介质阻挡放电等离子体老化微塑料及对Zn(II)吸附的影响
卢 伟,桑稳姣*,李 敏,张文斌,贾丹妮,占 诚,贺永健,陈翠珍,向雪莲
介质阻挡放电等离子体老化微塑料及对Zn(II)吸附的影响
卢 伟1,桑稳姣1*,李 敏2,张文斌1,贾丹妮1,占 诚1,贺永健1,陈翠珍2,向雪莲3
(1.武汉理工大学土木工程与建筑学院,湖北 武汉 430070;2.武汉市水务科学研究院,湖北 武汉 430014;3.宜昌市城市规划设计研究院有限责任公司,湖北 宜昌 443001)
为了在实验中缩短微塑料的老化时间,更真实地模拟自然老化条件,采用介质阻挡放电(DBD)等离子体老化聚乙烯微塑料(PE-MP)和聚丙烯微塑料(PP-MP),同时研究了老化前后PE-MP和PP-MP对Zn(II)的吸附过程和机理.随着放电时间延长和输入电压升高,微塑料表面出现微小裂纹或孔洞,形成含氧官能团.老化后PE-MP和PP-MP对Zn(II)的吸附容量分别提高了22.7%和14.8%.老化前后微塑料对Zn(II)的吸附均符合准二级动力学模型.颗粒内扩散模型表明,Zn(II)在微塑料上的吸附过程可分为快速吸附,慢速吸附和吸附平衡3个阶段.同时,老化前后微塑料对Zn(II)的吸附均符合Langmuir吸附等温线模型.热力学结果表明,微塑料对Zn(II)的吸附是自发的吸热过程. Ca2+、腐殖酸和低pH值不利于微塑料对Zn(II)的吸附.
微塑料;老化;等离子体;吸附;Zn(II)
近年来,尺寸小于5mm的微塑料引起的环境问题越来越受到人们的关注[1-3].目前,在海洋[4-5],湖泊[6],河流[7],甚至南极洲[8]和空气[9]中都发现了微塑料.微塑料具有较大的比表面积和疏水表面,容易在其表面富集一些有机污染物和重金属离子,从而导致这些有毒污染物在生物链上传递[10].另一方面,环境中的微塑料随着时间的推移不断老化[11],这将改变微塑料的表面性质,促进各种污染物与微塑料之间的相互作用,并进一步增加环境污染的风险[12]. Liu等[13]发现与聚乙烯和聚苯乙烯相比,聚丙烯的表面老化性能发生了较大的变化,表现为快速碎裂和表面氧化.聚乙烯和聚苯乙烯对Co(II)的吸附容量随老化程度的增加而增加,而不同老化程度的聚丙烯对Co(II)的吸附性能变化不大.Lin等[14]发现PP-MP在氙灯照射28d老化后,对Pb2+的吸附量是原始的1.7~2.5倍.
在自然条件下,微塑料的老化非常缓慢,极大地限制了对微塑料老化行为的研究.因此,许多研究人员用光辐照,化学氧化,热处理和γ-射线辐照等技术代替自然条件来探索微塑料的老化.高级氧化法因其产生的活性物质(·OH,H2O2,·SO4-等)能加速微塑料老化而在实验室研究中应用最为广泛.Fan等[15]研究发现老化后轮胎磨损颗粒和PE-MP的表面出现了小孔和裂纹,轮胎磨损颗粒和PE-MP的比表面积也有所增加.Lang等[16]用H2O2和Fenton试剂研究了聚苯乙烯(PS)的老化性能,发现老化不仅增加了PS的比表面积,而且氧化了PS表面的官能团,显著增加了PS表面的羰基和羧基的含量.然而,这些人工加速老化技术也存在条件过于单一及无法模拟自然环境中复杂的老化过程等缺点.DBD等离子体可以产生多种活性物质(O3,·OH,H2O2等),以及紫外可见光和局部高温效应[17-18],可以更合理地模拟自然环境.Galmiz等[19]研究发现,等离子体处理改变了塑料管的表面化学成分和性能.因此,推测DBD等离子体可能是模拟微塑料在自然环境中老化的潜在技术.
Zn(II)属于典型的二类重金属污染物,对水体中的鱼类和水生植物有一定的毒害作用,并且能够在动植物体内积累,进而影响生态系统[20-21].本研究利用DBD等离子体技术对PE-MP和PP-MP进行老化处理,并对其吸附Zn(Ⅱ)的性能进行研究.以期为探究微塑料的老化和微塑料作为重金属离子载体给环境带来的风险提供参考.
