Bimetallic two-dimensional materials for electrocatalytic oxygen evolution
Xiaojie Li, Qi Hu, Hengpan Yang, Tao Ma, Xiaoyan Chai, Chuanxin He
College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen 518060, China
ABSTRACT Electrocatalytic oxygen evolution reaction (OER) is one of the important half reactions of electrocatalytic water splitting.However, the slow kinetic process involving four-electron transfer severely limits its reaction efficiency, which in turn limits the overall electrocatalytic hydrolysis efficiency.In order to improve the activity of the electrocatalytic OER, researchers mainly update the catalyst from three aspects,that is, increase the conductivity of the electrocatalyst, and the quantity and quality of active sites.Twodimensional (2D) engineering can effectively reduce the resistance of the materials and greatly increase the number of electrochemically active sites, while heterometal doping, or the bimetal strategy, can improve the quality of active sites via changing the electronic structure of the material.Thus, the combination of the two can enhance the activity of electrocatalytic OER in all three aspects: conductivity, number and quality of active sites.However, there is currently no review on this topic.Therefore, in this review,we summarize the application of bimetallic 2D materials in electrocatalytic OER from four aspects: the structure, synthesis strategy, catalytic efficiency, and reaction mechanism.
Keywords:Bimetal/bimetallic Two-dimensional/2D Oxygen evolution Electrocatalytic MOF LDH
1.Introduction
Sustainability and environmental friendliness have become the two major themes of energy issues in the world today [1–7].Hydrogen energy, which is most in line with these two themes, has continuously aroused researchers’ attention in the past decades[8–14].The electrocatalytic hydrogen evolution reaction (HER)viawater splitting that can be coupled with photovoltaic cells is undoubtedly one of the hydrogen production methods that best meet these two themes.In this reaction, HER is only one of the halfreactions.The other half is the electrocatalytic oxygen evolution reaction (OER), also known as electrocatalytic water oxidation [15–20].OER involves a four-electron transfer and its kinetic speed is quite slow, much slower than the opposite half-reaction, HER [21–26].As a result, the rate of overall electrocatalytic water splitting is controlled by the rate of electrocatalytic water oxidation.Thus,solving the problem of slow kinetic speed of OER means that the reaction rate of the entire electrocatalytic water splitting can be increased, thereby increasing HER efficiency.Similar to HER in water splitting, the anode reaction of electrocatalytic CO2reduction(CO2RR) is also an OER process, so in CO2RR, OER is expected to have good performance as well [27–30].Besides, in rechargeable metal-air batteries, the anode of the charging reaction (i.e., the positive electrode of the battery) also involves the OER reaction[31–34].Hence, OER plays a significant role in the energy field.
Among the many factors affecting electrocatalytic water oxidation, the electrocatalyst is at the core [6,26,35,36].The key to determining the oxygen production efficiency of the electrocatalyst is its conductivity, and the quantity and the quality of its active sites[37–39].Specifically, the quality of active sites can be reflected by Gibbs free energy barrier (ΔG) for generating its oxygen-producing intermediate [40–42].Generally, the lower theΔG, the better.The most classic OER electrocatalysts are two noble metal-based oxides,i.e., RuO2and IrO2.However, their high prices have severely limited their practical applications, and they will be oxidized to RuO4and IrO3,respectively in an alkaline solution after a long period of working time, and then dissolved in the aqueous solution[43–45].Therefore, it is the core task of electrocatalytic OER to find inexpensive, efficient, and stable electrocatalysts.
In the past few decades, researchers have developed a series of less-expensive materials such as non-noble transition metal oxides[46,47], sulfides [48,49], selenides [50,51], nitrides [52,53], phosphides [54,55], oxyhydroxides [56,57], hydroxides [58,59], Prussian blue analogues (PBAs) [60,61].However, many shortcomings such as weak conductivity, few active sites, and poor quality of active sites (highΔG) still limit their practical applications [62–64].Whereupon, researchers have taken lots of measures to improve their performance.
Among them, the two-dimensional (2D) strategy is one of the most promising tactics.As we know, since graphene was first obtained in 2004, 2D nanomaterials have received extensive attention in scientific area due to their ultra-thin nanostructures and unique physical and chemical properties [65–69].In detail, compared with bulk materials, 2D nanomaterials have a large quantity of advantages.(1) The specific surface area and the exposed active sites of 2D nanomaterials have improved significantly.(2)Since most of the atoms of 2D nanomaterials are exposed on the surface, it is much easier to form defects, which are conducive to the increase of edge active sites and coordinated unsaturated metal active sites.(3) Ultra-thin 2D nanomaterials usually have merits in electrical conductivity.This is because electrons are mainly transferred within a thin layer for 2D nanomaterials, while bulk materials have both intra-layer transfer and inter-layer transfer.As a result, the interaction between the layers of bulk materials often causes a certain resistance to the electron transfer in a layer, leading to weak conductivity of bulk materials.Besides, among the advantages of 2D nanomaterials over bulk materials, the second and third points are also the edges of 2D nanomaterials over other non-2D nanomaterials, which is because the ultra-thin and ultra-large 2D nanomaterials cannot only provide a large enough hotbed that is easy to generate defects, but also provide a stable channel within a layer for charge transfer.Therefore, in the past decade or so, a large number of 2D nanomaterials have been applied in the field of electrocatalytic OER [70–77].
Moreover, another very important strategy is the heterometal doping strategy, that is, the bimetallic strategy.Introducing another metal into the original transition metal compound can change the morphology, add defects, change the valence of the original metal,and thus change the quantity and quality of active sites, or even change the location of active sites [35,64,78–85].
It can be seen from the above that combining the 2D strategy and the bimetallic strategy can not only improve the conductivity,but also increase the number and quality of active sites.In particular, when the bimetallic strategy fully improves the activity of the active sites and the 2D strategy fully exposes these active sites on the surface of the material, the synergy between the two strategies can be completely manifested.
Currently, researchers have developed a series of bimetallic 2D nanomaterials for electrocatalytic OER.However, there is no review that specifically summarizes the relevant content on the topic, although some excellent reviews have detailedly discussed the application of 2D nanomaterials in the field [75,77,86–91].Therefore, in this review, we sum up the electrocatalytic OER application of bimetallic 2D nanomaterials from four aspects: material structure, synthesis method, electrocatalytic performance, and reaction mechanism.The involved materials contain bimetallic 2D metal organic frameworks (MOFs), layer double hydroxides (LDHs), oxides,phosphides, phosphates, PBAs and oxyhydroxides.At the last, we anticipate the development direction and prospect of the field.
2.Materials structure
The transition metal elements in bimetallic two-dimensional nanomaterials are mainly concentrated in the fourth period of the periodic table (the first row of transition metals), which is because the metal elements in this line are cheap and more lively.Among them, the eighth group of iron, cobalt and nickel have similar atomic radii and chemical properties, so they are often utilized to dope with or partially replace each other.Therefore, we often see bimetallic iron-cobalt, iron-nickel or cobalt-nickel two-dimensional materials used in electrocatalytic OER.In addition, OER’s bimetallic two-dimensional electrocatalyst also involves other fourth period elements, such as copper, zinc, vanadium and manganese.What is more, it is worth noting that sometimes we can also see the participation of some main group metal elements, such as indium, which is at the fifth period, the third main group.Here, we summarize the structures of representative bimetallic 2D MOF, LDH, oxides,phosphides, and oxyhydroxides as follows.
In the ultrathin NiCo MOF nanosheets prepared by Zhaoet al.[92], the organic ligand used is benzenedicarboxylic acid (BDC).As shown in Fig.1a, the statuses of nickel and cobalt are equal in the material structure.They all coordinate with the six oxygen atoms from hydroxyl groups and carboxyl groups to form a virtual octahedral structure.However, on the surface of the two-dimensional MOF, due to the limited growth, some metal atoms have only five coordination bonds, which is the so-called unsaturated coordination state, thus forming a virtual tetragonal pyramid structure.Coordination unsaturated metal atoms are extremely significant in catalytic reactions because they are easily combined with solvents or reactant molecules and become active sites for catalytic reactions.
Most of the bimetallic two-dimensional MOFs are similar to the above-described NiCo MOF nanosheets, that is, the two metals have equal positions and form apseudo-octahedral structure with coordination atoms, and there are many coordination unsaturated metals on the material surface.However, in the MOF prepared by Liet al.[93], the bimetallic statuses are unequal.As shown in the Fig.1b, the bimetallic 2D MOF they prepared is formed with metal phthalocyanine (MPc) as the organic ligand and another metal as the metal junction (Fig.1b).Therefore, unlike the metal M’, the metal M is at the center of the MPc, so the statuses of the two metals differ from each other.As a result, the coordination environment of M is a plane cube composed of four M-N bonds, and that of M’is a plane cube composed of four M-O bonds.The assynthesized bimetallic MOF nanosheets contain ZnPc-Ni and NiPc-Zn.It is worth noting that because phthalocyanine is a compound with a large conjugated system of 18 electrons, the as-fabricated materials have good conductivity.
Like most bimetallic two-dimensional MOFs, the positions of the two metals are also equal in LDH.In the NiFe LDH prepared by Wanget al.[94], both Ni and Fe are connected to six oxygen atoms,so that the metal and the coordinated oxygen atoms form an octahedral structure (Fig.1c).Besides, similar structures also exist in oxides and oxyhydroxides.For example, in the bimetallic 2D irondoped nickel oxide nanosheets prepared by Zhuanget al.[95], both nickel and iron are connected with six O atoms to form an octahedral structure (Fig.1d).In the bimetallic oxyhydroxide nanosheet Fe0.33Co0.67OOH prepared by Yeet al.[96], the octahedral coordination structure of FeO6and CoO6can be found (Fig.1e).At the same time, there are also CoO4tetrahedrons in Fe0.33Co0.67OOH nanosheets.In contrast, in the iron-containing cobalt phosphide synthesized by Huet al.[97], iron partially replaced phosphorus,forming iron-cobalt bonds (Fig.1f).
To sum up, in most oxygen-containing bimetallic twodimensional materials, the statuses of the two metals are equal,and they coordinate with six oxygen atoms (MO6) to form apseudo-octahedral structure.It is reported that this structure of MO6may be beneficial to the performance of electrocatalytic OER process.
