and 0.044 min−1, respectively, when the concentrations increased to 20 mg/L, as shown in Fig.4a.The higher concentration of NSAIDs and concentration of their degradation intermediates could compete for the constant production of•OH at 10 mA/cm2.Linear increases of thekvalues were observed when current density was changed from 5 mA/cm2to 20 mA/cm2(Fig.4b), in which thekvalues were increased from 0.047 min−1, 0.030 min−1and 0.036 min−1to 0.172 min−1, 0.124 min−1and 0.116 min−1for ACT-M, ASP-M and IBP-M, respectively.There could be higher•OH production at higher current density because of the faster electron transfer and higher anode potential, which caused the more efficient degradation of NSAIDs mixture.The presence of carbonate slowed down the degradation performances of NSAIDs as shown in Fig.4c, in which the pH value of the solution was not adjusted after the addition of carbonate.After the addition of 1 mmol/L carbonate, thekvalues were sharply decreased by 18.4%, 14.3% and 19.3% for ACT-M, ASP-M and IBP-M, respectively.However, there were slow decreases ofkvalues when carbonate concentration was increased from 1 mmol/L to 100 mmol/L.There was greater inhibition of ASP-M degradation than ACT-M and IBP-M at higher carbonate concentration.Carbonate can quench•OH in the system, which produces CO3•−.Although the production of CO3•−could accelerate the elimination of oxytetracycline and oxcarbazepine [22], there were inhibitions of norfloxacin and ciprofloxacin degradation in advanced oxidation processes in the presence of carbonate [23,24].The different effects were mainly because of the different reaction kinetics between the organic compounds and CO3•−.The presence of humic acid, a typical dissolved organic matter, also inhibited electrochemical degradation performances of NSAIDs as shown in Fig.4d.Thekvalues were decreased by 46.6%, 40.3% and 54.4% for ACT-M, ASP-M and IBP-M,respectively, after the addition of 40 mg/L humic acid.The quenching of•OH and decrease of electroactive sites on anode surface (adsorption of humic acid) might be the reasons for the inhibitions.
In summary, the NSAIDs mixture could be degraded and mineralized simultaneously in the electrochemical advanced oxidation process.The electrochemical degradation kinetics of ACT, ASP and IBP in their mixture condition were different from those in the alone condition.The produced ROS was more adequately utilized in the mixture condition than in the alone condition, with the similar mineralization efficiency of the NSAIDs mixture and higher MCE value in the mixture condition.Acetic acid and oxalic acid were the main products in the mineralization process for both the alone and mixture conditions.The presence of carbonate and humic acid could inhibit the electrochemical degradation of NSAIDs mixture.This work provides novel knowledge for understanding the synchronous elimination of aqueous NASIDs mixture in electrochemical advanced oxidation process.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financially supported by the National Science Fund for Distinguished Young Scholars (No.51625801), the National Natural Science Foundation of China (Nos.51878169 and 52000028), the Guangdong Innovation Team Project for Colleges and Universities (No.2016KCXTD023), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2017),and Guangdong Basic and Applied Basic Research Foundation (Nos.2019A1515110182 and 2019A1515110681).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.10.065.
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