1 材料与方法
1.1 材料
实验所用试剂: PE-MP,PP-MP,无水乙醇,盐酸(HCl),氯化钙(CaCl2),ZnSO4·7H2O (分析纯),氢氧化钠(NaOH)和腐殖酸(HA)购自上海麦克林生化科技有限公司,PE-MP和PP-MP的平均粒径为150 μm.PE-MP和PP-MP在去离子水和10%盐酸溶液中浸泡24h,然后用无水乙醇和超纯水多次洗涤以去除微塑料表面的有机杂质和金属离子并干燥.
HA储备液: 称取1.00g HA,投入到1L的去离子水中,滴加适量的0.1mol/L NaOH使其溶解,用0.45 μm滤膜滤去不溶物.
1.2 实验装置
DBD等离子体装置(南京苏曼电子有限公司)主要由接触式调压器(TDCG2-1),等离子体发生电源(CTP-2000K),DBD反应器(DBD-50)和数字示波器组成(DS1102E)[22](图1).接触电压调压器采用自耦合电压调节;等离子体发生电源属于控制电源输入为AC220V的低温等离子体实验电源,其主面板上可显示输入电流和电压;等离子体反应器为石英制成的双层介质阻挡反应器;数字示波器可显示放电过程中的放电电压和放电频率等参数.
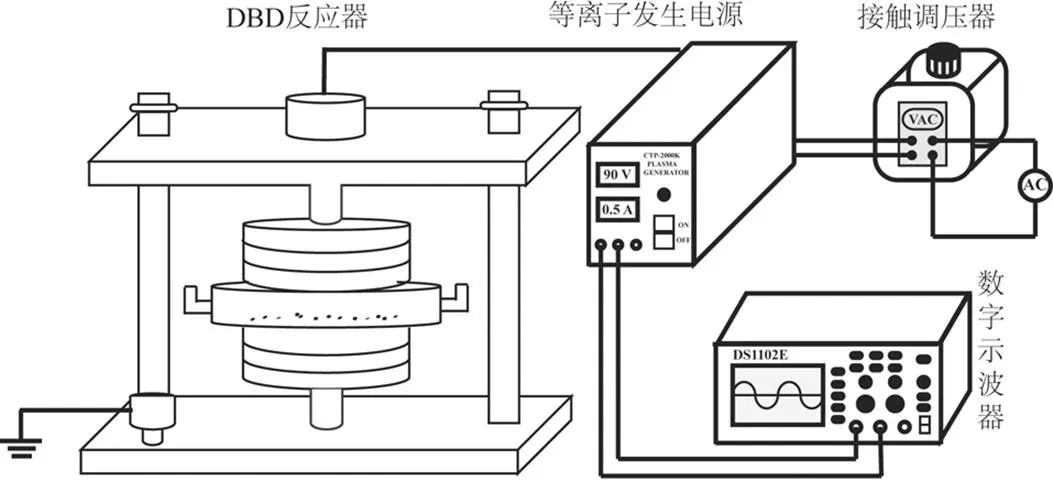
图1 实验装置示意
1.3 等离子体老化微塑料
将微塑料在DBD等离子体反应器中分批老化,每批将3g 微塑料(PE-MP或PP-MP)放入DBD反应器中,加入5mL去离子水后,将输入电压分别调整为30,60和90V,输入电流为0.5A,放电4,8,10和12h后取样进行清洗和干燥.
1.4 微塑料对Zn(II)的吸附
1.4.1 吸附动力学 分别取0.5g原始或老化微塑料放入锥形瓶中,加入浓度为5mg/L的Zn(II)溶液50mL(pH=6),在25℃,转速为160r/min条件下振荡48h.分别于0.25,0.5,1.0,2.0,4.0,8.0,12,24,48h取样,用0.2μm滤膜去除溶液中的微塑性颗粒.取上清液5mL,用原子吸收光谱仪测定Zn(II)含量.每个处理过程设3组平行,不加PE-MP(或PP-MP)组为对照组.通过测定对照组和处理组的Zn(II)含量差值,计算PE-MP(或PP-MP)对Zn(II)的吸附量.
对所得吸附动力学数据用准一级动力学,准二级动力学和颗粒内扩散模型拟合.