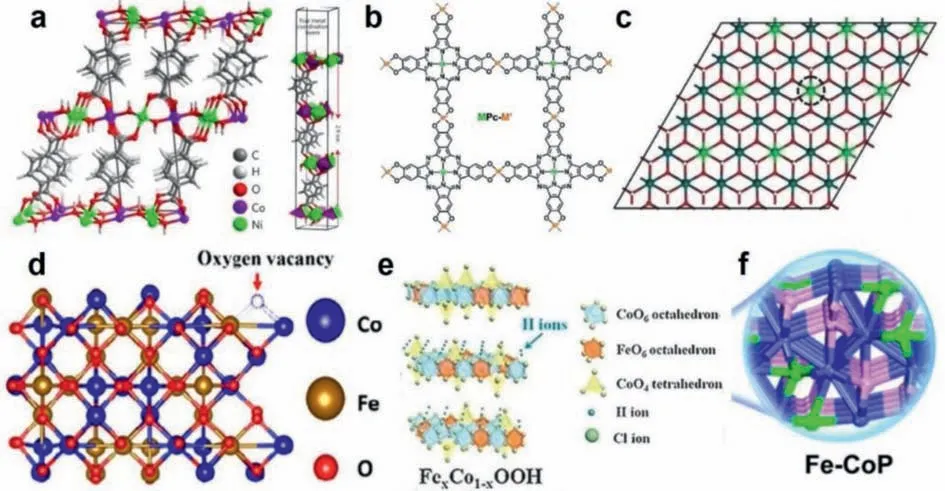
Fig.1.(a) The bimetallic ultrathin MOF nanosheets formed with benzenedicarboxylic acid (BDC) as the organic ligands and Co and Ni as the metal sites.Reproduced with permission [92].Copyright 2016, Springer Nature.(b) The conductive 2D MOF formed from metal phthalocyanine (MPc) and metal cations.M and M’stand for two different metal sites.Reproduced with permission [93].Copyright 2021, Royal Society of Chemistry.(c) 2D NiFe LDH nanosheets.The blue, green, red, and white represent Ni, Fe,O and H atoms, respectively.Reproduced with permission [94].Copyright 2018, Wiley-VCH.(d) The bimetallic 2D iron-doped nickel oxide nanosheets.Reproduced with permission [95].Copyright 2018, Springer Nature.(e) The bimetallic oxyhydroxide nanosheet Fe0.33Co0.67OOH.Reproduced with permission [96].Copyright 2018, Wiley-VCH.(f) The iron-containing cobalt phosphide.Green for Fe, blue for Co, and pink for P.Reproduced with permission.Reproduced with permission [97].Copyright 2018, Elsevier.
3.Synthetic strategies
In this review, we discuss the preparation of bimetallic twodimensional nanomaterials from two aspects: bimetallic synthesis strategy and two-dimensional synthesis strategy.In order to better make readers understand the writing logic of this part, we have specially made the following mind map (Fig.2).
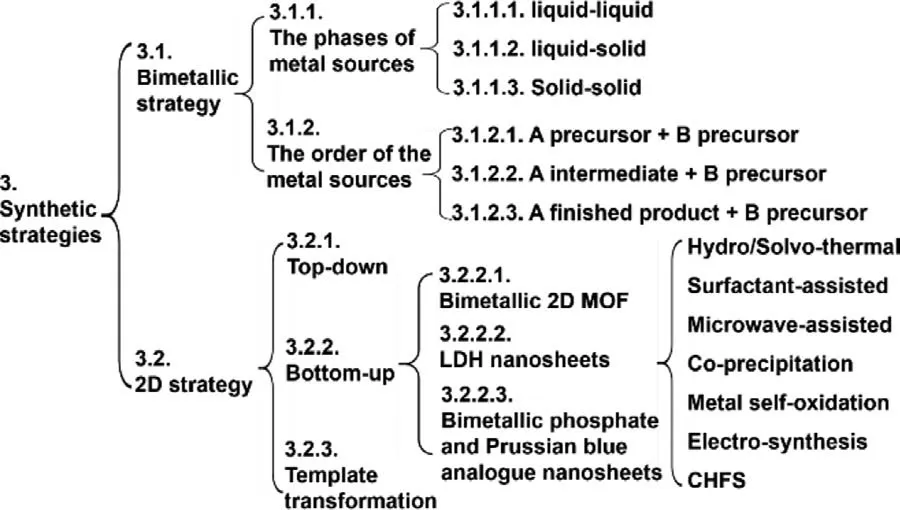
Fig.2.The mind map of Part 3.
3.1.Bimetallic strategy
3.1.1.The phases of metal sources
From the bimetallic viewpoint, there are two classification routes.According to the phases of the two metal sources, it can be classified as three modes: Liquid-liquid, liquid-solid, and solidsolid.Among them, the liquid-liquid method is the most common one since the homogeneous mixing is more uniform.For example, Zhaoet al.utilized a mingled solution of CoCl2and NiCl2to synthesize ultrathin NiCo bimetallic-organic framework nanosheets [92].Rosaet al.took a mixed aqueous solution of Ni(NO3)2and Fe(NO3)3as the precursor of the Ni-Fe layered double hydroxide 2D thin films [98].Lianget al.dissolved FeCl2and Ni(NO3)2together into an aqueous solution to prepare Fe-doped NiPS3nanosheets [72].
Liquid-solid mode is usually taken to directly fabricate the electrode for electrocatlaytic OER.Sunet al.prepared bimetallic 2D FeNi MOF by liquid-solid mode (Fig.3).They dissolved FeCl2·4H2O and terephthalic acid (C8H6O4) in a mixed solution ofN,N-dimethylformamide (DMF)/ethanol/water (14:1:1, v/v/v).After fully stirring, the mixed solution and nickel foam are placed in a Teflon-lined autoclave for solvothermal reaction.In this process, because the C8H6O4solution is acidic, the nickel atoms on the surface of the nickel foam will be partially oxidized to Ni2+,thereby participating in the formation of MOF.Consequently, the FeNi bimetallic MOF nanosheets attached to the surface of the nickel foam can be finally obtained.The advantage of liquid-solid mode is that the as-prepared electrode does not require a binder,and the synthesized bimetallic 2D material can be firmly attached to the surface of the base nickel foam [99].
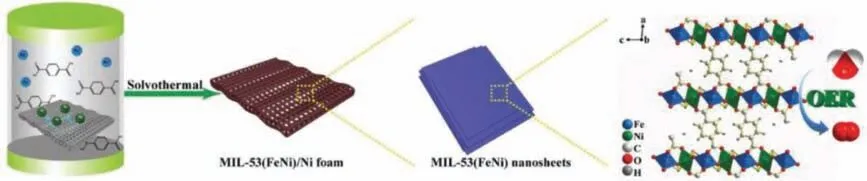
Fig.3.Schematic illustration of the fabrication of 2D NiFe MOF on nickel foam.Reprinted with permission [99].Copyright 2018, Wiley-VCH.
Panet al.used a very similar method to synthesize FeNi 2D MOF electrodes.However, they utilized FeCl3·6H2O as the iron source and 2-amino terephthalic acid as the organic ligand.In their synthesis path, Fe3+will oxidize Ni atoms to be Ni2+on the surface of Ni foam, and itself is reduced to Fe2+.Then due to the presence of air in the Teflon-lined autoclave, Fe2+is again oxidized to Fe3+.At last, the metal junctions of the final FeNi MOF nanosheets are still Ni2+and Fe3+.Besides, they took experiments to prove the stability of the bimetallic 2D MOF electrode synthesizedviasolid-liquid mode.After maintaining a current density of 50 mA/cm2under applied potential for 24 h, no significant current density loss (<10%) is observed.What is more, they also demonstrated that after the OER reaction, the electrode has undergone a certain change, that is, FeNi MOF nanosheets are partially converted intoγ-NiOOH and FeOOH.The former is a highly efficient electrocatalytic OER catalyst, while the latter has poor electrocatalytic performance due to its poor conductivity.However, owing to the direct growth on the Ni substrate, the conductivity of FeOOH is significantly improved, and the OER performance is thus enhanced[100].
Huet al.took a solid-solid metal source model to synthesize two-dimensional Fe-CoP/CoO (Fig.4) [97].They directly put the solid metal salts CoSO4·7H2O and FeSO4·7H2O in molten sodium hydroxide, and kept the reaction for 1 min to prepare FeCo LDH/CoO.Then they utilized FeCo LDH/CoO as the precursor for phosphating.In detail, FeCo LDH/CoO and NaH2PO2were placed in the same porcelain boat, which were then put into a tube furnace, with NaH2PO2in the upstream position.Afterwards, under the protection of Ar, Fe-CoP/CoO can be obtained after 2 h annealing at 300°C, while pure Fe-CoP can be acquired within 9 h.In this fabrication method, since molten sodium hydroxide can provide ultra-high concentration of exposed OH−, which will directly react with Co2+and Fe2+without traditional hydration [101,102], the reaction speed and material quality can be greatly improved.The FeCo LDH/CoO prepared by this method only takes 1 min, which is much faster than the traditional way [103].
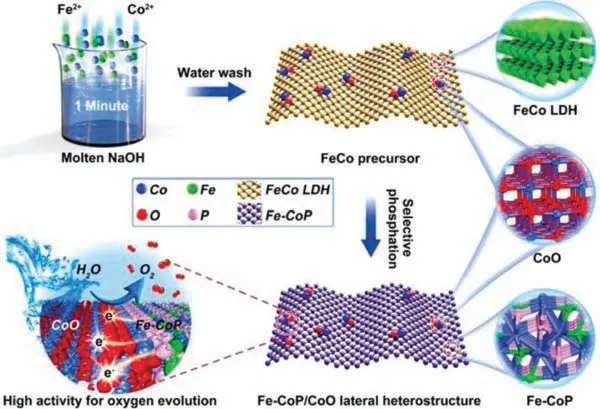
Fig.4.Schematic illustration of the synthesis of Fe-CoP/CoO nanosheets.Reprinted with permission [97].Copyright 2021, Royal Society of Chemistry.
3.1.2.The order of the metal sources
The other classification route from the bimetallic level is based on the orders of the incorporation of metals, which can be divided into three modes: the simultaneous addition of AB precursor, A intermediate+B precursor, and A finished product+B precursor.The first model is the most commonly used one without any particularity, and will not be discussed in detail here.
A intermediate+B precursor: Liet al.prepared bimetallic conductive MOF nanosheets (Fig.5) under the mode of A intermediate+B precursor.They first synthesized monometallic nickel phthalocyanine (NiPc), and then took NiPc (Ni intermediate) and zinc(II) acetate dihydrate (Zn precursor) to fabricate bimetallic 2D MOF (NiPc-Zn)viaa solvothermal method.In particular, as shown in Fig.1b, in the NiPc-Zn prepared by this strategy, the statuses of the two metals are unequal.Nickel is located in the center of phthalocyanine, and the coordination environment is Ni-N4, while zinc is the metal junction connecting NiPc, and the coordination environment is Zn-O4.As described above in the material structure, due to the 18-electron conjugation in the phthalocyanine, the as-fabricated bimetallic 2D MOF has good conductivity.Besides,they also fabricated ZnPc-Ni using the same synthetic strategy [93].
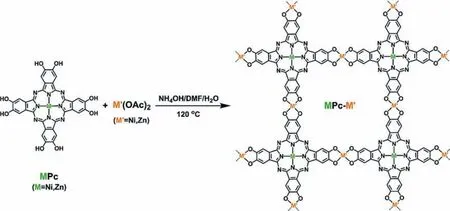
Fig.5.Schematic illustration of the construction of NiPc-Zn or ZnPc-Ni MOF nanosheets.Reprinted with permission [93].Copyright 2021, Royal Society of Chemistry.
Liet al.exploited the A intermediate+B precursor model to synthesize Fe-Co3O4nanosheets.They first took a simple precipitation method to fabricate 2D Co MOF film on carbon cloth (Co-MOF NS/CC), with Co(NO3)2·6H2O as the Co source, 2-methylimidazole(C4H6N2) as the organic ligand.Then the prepared Co-MOF NS/CC(Co intermediate) was immersed in the [Fe(CN)6]3−solution (Fe precursor) and the mixture was stirred for 2 h for anion exchange.After drying, the obtained Fe@Co MOF NS/CC was annealed at 350°C for 2 h to get Fe-Co3O4nanosheets.In this method, 2D Co-MOF NS assumes the role of a template.The prepared 2D Fe-Co3O4nanosheets have good stability and excellent electrocatalytic OER performance [104].
From the above examples, we can see that the strategy of A intermediate+B precursor can help to obtain 2D nanosheets with inconsistent statuses of two metals.Besides, this strategy always involves with ion-exchange reaction and has certain advantages in preparing products like bimetallic oxide nanosheets that require further calcination.
A finished product+B precursor: Penget al.took the mode of A finished product+B precursor to fabricate a range of bimetallic 2D MOF (Fig.6).They firstly prepared a 2D Fe-based MOF (HUST-8) with FeCl2as the iron source and tetrapyridinate as the organic ligand using a diffusion route.Then they employed a reflux means at 85°C to introduce a single metal atom into the unoccupied porphyrin center of HUST-8 to fabricate bimetallic 2D MOF materials,i.e., Ni@HUST-8, Co@HUST-8, Zn@HUST-8 and Mn@HUST-8.Obviously, in the bimetallic 2D MOF obtained by this method, the positions of the two metals are not the same.Iron assumes the metal joints linking the organic ligands, while the other metal is trapped in the porphyrin center of the ligands.They demonstrated that Ni@HUST-8 has the best OER performance due to the special synergistic effect of Fe and Ni centers.The advantage of this method is that the synthetic temperature is low since the unoccupied porphyrin centers provide appropriate space for external metal ions[105].
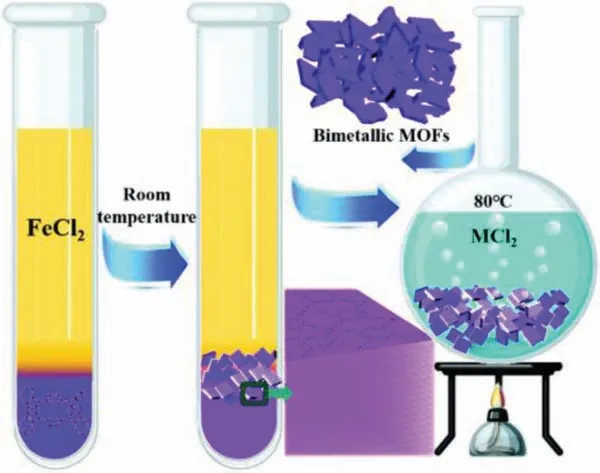
Fig.6.Schematic illustration of Ni@HUST-8.M stands for metal.Reprinted with permission [105].Copyright 2020, Royal Society of Chemistry.
Zhouet al.reported a cation exchange way to prepare irondoped Ni(OH)2and Co(OH)2nanosheets [106].This method can also be classified in the model of A finished product+B precursor.Take Fe doped Ni(OH)2as an example.They put NiOH nanosheets in 50 mL ethanol, and made the solution into a suspension with 3 h of ultrasonication and 1 h of stirring.After that, anhydrous FeCl3was added, and the mixture was stirred for 12 h at 50°C to obtain Fe doped Ni(OH)2nanosheets.The as-fabricated materials had higher electrochemically active surface area (ECSA) and improved surface wettability than that of FeNi LDH prepared by the traditional one-pot method, and thus had higher OER performance.This phenomenon is because that in the process of cation exchange,a chemical etching process occurs simultaneously.In detail, small amount of FeCl3can be converted into Fe(OH)3in water, and a little HCl will be produced concomitantly.Consequently, Ni(OH)2will be partially etched by HCl, resulting in a large number of defects and nanopores.
Like A intermediate+B precursor, A finished product+B precursor also contributes to the synthesis of bimetallic 2D nanosheets with unequal statuses of two metals.Similarly, this strategy often involves ion exchange reactions as well.The difference between the two strategies is that, after the ion exchange reaction, the A finished product+B precursor method directly obtains the final product without further processing such as calcination.
3.2.2D strategy
From the perspective of synthesizing two-dimensional nanomaterials, there are three strategies, namely top-down, bottom-up and template conversion strategies.Top-down means that the bulk precursor is synthesized first, which is then peeled off into a twodimensional nanosheet structure by various exfoliation methods.Bottom-up refers to using methods such as solvothermal way to directly prepare 2D nanosheets by controlling reaction conditions and adding modifiers.The template conversion method refers to the use of 2D nanomaterials as precursors and template, which are transformed into new 2D nanomaterials through reactions under certain conditions.
3.2.1.Top-down
Zhaoet al.employed the top-down strategy to fabricate the ultra-thin NiCo 2D MOF [92].They first prepared the bulk NiCo MOF using an ultrasound-assisted method.In detail, a mixed solution of DMF, water and ethanol was first prepared in a polytetrafluoroethylene (PE) tube.After that, under the assistance of ultrasound, the ligands, Benzenedicarboxylic acid (BDC), metal sources,CoCl2·6H2O and CoCl2·6H2O, and triethylamine (TEA) were added to the above solution in turn.After 5-min stirring, the bulk NiCo MOF was initially obtained.Then, after 8 h of strong ultrasonication, ultra-thin NiCo 2D MOF nanosheets were acquired.
It is worth noting that, apart from the above-mentioned case,we have not found more articles concerning the top-down strategy for the preparation of bimetallic 2D MOF nanosheets in recent year literature.This may be attributed to the shortcomings of the topdown way: (1) Due to the stability of the internal structure with interlayer chemical bonds, many bulk MOFs cannot be peeled off,such as MOF-74 [107], zeolitic imidazolate framework-67 (ZIF-67)[108], MOF-5 [109] and Materials Institute Lavoisier (MIL) [110].(2)Ultrasonic treatment for too long time will inevitably lead to morphological damage and structural deterioration.(3) High-power ultrasound is costly and will produce much noise.
3.2.2.Bottom-up
Bimetallic 2D MOF: Liet al.adopted a bottom-up solvothermal strategy to synthesize a series of bimetallic 2D MOFs, including NiFe, NiAl, NiCo, NiMn, and NiCd MOFs [111].In their scheme, the coordination polymer is restricted from growing in one directionviaa mixed solution, and the interaction between crystalline layers is successfully inhibited.Specifically, they dissolved the metal salts and the organic ligand (terephthalic acid) in the mixed solution ofN,N-dimethylacetamide (DMAC) and water, and then took a solvothermal reaction to fabricate the above-mentioned bimetallic 2D MOFs.In this process, the configuration of the mixed solvent is the key.Using water alone as the solvent will produce amorphous nanoflowers decorated with nanoparticles, while using DMAC alone will produce fluffy powder.Only when the solvent is the mixture of DMAC and water, can a 2D nanosheet with a good crystal form be produced.Moreover, the output of this route is very large, even reaching gram-level.The nickel-iron metal ion is coordinated with six oxygen atoms (O6), which come from carboxylate, hydroxyl and water ligands.Metal ions and O6form a geometric octahedral structure with the metal ion as the geometric center.These octahedral structures are connected into a chain by ligands, and they are then connected into a network by ligands.The thickness of the 2D MOF is 2–3 coordination layers.
Yanget al.took a method very similar to that of Liet al.to synthesize CoFe MOF nanosheets and CoNi MOF nanosheets [112].Different from Li, Yang used 5-((pyridin-4-ylthio)methyl)isophthalic acid as the organic ligands.Therefore, in the 2D MOF they fabricated, the coordination environment of cobalt and iron is four oxygen and two nitrogen atoms, rather than O6.
Geet al.also successfully synthesized FeCo MOF nanosheets.However, unlike the above method, he added triethylamine (TEA)to the traditional solvent of DMF, ethanol and water, thus eliminating the solvothermal steps, and fabricating the FeCo 2D MOF by simply stirring at room temperature.The ligand they exploited was 1,4-benzenedicarboxylic acid (1,4-BDC) and the metal sources were Co(NO3)2·6H2O and FeCl3·6H2O.Herein, without TEA, that is, using the mixture solvent of DMF, ethanol and water, only bulk MOF could be obtained; without water, the final product was merely three-dimensional MOF.Only with the combination solvent of the four liquids, can an excellent two-dimensional MOF structure be obtained.Among the mixture solvent, TEA can not only promote the deprotonation of the carboxylic acid in the ligand, but also accelerate the coordination of the ligand and the metal ion.In the presence of water, a small amount of hydroxide ions from the water can help stabilize the formation of the edge of the twodimensional material.Compared with bulk materials and threedimensional materials, the obtained 2D material can significantly improve its electrical conductivity and obtain more unsaturated metal ligands, thereby efficiently reducing its overpotential.Besides, both Fe3+and Co2+coordinate with O6, and Fe3+can transfer part of the electrons to Co2+through the ligand, thereby changing the electronic structure of the entire MOF and improving the activity of the catalytic sites [113].
LDH nanosheets: Fanet al.utilized a one-step hydrothermal method to synthesize monolayer NiV LDH [114].They prepared a mixed aqueous solution of NiCl2and VCl3, then added a certain amount of urea into the mixture.After transferring the mixture into a Teflon-lined autoclave, they exploited a hydrothermal reaction at 120°C for 12 h to obtain the final LDH.In this process,weakly alkaline urea provides slow-release OH−, which is beneficial to the synthesis of thin-layer LDH.The as-prepared monolayer Ni0.25V0.75-LDH has electrocatalytic OER activity comparable to NiFe-LDH.
Gonget al.used a two-step solvothermal method to adhere NiFe LDH to the outside of multi-walled carbon nanotubes(MWCNT) [115].They first dispersed MWCNT in anhydrous DMF by ultrasound, and then stirred the mixture vigorously at 85°C for 4 h.After that, the final product is obtained by two-step solvothermal method (120°C, 12 h; 160°C, 2 h).