1.4.2 等温吸附实验 用分析纯ZnSO4·7H2O配制50mg/L的Zn(II) 储备液,将Zn(II) 储备液分别稀释为0.1,0.2,0.5,1.0,3.0,5.0mg/L.将原始或老化PE- MP(或PP-MP)样品0.5g放入锥形烧瓶中,加入浓度为0.1~5mg/L的Zn(II)溶液50mL.在25℃,转速为160r/min条件下振荡48h.后续实验步骤与吸附动力学实验相同.
PE-MP和PP-MP对Zn(II)的吸附等温线可用Langmuir方程和Freundlich方程描述.
1.4.3 吸附热力学 采用van't Hoff方程考察热力学参数,即吉布斯自由能Δ0,焓变Δ0和熵变Δ0的变化,以确定微塑料对Zn(II)吸附过程的热变性和自发性,试验温度分别为 288,298和 308K.
1.5 分析方法
用扫描电子显微镜(SEM,JEM-7500F,日本)观察老化前后PE-MP和PP-MP的形态变化.采用傅立叶变换红外光谱(FTIR,Nexus,美国),X射线光电子能谱仪(XPS,ESCALAB 250Xi,美国)和X射线衍射(XRD,D8Advance,德国)对其表面官能团,表面元素和晶体结构进行分析.用原子吸收光谱仪(CONTRAA-700,德国)测定溶液中的Zn(II)浓度.
2 结果与讨论
2.1 等离子体老化PE-MP和PP-MP
2.1.1 老化前后PE-MP和PP-MP的形貌特征 如图2所示,原始PE-MP和PP-MP的表面相对较光滑,随着放电时间延长和输入电压升高,微塑料表面变得越来越粗糙.当电压和老化时间增加到一定程度时,老化后PE-MP和PP-MP的表面出现细小的裂纹或孔洞.结果表明,等离子体有效促进了PE-MP和PP-MP的老化,增加了PE-MP和PP-MP的比表面积,这可能会产生更多的吸附位点,有利于污染物的吸附[23].同时,在输入电压为 90V,放电时间为10h时,老化效果已经很明显;继续延长放电时间,老化效果的提升并不明显,因此后续实验所用的老化微塑料均在此条件下制备.其他类似研究也观察到了老化微塑料表面的裂缝和孔洞[15,24-25].Ge等[26]发现等离子体处理不会引起淀粉颗粒完整性的变化,但会导致淀粉颗粒表面的开裂,腐蚀和微沉积.在老化过程中,等离子体产生的活性物质和紫外线会导致微塑料分子链的断裂和交联反应,从而破坏微塑料分子中的化学键,导致分子链断裂[15].这可能是老化后PE-MP和PP-MP表面形态发生变化的原因.

图2 输入电压和放电时间对微塑料表面形貌的影响
´5000
2.1.2 老化前后PE-MP和PP-MP的官能团和表面元素分析 如图3所示,与原始PE-MP相比,等离子体老化后的PE-MP在3420cm-1处出现一个新峰,代表O-H基团的拉伸振动.此外,在老化PE-MP的1473和2919cm-1处观察到C-H和C-H2拉伸振动[17].等离子体老化后PP-MP的FTIR谱中没有观察到明显的O-H基拉伸振动,但1377,1473和2918cm-1处的峰比原始PP-MP更尖锐,这可以归因于C-OH,C-H和C-H2的拉伸振动[27-28].同时,老化PP-MP在1700~1800cm-1处产生了一个代表羰基的新峰.这些现象表明,等离子体可以氧化PE-MP和PP-MP的表面,并引入含氧官能团,这在微塑料的光照或自然老化过程中也会发生[14,29].PE-MP和PP-MP的表面氧化可能是等离子体产生的自由基和紫外线的共同作用.研究发现,自由基可以攻击聚苯乙烯等聚合物中的氢原子,破坏C-H键.断裂的C-H很容易与氧气反应形成C-O键,然后与空气中的氢反应形成过COOH基团[30-31].含氧官能团的产生将增加PE-MP和PP-MP的亲水性,这会增加微塑料与溶液中重金属接触的机会[30].
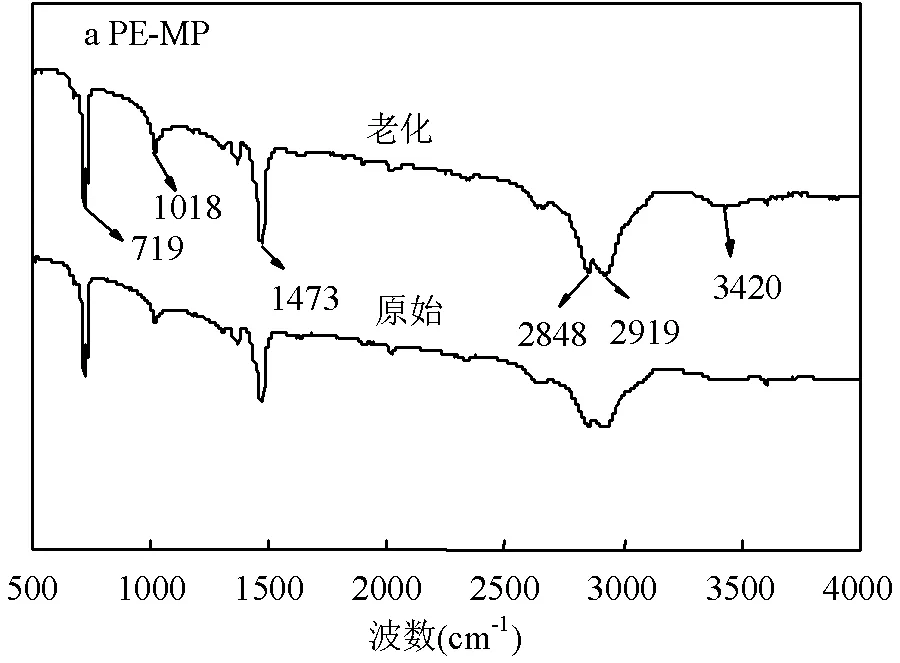

如图4所示,经等离子体老化处理后,C-O键增加,而C-C键逐渐减少,表明PE-MP和PP-MP已经被氧化.原始PE-MP和PP-MP中所含的氧原子可能是由加工过程中加入的添加剂引起的[30,32],老化后氧原子的增加是微塑料被等离子体老化的结果,这与FTIR的结果基本一致.
2.1.3 老化前后PE-MP和PP-MP的结晶度分析 如图5所示,老化的PE-MP在30°~35°之间出现多个新峰,而老化PP-MP在20°~25°之间的特征峰变得更加尖锐,这表明等离子体老化后PE-MP和PP-MP的结晶度增加[30,33].结晶度的提高意味着微塑料更脆,容易产生更小的颗粒,这也是环境中的塑料更脆弱的原因[34].同时,老化后微塑料的峰与原始微塑料相比没有明显的移动,说明PE-MP和PP-MP的尺寸在老化过程中变化不大.
2.1.4 PE-MP和PP-MP的表面能谱分析 如图6所示,PE-MP和PP-MP的氧含量在等离子体老化后有所增加,表明微塑料的表面在等离子体处理过程中与活性物质发生反应产生了含氧官能团.另一方面,吸附Zn(II)后的微塑料EDS结果显示老化后微塑料表面Zn(Ⅱ)的占比增大,说明老化在一定程度上促进了微塑料对Zn(Ⅱ)的吸附.这可能是因为含氧官能团的产生提高了微塑料的亲水性,增加了微塑料与溶液中Zn(Ⅱ)接触的机会.
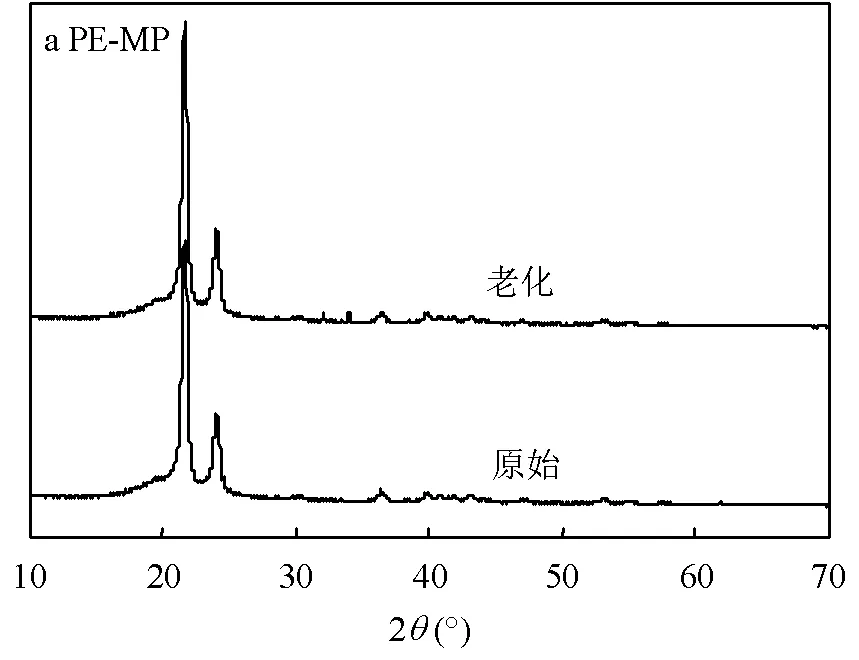
图5 老化前后微塑料的XRD图

2.2 吸附动力学
从图7可知,当达到吸附平衡时,放电等离子体老化后的PE-MP和PP-MP对Zn(II)的吸附量分别比原来的PE-MP和PP-MP提高了22.7%和14.8%.这可归因于等离子体体系中产生的各种活性物质和强紫外线诱导了PE-MP和PP-MP主链上C-C和C-H键的解离,并产生了含氧官能团,使PE-MP和PP-MP老化后表面变得粗糙,结晶度增加,表面亲水性提高.