Jianget al.took a surfactant-assisted solvothermal method to synthesize 2D CoNi LDH on the surface of nickel foam[116].Typically, they dissolved Co(NO3)2·6H2O, Ni(NO3)2·6H2O and cetyltrimethyl ammonium bromide (CTAB) in a mixed solution of water and methanol.Then the mixture was transferred to a Teflonlined autoclave, and a piece of nickel foam was partially immersed in the solution.After 24 h of solvothermal reaction (180°C), NiCo-LDH nanosheet arrays attached to nickel foam were obtained.The strategy ofin-situsynthesis of 2D LDH directly on nickel foam can avoid the use of binders and conductive additives, and thus can greatly reduce the dead volume of the electrode.
Qiaoet al.used a one-step microwave-assisted method to synthesize ZnCo LDH nanosheets [117].They dissolved Zn(NO3)2·6H2O,Co(NO3)2·6H2O, and urea in water, and then added ethylene glycol to the solution.After transferring the mixture in a round-bottom flask to the microwave oven, they began the microwave irradiation for 20 min to acquire ZnCo LDH nanosheets.Compared with the hydrothermal method, the microwave-assisted route is more convenient, and the required synthesis time is greatly shortened.
Beraet al.took a one-step co-precipitation method to synthesize CoMo LDH nanosheet and nanoflake, respectively [118].The former was synthesized by using CoCl2and Na2MoO4solution as the metal source, dropping NaBH4as the alkali source, and stirring for 20 min.The latter is formed by adding dropwise of a mixed solution of Na2MoO4and NaOH to the CoCl2aqueous solution and stirring for 15 min.Experimental results proved that the OER activity of CoMo LDH nanosheet is better than that of CoMo LDH nanoflake.
Chenet al.synthesized Cu doped cobalt hydroxide (Cu–Co(OH)2) nanosheetsviaa metal self-oxidation method [119].They first dissolved CoCl2and CuCl2in a mixed solution of water and ethanol.Then, under the protection of argon, an ethanol solution of NaBH4is added to the above solution to reduce Co2+and Cu2+to CoCu alloy particles.After that, the particles were washed and put into water, and after two days of auto-oxidation process in the air, Cu–Co(OH)2nanosheets were obtained.In addition, they used the same method to synthesize Mn–Co(OH)2nanosheets.
Liet al.synthesized MFe-LDH (M=Ni, Co and Li) on the surface of nickel foam by electrochemical synthesis [120].Specifically, they took nickel foam as the working electrode, and Pt wire and saturated calomel electrode (SCE) as the counter and reference electrodes, respectively.An aqueous solution of Ni(NO3)2(or Co(NO3)2or Li(NO3)2) and Fe(SO4)2was used as the electrolyte, and the electrochemical synthesis was conducted at −1 Vvs.SCE under nitrogen protection and stirring.Then, after autooxidation in the air, MFe LDH containing Fe3+is obtained.It should be noted that during the electrosynthesis process, a reaction of NO3−+H2O+2e−→NO2−+2OH−occurs on the surface of the working electrode.The generated OH−can be combined with metal cations to form LDH.
Rosaet al.prepared NiFe-LDH nanoplatelets by the continuous hydrothermal flow synthesis (CHFS) method (Fig.7) [98].In brief,they introduced a mixed aqueous solution of KOH and K2CO3at 170°C from the upper side of the reactor, and another mixed aqueous solution of Ni(NO3)2and Fe(NO3)3at room temperature from the lower side of the reactor.The mixture obtained after the reaction flowed into a re-heater (80°C) and stayed for 37 s.Then the obtained products were separated and purified.During the CHFS process, the pressure in the reactor must always be maintained at 200 bar, which is essential for the stability of the flow rate.The CHFS method has the advantages that the reaction conditions are stable and uniform, and stable super-saturation conditions are provided in the material mixing stage, thereby reducing the heterogeneity of the obtained nanomaterials.
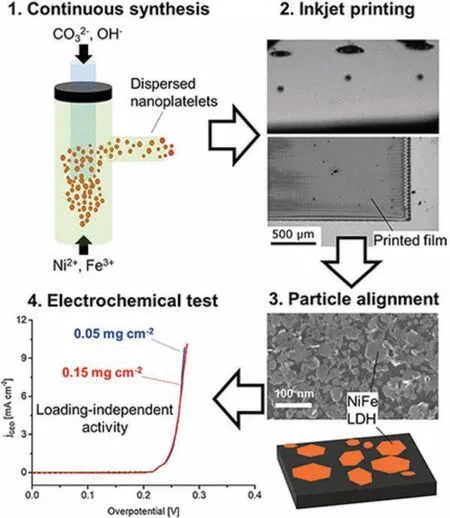
Fig.7.The fabrication and application of NiFe-LDH nanoplatelets with a continuous hydrothermal flow synthesis (CHFS) method.Reprinted with permission [98].Copyright 2020, American Chemical Society.
It can be seen from the above that bimetallic 2D LDH nanosheets can be synthesized by various methods.We have summarized the advantages and disadvantages of these methods in Table 1 for reference [121].In fact, the preparation of bimetallic 2D LDH is essentially a process of making a weak base from a strong base.The bimetals mentioned in this review contain either cobalt or nickel.Generally, most of the cobalt and nickel salts used are chlorides or nitrates with good solubility, and sometimes acetate is also utilized.For V, Mo, and other non-group-VIII transition metals,we often use vanadate, molybdate, or like as precursors.The alkali taken includes sodium hydroxide (potassium hydroxide), sodium borohydride and urea (from strong to weak).Actually, the methods listed in Table 1 are the conditions required for the reaction, including temperature, pressure, electric field (electrodeposition) and electromagnetic field (microwave).The key to preparing bimetallic 2D LDH is to select suitable precursors, alkali sources, and reaction conditions, so that a sufficiently thin 2D nanosheet can be ob-tained.For how to choose reaction conditions or reaction methods,Table 1 can be referred to.
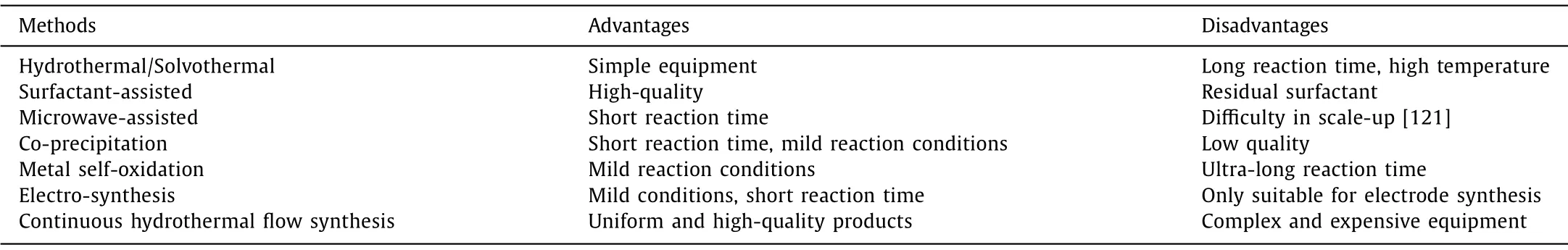
Table 1 The advantages and disadvantages of bimetallic 2D LDH synthesis methods.
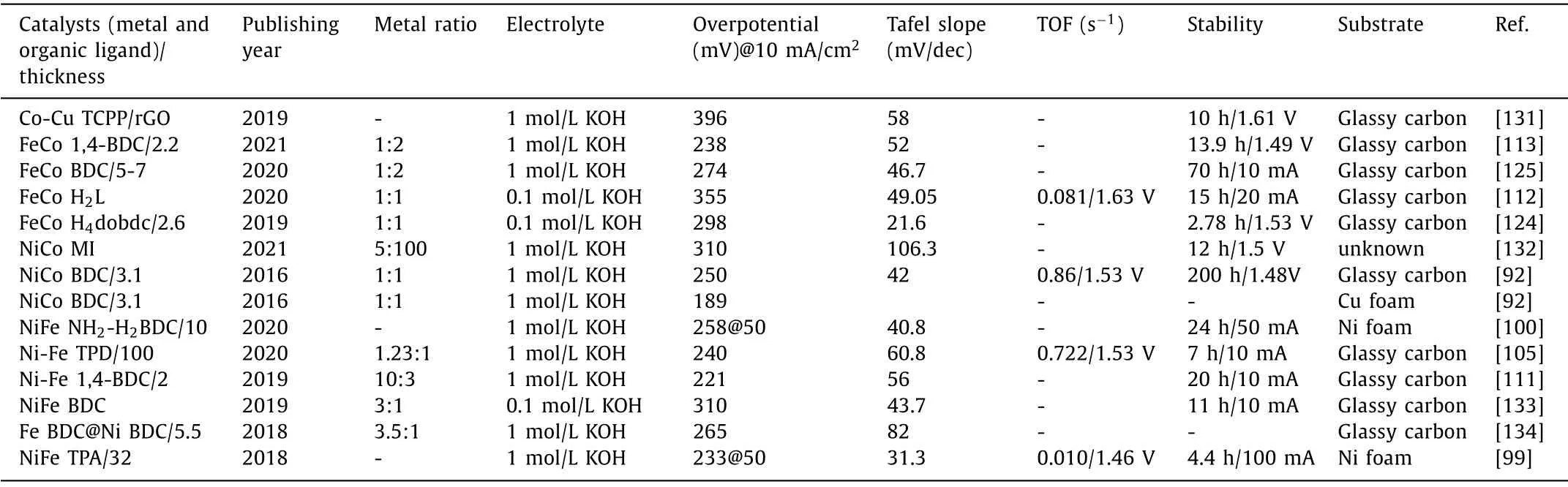
Table 2 Electrocatalytic OER performance of bimetallic 2D MOF nanosheets.
Bimetallic phosphate and Prussian blue analogue nanosheets:Hanet al.synthesized 2D amorphous Fe-doped indium phosphate nanosheets by combining two-phase colloid and solvothermal methods [122].They first dissolved In(NO3)3·4.5H2O and Fe(NO3)3·9H2O in oleylamine (OM) and 1-octadecene (ODE) oil solvents to obtain solution A.Then the NaH2PO4aqueous solution was poured into solution A.After mixing, the mixed solution was transferred to a teflon-lined autoclave, and solvothermal reaction was carried out at 180°C for 24 h.In the process, the oil phase and the water phase are located at the upper and lower parts of the reactor, respectively.The chemical reaction occurs at the interface of the oil phase and the water phase, which is the key to ensuring that the synthesized two-dimensional material is sufficiently thin and amorphous.
Wanget al.used a two-step method to synthesize 2D S-CoFe-PBA/carbon fiber paper (CFP) [123].In the first step, they slowly poured the aqueous solution of Na2Fe(CN)5NO·2H2O into the aqueous solution of Co(NO3)2·6H2O.Then added CFP to the mixed solution and let it stay for 24 h to obtain CoFe-PBA/CFP.In the second step, they took a solvothermal method to prepare S-CoFe-PBA/CFP.In brief, they put thioacetamide (TAA) and CoFe-PBA/CFP in ethanol and transferred the mixture to a Teflon-lined autoclave, and then kept it at 90°C for 1.5 h.
3.2.3.Template transformation
Yaoet al.transformed FeCo oxide nanosheets into 2D FeCo MOFviaa solvothermal method.They first synthesized FeCo bimetallic nanosheets, then placed them in a mixed solvent of DMF, water and ethanol, and added the ligand 2,5-dihydroxyterephthalic acid to convert the oxide into a two-dimensional MOF in a Teflonlined autoclave (80°C, 12 h).The 2D MOF prepared by this method is very thin, only 2.6 nm, thanks to its ultra-thin precursor (FeCo oxide nanosheets, 1.2 nm).In the process, the precursor serves as a template, which can limit the growth of MOF only on a twodimensional plane, instead of longitudinal direction.Besides, no need for surfactants is another advantage.The resulting product has an overpotential of 298 mV for electrocatlaytic OER.In particular, it has an ultra-low Tafel slope, only 21.6 mV/dec.Unsaturated metal sites are the merits of the 2D FeCo MOF, and the electron transfer from Co2+to Fe3+is a key for the excellent OER performance [124].
Caiet al.used a simple ligand-assisted synthesis method to convert the CoFe LDH into 2D CoFe MOF.Specifically, they put CoFe LDH and MOF organic ligand (terephthalic acid) into DMF solvent with ultrasonication, and then took solvothermal means to transform LDH into 2D MOF.In the process, LDH can continuously provide metal ions, and its 2D structure serves as a template for generating 2D MOF.Besides, their experimental results proved that the product obtained by the precursor conversion method is more catalytically active than that acquired from the traditional top-down or bottom-up synthesis routes [125].
Zhanget al.took the template transformation way for the fabrication of LDH.They first synthesized 2D FeNi MOF on the surface of Cu foil [126].In brief, they placed the Cu foil in the mixed solution of potassium(III) trihydrate trioxalate (Fe source), NiCl2·6H2O(Ni source) and tetrapentyl ammonium bromide (organic ligand).After staying for 10 min, the Cu foil was taken out, washed and dried, and a 2D FeNi MOF supported on the Cu foil (FeNi MOF/Cu)was obtained.Then they placed FeNi MOF/Cu in water and added potassium hydroxide solution dropwise to the water.After 30 min,the 2D MOF was completely converted to 2D LDH.After washing and drying, 2D FeNi LDH/Cu foil was obtained.They also obtained FeCo LDH/Cu foil using the same method, and some other 2D trimetal FeNiCo LDH/Cu foil.
Donget al.converted 2D NiFe LDHs into two-dimensional NiFe oxides by calcination (Fig.8) [127].They first synthesized 2D NiFe LDH by solvothermal method.In particular, they chose polyethylene glycol 200 (PEG-200) as the solvent.PEG-200 is a good morphology control agent, and can control the formation of nanostructures.This is because the two hydroxyl groups of PEG-200 can be selectively adsorbed on certain surfaces to promote the growth rate of these surfaces.Typically, they took Ni(NO3)2·6H2O and Fe(NO3)2·9H2O as the metal sources, and anhydrous sodium acetate (CH3COONa) as the alkali source, and fully dissolved the three in PEG-200.Then the solvothermal reaction was carried out at 200°C for 16 h.After drying, 2D NiFe LDH nanosheets were obtained, which was then annealed at 350°C to obtain Fe doped NiO nanosheets.In the transformation process (annealing), NiFe LDH assumes the role of template and the precursor of Fe doped NiO nanosheets.
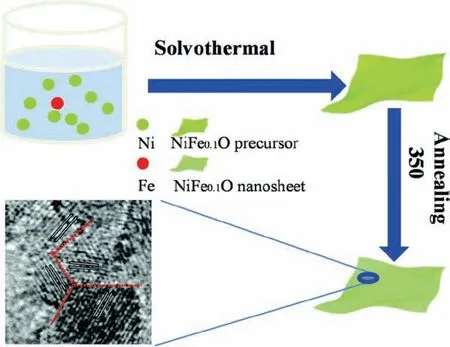
Fig.8.Schematic illustration of the synthesis of NiFe oxides.Reprinted with permission [127].Copyright 2020, Royal Society of Chemistry.
Liet al.used 2D Fe@Co-MOF as a template and calcined it to obtain 2D Fe-Co3O4[104].They first synthesized Co MOF nanosheets on the surface of carbon cloth (Co-MOF NS/CC)by a simple precipitation method.The Co source taken was Co(NO3)2·6H2O, and the organic ligand was 2-methylimidazole(C4H6N2).Then Co-MOF NS/CC was immersed in [Fe(CN)6]3-solution for 2 h to obtain Fe@Co-MOF NS/CC.After heating at 350°Cfor 2 h in the air, Fe-Co3O4nanosheets supported on carbon cloth were obtained.
Chaviet al.synthesized CoFe2O4and NiFe2O4nanosheets through the way of precursor template conversion [128].They first took a wet chemical route to fabricate CoFe hydroxide.Specifically,they added urea and glucose to a mixed solution of Co(NO3)2·6H2O and FeSO4·7H2O.After thoroughly stirring, a solvothermal process was conducted at 140°C for 6 h to obtain soft black CoFe hydroxide.The CoFe2O4nanosheets were acquired after the calcination of CoFe hydroxide at 500°C for 10 h.The same method was utilized to obtain NiFe2O4nanosheets.In this fabrication route, glucose played the role of carbon source and can be used as the template for generating the 2D products.In the subsequent calcination process, the carbon template could be removed.
Jianget al.prepared bimetallic CoFe phosphide (Co1−xFexP)ultra-thin nanosheets by a two-step conversion route [129].They first prepared Co1-xFex(CO3)0.5(OH)·0.11H2O nanosheets using a surfactant-assisted solvothermal method.In the process, they took cobalt acetate and iron acetate as metal sources, CTAB as a surfactant, and the reaction was realized in a mixed solvent of water and ethanol at 180°C for 24 h.After that, Co1-xFex(CO3)0.5(OH)·0.11H2O was calcined at 320°C for 5 min to obtain Co3–3xFe3xO4.Then, in a tube furnace, Co3–3xFe3xO4was converted to Co1-xFexP ultra-thin nanosheets with NaH2PO2as P source at 300°C for 2 h under the protection of argon.It is worth noting that the material had both a crystalline phase and an amorphous phase, and this structure might be beneficial to electrochemical catalytic activity [130].
In summary, the bimetallic 2D MOF and LDH can be transformed into each other, while bimetallic 2D oxides generally need to be converted from LDH by calcination, and phosphides need to be obtained by phosphating LDHs or oxides.
4.Performances
The two most important parameters that characterize the performance of electrocatalytic OER are overpotential and Tafel slope.Overpotential (η) generally refers to the difference between the potential (vs.RHE) required to reach a certain current density and the hydrolysis potential (1.23 Vvs.RHE).In the literature, the overpotential with a current density of 10 mA/cm2is usually used as a reference for parallel comparison.The Tafel slope is employed to reveal the electrocatalytic kinetics and activity mechanism, and the smaller the Tafe slope, the faster the reaction kinetics rate.For the specific calculation methods of the two, please refer to the review[45] by Lvet al.
In this review, we have listed the relevant results of bimetallic two-dimensional nanomaterials in Tables 2–4.It can be observed from Table 2 [92,99,100,105,111–113,124,125,131–134] that the lowest overpotential of the bimetallic two-dimensional MOF is 189 mV@10 mA/cm2.It needs to be noted that this value is realized when Cu foam is the substrate.When glassy carbon electrode serves as the base material, the lowest overpotential is 221 mV,which is achieved by the classic bimetallic 2D MOF:NiFe-MOF with 1,4-benzenedicarboxylate as organic ligands.The thickness of the two-dimensional NiFe MOF is only 2 nm, and the bimetallic ratio is Ni:Fe=10:3.Besides, the lowest Tafel slope of the bimetallic 2D MOF is only 21.6 mV/dec, which is realized by the 2D FeCo MOF with 2,5-dihydroxyterephthalic acid as organic ligands.The thickness of the MOF is 2.6 nm, and the molar ratio of Fe:Co is 1:1.
From Table 3 [94,98,106,114–120,135–140], we can know that the smallest overpotential and the smallest Tafel slope are focused on NiFe LDH, with the thickness of 3 nm, and the NiFe ratio of 6:1.We need to pay attention to the fact that this NiFe LDH has been amorphized and it is foreseeable that amorphization may be a direction to improve OER activity in the future.
In Table 4 [72,95,97,104,118,122,123,128,129,141–143] are 2D oxides, phosphides and Prussian blue analogues, some of which also have a very unmissable potential and Tafel slope, such as Fe1Co1Oxwith a thickness of only 1.2 nm, and Co0.7Fe0.3P supported on carbon nanotubes.
In addition, some scholars believe that the mass activity, which is the current density per unit mass of a catalyst at a certain potential, can more truly reflect the catalytic ability of the electrocatalyst.However, because there is no uniform standard for the selected specific potential, the concept of mass activity has not yet been widely applied.It may be an essential catalytic performance parameter in the future.Turnover frequency (TOF) refers to the number of times that a unit mole of metal atoms participates in the reaction per unit time (within per second) under a specific potential or current.Its calculation method can refer to the previous literature [144].However, TOF is also difficult to use for a wide range of lateral comparisons because researchers use different specific potentials or currents.
Moreover, there are two ways to characterize the stability of the catalyst.One way is to compare the polarization curves before and after a certain number of CV cycles (e.g., 1000).The other is to test the lasting time under a certain current or voltage.After comprehensive comparison of Tables 2–4, we found that both FeCo-BDC MOF and NiCo-BDC MOF have reached ultra-long-time curve, indicating that BDC-related MOF might be quite stable.Besides that,most of bimetallic 2D materials can maintain a good current level within a certain period of time, although the overall stability results of them are irregular.After further analysis, it should be noticed that almost all of the studies investigated in this review involve Ni or Co element, which might be a fundamental reason for the good stability of these materials in alkaline solutions.This is because Co or Ni-related materials can often be oxidized to highvalence NiOOH or CoOOH during the electrocatalytic OER process,and both of NiOOH and CoOOH are reported to be good and stable OER catalysts.Actually, this is the advantage of Ni- or Co-related materials over the traditional precious metals Ru or Ir based materials.According to previous reports, Ru- and Ir-related materials are easily oxidized to RuO4and IrO3in the OER process under alkaline conditions, thereby being dissolved in the aqueous solution and leading to weak stability [43–45].Therefore, theoretically speaking, the Ni or Co-2D material can help expose more Ni or Co sites, so that it is logically easier to obtain NiOOH and CoOOH for Ni or Co-2D materials.However, since most articles did not compare 2D materials with non-2D materials in terms of stability, it is difficult to make a more accurate summary at this point.We hope that this content can be added in the future studies about the application of bimetallic 2D materials in electrocatalytic OER.
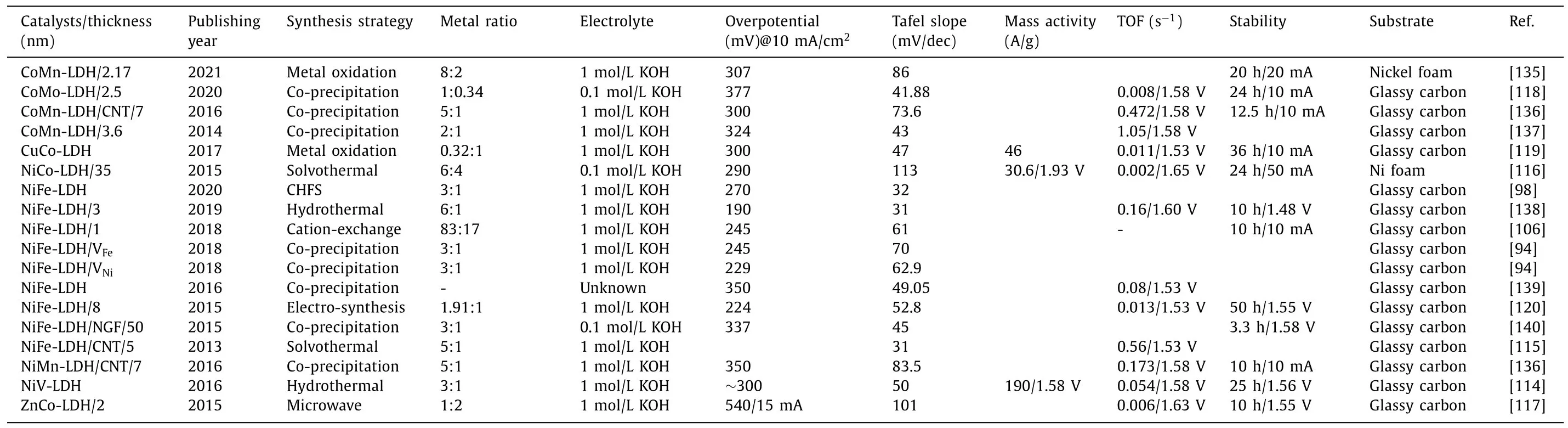
Table 3 Electrocatalytic OER performance of 2D LDH.
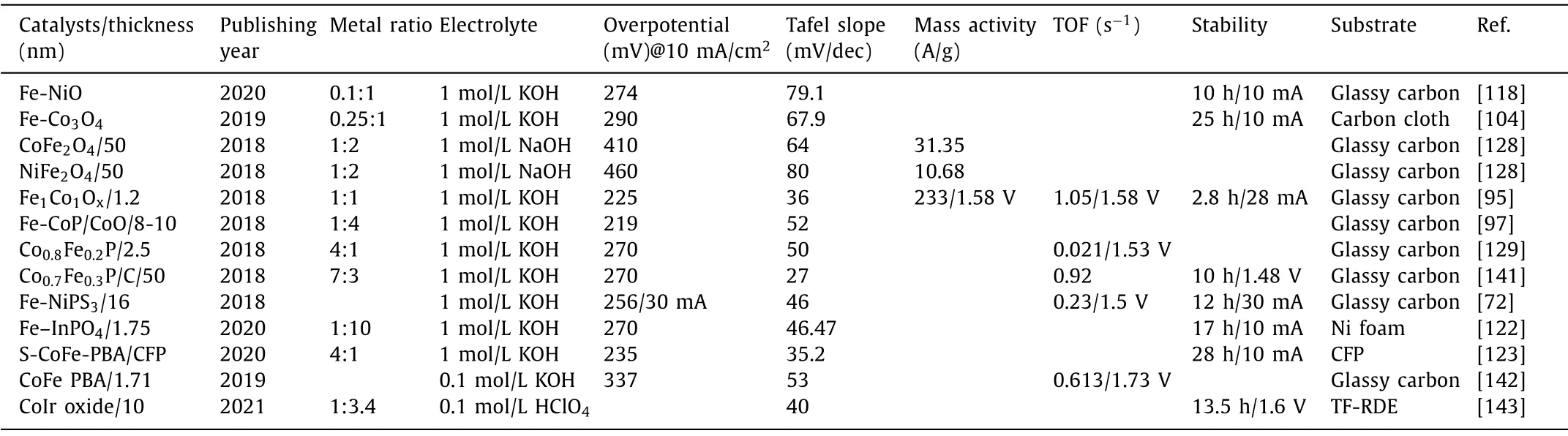
Table 4 Electrocatalytic OER performance of bimetallic 2D oxide, phosphide, phosphate, PBA.
In the tables of this review, we have summarized the above series of performance characterizations.At the same time, we also summarized the thickness of the two-dimensional nanomaterials,the ratio of the bimetal and the year of publication in the table.It can be found from the tables that the thickness of twodimensional nanomaterials mostly reaches about 1–10 nanometers.As to the use of metals, the three elements of iron, cobalt and nickel dominate more than half of the previous work.The ironnickel combination is the most frequently exploited one, which is followed by the iron-cobalt combination.The ratio of iron to nickel is usually 3:1.However, the ratio of iron to cobalt is more various,but in most cases, cobalt content is greater than that of iron.The reason is because, usually, nickel and cobalt are the active sites of OER, and the addition of iron enhances the OER activity of nickel or cobalt through the electronic interaction.
At last, we focused our attention on the only article [143] that reported bimetallic 2D nanomaterial for OER reaction in an acidic solution.In this research, CoIr bimetallic 2D nanoframe was preparedviaa microwave-assisted route, and then the OER test was conducted in a 0.1 mol/L HClO4electrolyte.The authors pointed out that the 2D nano-frame structure can provide more sufficient active space for reactive molecules.The bimetallic design can optimize the Gibbs free energy of the reaction intermediate on the one hand, thereby reducing the overpotential.On the other hand,it can reduce the amount of precious metal Ir used.
5.Mechanisms
Many characterizations and density functional theory (DFT) calculations have been used to explore the reaction mechanism of bimetallic 2D nanomaterials in electrocatalytic OER.Among them[111], the ECSA can reflect the quantity of active sites, and the electrochemical impedance spectroscopy (EIS) is related to the charge transfer ability of the catalyst.Besides, since the electrocatalytic OER reactions involved in this review are mostly carried out in alkaline solutions, we present the typical reaction steps of electrocatalytic OER under alkaline condition as follows Eqs.1–4:
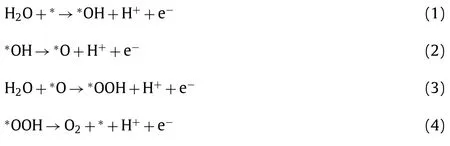
In the process, the Gibbs free energy of the four reaction equations areΔG1-ΔG4, respectively.The reaction step corresponding to the maximum of the fourΔGis the rate determination step(RDS).Generally, RDS is the maximum value ofΔG2andΔG3.It is reported that the valence state of the metal active site has a great influence on the electrocatalytic OER activity, which is a key factor in determining the value ofΔGmax[45].
It is worth noting that the formation of the O–O bond in Eq.3 belongs to the water nucleophilic attack (WNA) mechanism,also known as the acid-base mechanism.Typically, the active site(∗) is electrophilic, and the∗O obtained by adsorbing O through the double bond is still electrophilic.The nucleophilic water molecules attack the electrophilic oxygen in∗O, thereby forming an O–Oσbond, and at the same time destroying aπbond in∗O, and finally forming∗OOH.Moreover, regarding the formation of O–O bonds,there are also coupling between two metal oxygen/oxyl units (I2M)mechanisms, bimolecular hydroxyl coupling (BHC) mechanisms, intramolecular hydroxyl coupling (IHC) mechanisms, intramolecular oxygen/oxy coupling (IOC) mechanisms, and redox isomerization(RI) mechanisms [145–149].Whether these O–O bond formation mechanisms exist in bimetallic 2D nanomaterials for OER remains to be explored.
Additionally, we also need to notice that transition metal compounds, especially iron or nickel or cobalt-related materials, are often oxidized into oxyhydroxides, such as NiOOH, CoOOH, and FeOOH, during electrocatalytic OER process.According to previous reports, the electrocatalytic OER activity of those oxyhydroxides is very impressive.Some studies believe that they are the “true”effective electrocatalytic OER catalysts, and their performance is much better than other materials [150,151].As to bimetallic 2D nanomaterials, the same oxidation phenomenon occurs.For example, in the 2D FeNi MOF (the ligand is 1,4-BDC) prepared by Liet al.[111], after 1000 CV cycles, it can be seen from the XPS data that Ni3+peaks appear, indicating the formation of NiOOH.Similarly, they also discovered the formation of FeOOH.In the 2D NiCo LDH fabricated by Jianget al., they found that after a period of electrocatalytic OER, the green NiCo LDH nanosheets will turn to brown-black, which implies the formation of NiOOH and CoOOH[116].Moreover, in the 2D Fe-NiPS3prepared by Lianget al., Fe doped NiOOH was also generated in the OER process [72].
Afterwards, we will analyze the benefits of bimetallic 2D nanomaterials to the electrocatalytic OER from the perspectives of “2D”and “bimetal”, respectively.
5.1.2D mechanism
Two-dimensional nanomaterials can reduce the resistance of charge transfer and bring a larger electrochemically active surface area compared with bulk counterparts due to their ultra-thin characteristics, and most of the studies have measured EIS and ECSA to prove this viewpoint.
Besides, in terms of two-dimensional MOF, researchers believe that 2D MOF has more unsaturated metal active sites than bulk counterparts, which is beneficial to the OER process.Zhaoet al.put forward and profoundly proved this point for a ultrathin bimetal 2D NiCo MOF (BDC as organic ligand) nanosheets (NiCo-UMOFNs)[92].They experimentally demonstrated that the overpotential of NiCo-UMOFNs was reduced from 317 mV to 250 mV, and the Tafel slope decreased from 61 to 42 mV/dec compared to bulk NiCo MOFs.Besides, they logically speculated that due to the partially terminated BDC coordination bonding with surface metal atoms,the ultra-thinning of NiCo-UMOFNs will produce many unsaturated metal sites.Then, the synchrotron radiation-based X-ray absorption spectra (XAS) and corresponding DFT simulation calculations made it clear that NiCo-UMOFNs do have a large number of unsaturated metal sites.In particular, compared with bulk NiCo MOFs, the M-O coordination number of NiCo-UMOFNs has decreased significantly.Additionally, they explained why the unsaturated metal sites are conducive to the OER reaction through partial state density (PDOS)calculations.In detail, the previous literature proves that, within a certain range, the more filled the 3d egstate of the metal, the more favorable the OER reaction [152].PDOS proves that the 3d egstate of the unsaturated coordination metal of NiCo-UMOFNs is more filled than the 3d egstate of the saturated coordination metal of the bulk NiCo MOFs, so the OER capability of NiCo-UMOFNs is much stronger.
From the above discussions, we can find that the contribution of “2D” to bimetallic 2D nanomaterials lies in the advantages of the 2D structure in conductivity, the number of active sites, and the formation of coordinated unsaturated metal active sites.In particular, the acquirement of coordinated unsaturated metal active sites can be regarded as a unique contribution of 2D structure, which is rarely reported in other structures.
5.2.Bimetallic mechanism
In Zhao’s work, they also revealed how the synergy of NiCo bimetals makes the OER efficiency of NiCo UMOFNs greater than that of Ni UMOFNs and Co UMOFNs [92].First, their calculation results (Figs.9a and b) show that theΔGmaxof the unsaturated Ni site in NiCo UMOFNs isΔG3, 1.55 eV, and that of the unsaturated Co site is alsoΔG3, which is 1.54 eV.Besides, theΔGmaxof the unsaturated Ni site in Ni UMOFNs is stillΔG3, 1.63 eV,whereas that of the unsaturated Co site in Co UMOFNs isΔG1,1.77 eV.Obviously, theΔGmaxof NiCo UMOFNs is better than that of single-metal-based Ni UMOFNs and Co UMOFNs.Then, the XPS data shows that some electrons are transferred from Ni2+to Co2+through the oxygen of the ligand, and they explained this transfer processviacrystal field theory.In detail, since the valence band structure of Co2+is 3d7and has high spin, Co2+owns unpaired electrons on theπsymmetric (t2g) d orbital, which will interact with O2−throughπ-donation (Fig.9c).On the contrary, theπsymmetry (t2g) d orbital of Ni2+is completely occupied, and it interacts with the connected O2−through e−–e−repulsion.However,for NiCo UMOFNs, O2−in the middle interacts with Co2+via πdonation on the one side and has e−–e−repulsion interaction with Ni2+on the other side, thus causing part of Ni2+electrons pass to Co2+through O2−.After that, they took electrochemical experiments to demonstrate NiCo UMOFNs perform much better than the mixture of Ni UMOFNs and Co UMOFNs, disclosing that NiCo UMOFNs with electron transfer from Ni2+to Co2+have better electrochemical activity.
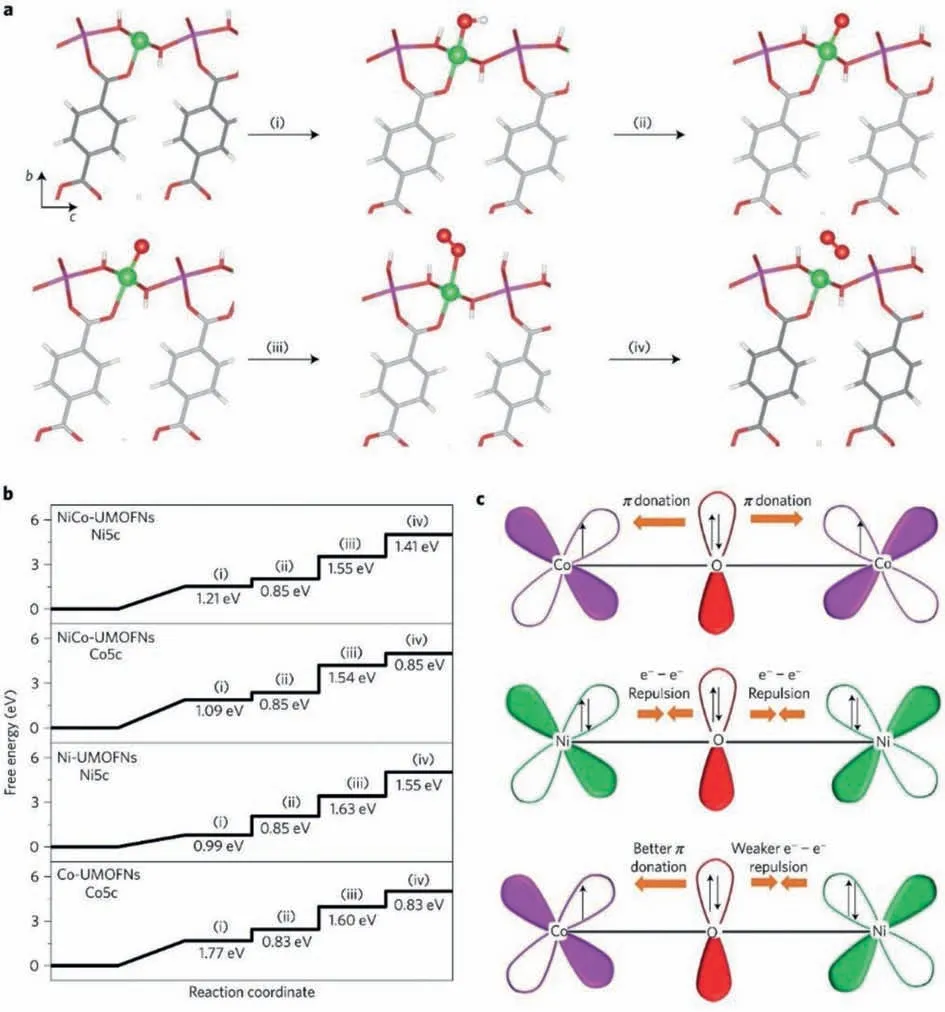
Fig.9.(a) Electrocatalytic OER steps of UMOFNs.Green for unsaturated metal sites (Ni/Co) coordinated by 5 O atoms, purple for saturated metal sites (Ni/Co) coordinated by 6 O atoms, red for O, grey for C, and white for H.(b) Gibbs free energy of the OER process on UMOFNs surfaces.(c) Schematic illustration of the electronic interaction between Co and Ni in UMOFNs.Reprinted with permission [92].Copyright 2016, Springer Nature.
Caiet al.took a simple ligand-assisted (terephthalic acid) synthesis method to convert the CoFe LDH into 2D CoFe MOF.XPS data display that the presence of bimetals of the MOF causes some electrons from Co2+to be transferred to Fe3+through theπconjugation produced by the bridged oxygen, so that Co gains a higher valence state, which may be beneficial to the OER reaction[125].
In the NiFe MOF reported by Sunet al.(BDC as the ligand), the DFT optimization result is that Ni is five-coordinated and Fe is sixcoordinated.Similar to Zhao and Cai’s report, XPS data prove that part of the electrons are transferred from Fe2+to Ni2+through the coordination ligand, resulting in higher OER performance[99].
Liuet al.synthesized 2D 5% NiCo MOF wtih 2-methylimidazole as the organic ligand.It should be noted that during the synthesis process of ZIF-68 containing no Ni and only Co, small metal particles were produced, and after the addition of Ni, the resulting MOF does not contain nanoparticles.This phenomenon shows that during the synthesis of MOF, the interaction of bimetallic ions is beneficial to resist the formation of metal particle agglomerates.Moreover, the addition of Ni will slightly change the overall bond length and bond angle in the MOF, which may also be one of the reasons that ultimately promote the performance of OER.Besides,the XPS results again show that the addition of Ni can change the electronic structure of Co, thereby increasing the valence state of Co [132].
In the CoCu bimetallic Porphyrin MOF/rGO (rGO for reduced graphene oxide) designed by Menget al., Cu is embedded in the center of the Porphyrin ligand, and Co is used as the metal node of the MOF.In their proposed reaction mechanism, as shown in Fig.10, both Cu and Co are the active sites.When the Co2+adsorbed on the hydroxyl group is deprotonated, the released electrons can be transferred to the copper porphyrin center, reducing Cu2+to Cu+, so that the OER activity of the copper porphyrin center will increase [131].
In the Cu-doped cobalt hydroxide (CuCo LDH) prepared by Chenet al., the results of XPS indicate that the incorporation of Cu promotes the valence of Co to increase to more than 2.Particularly,compared with Co(OH)2, the binding energy of Co 2p3/2and Co 2p1/2of CuCo LDH increase by 1.28 eV and 0.65 eV, respectively.This change in electronic structure ultimately promotes the enhancement of the electrochemical OER activity of the Co site.They proved this by the fact that CuCo LDH has lessΔGthan Co(OH)2in each step in the DFT calculation [119].
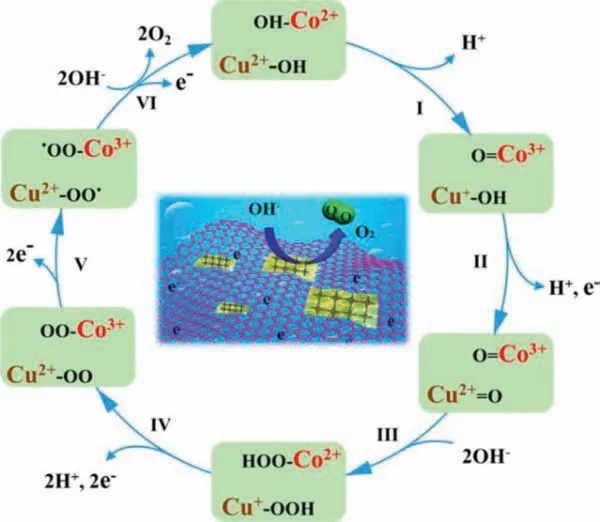
Fig.10.Schematic illustration of the proposed mechanism for CoCu bimetallic porphyrin MOF/rGO.Reprinted with permission [131].Copyright 2019, Wiley-VCH.
Fanet al.prepared NiV LDH, and they found that the introduction of vanadium on the one hand can effectively improve the charge transport ability of the material, and on the other hand can increase the activity of electrochemically active sites.The Nyquist plots of EIS show that the diameter of Ni0.75V0.25-LDH is much smaller than that of Ni0.75Fe0.25-LDH, and its charge transfer resistance (Rct) is just 62Ωcm2, while Ni0.75Fe0.25-LDH is 94Ωcm2.Besides, in the DFT calculation, theΔGmaxof Ni0.75V0.25-LDH is also much smaller than that of Ni0.75Fe0.25-LDH [114].
2D Fe-containing cobalt phosphide/cobalt oxide was prepared by Huet al.Through electronic density of states (DOS) calculation they proved that the incorporation of Fe is advantageous to the improvement of the charge transport ability of the material.They compared the DOS (Fig.11a) and charge distribution (Fig.11b) of CoP and Fe-CoP, and found that the electronic state of the latter is closer to the Fermi level (refer to the increase in the yellow area in the figure).Obviously, this will facilitate the optimization of OER overpotential and Tafel slope.What is more, they also demonstrated that theΔGmaxof Fe-CoPO is 0.07 eV lower than that of CoPO, indicating that the incorporation of Fe is beneficial to improve the OER ability of the compound active sites (Fig.11c) [97].
Additionally, Lianget al.fabricated Fe-doped NiPS3and calculated the adsorption energy of NiPS3and Fe-doped NiPS3for hydroxyl groups (ΔGOH) through DFT calculations (theΔGOHis also a very important factor in the OER process).Through the optimization of the model, they found that the hydroxyl group will be adsorbed on the phosphorous site, rather than on the metal site.The calculation results display that theΔGOHof NiPS3is 1.17 eV,while that of Fe-doped NiPS3is 1.43 eV.According to previous reports, the best value should be 1.6 eV [153].Obviously theΔGOHof Fe-doped NiPS3is much closer to 1.6 eV, making it clear that the electrochemical activity of transition metal phosphides can be improved by heterometal doping [72].
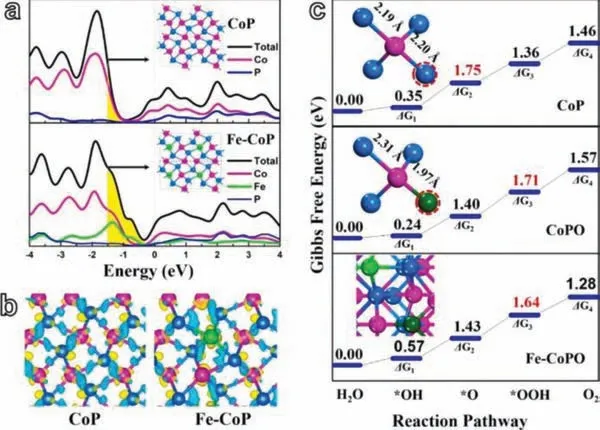
Fig.11.(a) DOS for CoP and Fe-CoP.(b) The charge density distribution of CoP and Fe-CoP.(c) Gibbs free energy of CoP, CoPO and Fe-CoPO.Purple for Co, blue for P,green for Fe, and dark green for O.Reprinted with permission [97].Copyright 2018,Elsevier.
In the iron-substituted CoOOH (i.e., FexCo1-xOOH) designed by Yeet al., part of the CoO6octahedral structure in CoOOH was replaced by FeO6octahedrons during thein-situtransformation fromα-Co(OH)2to FexCo1-xOOH.DFT calculations present that adding Fe into the CoOOH lattice can significantly reduceΔG1ΔG4, because FeO6has a higher OER activity.Besides, Fe0.33Co0.67OOH porous nanosheet arrays grown on carbon fiber cloth (PNSAs/CFC)has high conductivity, high TOF value and large ECSA.Benefiting from the above advantages, FexCo1-xOOH PNSAs/CFC shows greatly enhanced electrocatalytic performance for OER [96].
In short, compared with single-metal 2D nanomaterials, the two metals of bimetallic 2D nanomaterials can help optimize the adsorption energy and Gibbs free energy of the intermediates during OER processes through electron transfer and interaction,thereby improving catalyst activity.In addition, in some cases,bimetallic 2D nanomaterials can also have the effects of improving conductivity and inhibiting agglomeration, which are their advantages over single-metal 2D nanomaterials as well.Moreover,it should be noted that, similar to bimetallic 2D nanomaterials,polymetallic 2D nanomaterials can perform the above-mentioned functions, either.However, because polymetallic materials involve many parameters and the proportion adjustment is more complicated, the optimization difficulty is relatively high.As a result,there are very few reports on the application of polymetallic 2D nanomaterials in electrocatalytic OER.This also highlights the special advantages of bimetallic 2D nanomaterials.
5.3.The synergy of 2D and bimetal effects
To sum up, the two-dimensional characteristics can improve the charge transfer ability of nanomaterials, increase the electrochemical specific surface area and thus increase the quantity of active sites, and can also increase the unsaturated coordination metal sites in the MOF.The characteristics of bimetals can change the electronic structure, thereby improving the quality of active sites.In addition, in some cases, bimetals also contribute to the formation of two-dimensional structures, the improvement of charge transport capabilities, and the increase in the number of active sites.Obviously, when the advantages of the two are combined, the synergy will be manifested.In particular, when the twodimensional properties improve the electrical conductivity of the material and the number of active sites, and the bimetal enhances the quality of the active sites, the combination of the two can elevate the electrocatalytic OER performance of the material in an all-round way.
6.Summary and outlook
In summary, we reviewed the application of bimetallic 2D nanomaterials in electrocatalytic OER.The materials contain MOF,LDH, oxides, phosphides, phosphates and oxyhydroxides.The metals involved include Fe, Ni, Co from the eighth group and some other elements of the fourth and fifth period, such as Zn, Co, Mn,V and In.From the perspective of bimetals, there are two classifications of the synthesis strategies.One is according to the different states of the metal precursors, and the other is based on the order in which the metal precursors are added.The former has three modes: liquid-liquid, liquid-solid, and solid-solid, while the latter also owns three modes: A precursor+B precursor, A intermediate+B precursor, and A final product+B precursor.When mentioned two-dimensional nanomaterials, the synthesis strategies can be divided into three categories, namely top-down, bottom-up, and template transformation methods.Among them, the top-down way has been seldom used in recent years due to a series of shortcomings.The bottom-up routes contain hydrothermal or solvothermal methods, surfactant-assisted methods, microwave-assisted methods, co-precipitation, metal auto-oxidation, electrochemical methods, CHFS way, ion exchange route,etc.As to the template transformation strategy, the bimetallic two-dimensional MOF and LDH can be transformed into each other, while oxides usually need to be converted from hydroxides, and phosphides need to be obtained by phosphating precursors.In regard of reaction mechanisms, the two-dimensional characteristics can mainly increase the charge transport ability and improve the number of electrochemical active sites of the electrocatalyst, while the bimetal can mainly upgrade the quality of the active sites.Therefore, the combination of the two can enhance the electrocatalytic activity of the material in all stages of electrocatalysis.
Although as summarized in this review, the current scientific researchers have made excting achievements in the application of bimetallic 2D nanomaterial for electrocatalytic OER, there are still some sufficiencies that are worthy of our consideration.One is the stability.Although in most of the articles, stability tests have been conducted, few of them compare the stability of bimetallic 2D nanomaterials with non-bimetallic or non-2D nanomaterials, which makes it difficult for readers to understand the special features of bimetallic 2D materials in terms of OER stability.The second point is the acid and neutral test.Judging from the literature in this review, most of the current research on this type of material is concentrated in alkaline solutions, but there are few reports that are under acidic and neutral conditions.Actually, under acidic conditions for water splitting, there are many advantages such as high proton conductivity, high current density, and high energy efficiency.Besides, in the CO2reduction reaction, only under neutral conditions, CO2will neither be neutralized by alkali, nor will its solubility be reduced by acid.In addition, in the microbial electrolytic cell, neutral conditions are also required to ensure the activity of the microorganisms.Therefore, electrocatalytic OER under acidic or neutral conditions also has great application prospects.However, perhaps since the OER application of bimetallic 2D nanomaterials is in the initial stage, the studies under acidic and neutral conditions are rarely reported, which should be focused on and expanded in the future.Third, as of now, there are no OER reports of bimetallic 2D nanomaterials in pilot application and practical application.However, some of this type of materials have shown high activity and stability in OER, and are even far superior to the performance of existing industrial anode electrodes.With the rapid development of science and technology today, the cycle of a new product from the laboratory to the actual application is also shortening.Therefore, the practical application of bimetallic 2D nanomaterials for OER should also be accelerated.
In addition to the above, the application of relevant materials in electrocatalytic OER can be furtherly promoted from the following aspects: (1) The successful application of bimetallic materials shows that 1+1>2 effects can be realized between different metals.Therefore, whether the combination of three metals and poly-metals in different proportions can produce good results is worth pondering and exploring.(2) From the viewpoint of twodimensional materials, there is still much space for improvement in ultra-thinness.In addition, the free superposition of different ultra-thin two-dimensional nanomaterials can form a so-called van der Waals heterojunction which usually has some unique properties.Apparently, it is a great way for future exploring.(3) From the review, we can find that in the bimetallic combination, Fe, Co and Ni are often combined with each other.Among them, the NiFe combination is used the most frequently in the two-dimensional MOF, LDH, oxide, phosphide and oxyhydroxide.Besides, the effect of NiFe combination is also in the highest level.However,the application of NiFe bimetal-based single-atom two-dimensional nanomaterials in the field of electrocatalytic OER has not been reported.Therefore, this is also one of the most worthwhile projects.(4) In new material design and mechanism exploring, DFT calculations are often used to assist in explanation.However, we often find that in some cases, DFT calculation alone is far from enough.Currently, there are applications of finite element calculations in electrocatalysis.For bimetallc 2D nanomaterials, the electron and ion distribution in the unique area between the material edge and the electrolyte will affect the experimental results significantly.The finite element calculation can simulate the specific charge distribution.As a result, the application of this calculation will make the research of bimetallc 2D nanomaterials for OER more powerful and accurate.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
We appreciate the financial support of the National Natural Science Foundation (NNSF) of China (Nos.21975162,51902208, 51902209, 22172099), the Natural Science Foundation of Guangdong (No.2020A1515010840) and Shenzhen Science and Technology Program (Nos.JCYJ20200109105803806,RCYX20200714114535052, RCBS20200714114819161,JCYJ20190808111801674).
 Chinese Chemical Letters2022年8期
Chinese Chemical Letters2022年8期
- Chinese Chemical Letters的其它文章
- Adsorptive removal of PPCPs from aqueous solution using carbon-based composites: A review
- A review on hollow fiber membrane module towards high separation efficiency: Process modeling in fouling perspective
- Recent advances in DNA glycosylase assays
- Chiral pillar[n]arenes: Conformation inversion, material preparation and applications
- Recent progress in carbon-based materials boosting electrochemical water splitting
- Working principle and application of photocatalytic optical fibers for the degradation and conversion of gaseous pollutants