这些物理化学性质的变化显著促进了老化PE-MP和PP-MP对Zn(II)的吸附[25,35].从表1可知,等离子体老化前后PE-MP和PP-MP对Zn(II)的吸附更符合准二级动力学模型.结果表明,PE-MP和PP-MP对Zn(II)的吸附是一个复杂的过程,可能包括物理吸附和化学吸附[16].同时,PE-MP和PP-MP对Zn(II)的2值分别从4.735g/(mg×min)降至3.585g/(mg×min)和从3.563g/(mg×min)降至3.131g/ (mg×min),表明老化PE-MP和PP-MP对Zn(II)的吸附速率降低.
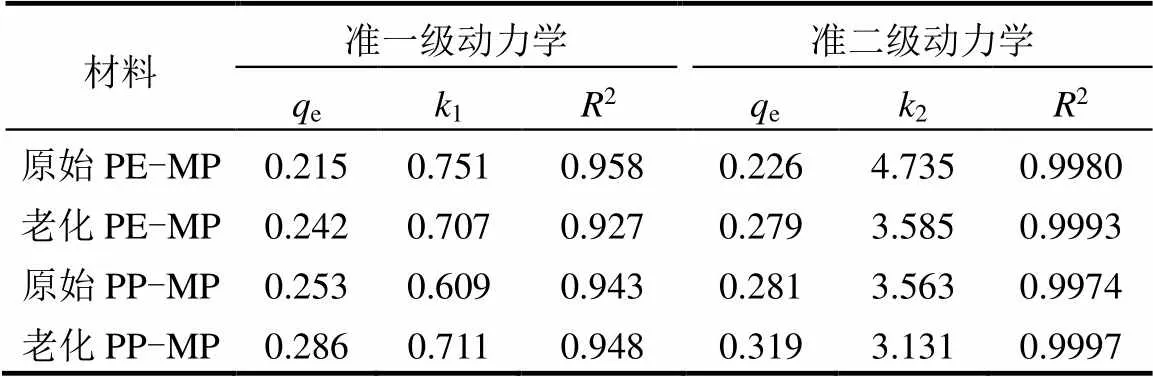
表1 原始和老化PE-MP及PP-MP吸附Zn(II)的动力学参数
注:e是微塑料在吸附平衡时对Zn(II)的吸附量,mg/g;1是准一级吸附速率常数h-1;2是准二级吸附速率常数,g/(mg·h),下同.

是吸附时间,h;q是时间时对Zn(II)的吸附容量,mg/g,下同
如图8和表2所示,原始及老化PE-MP和PP- MP的颗粒内扩散模型具有良好的线性关系.直线未通过原点,表明PE-MP和PP-MP对Zn(II)的吸附速率受粒内扩散和外扩散控制,表明对Zn(II)的吸附是一个多阶段吸附过程[36-38].吸附过程可分为3个阶段:第一阶段为快速吸附阶段,在吸附物浓度差和静电引力的作用下,Zn(II)迅速占据PE-MP和PP-MP表面的吸附位点,吸附1h后,Zn(II)的吸附量达到吸附平衡的50%左右;第二阶段为慢吸附阶段,Zn(II)与PE-MP和PP-MP的剩余吸附位点缓慢结合,直至达到第三阶段(吸附平衡阶段).老化微塑料在达到吸附平衡前的2个阶段截距C值大于原始微塑料,这是因为老化后微塑料表面吸附位点的增加增大了Zn(II)在微塑料颗粒内部扩散的传质驱动力.
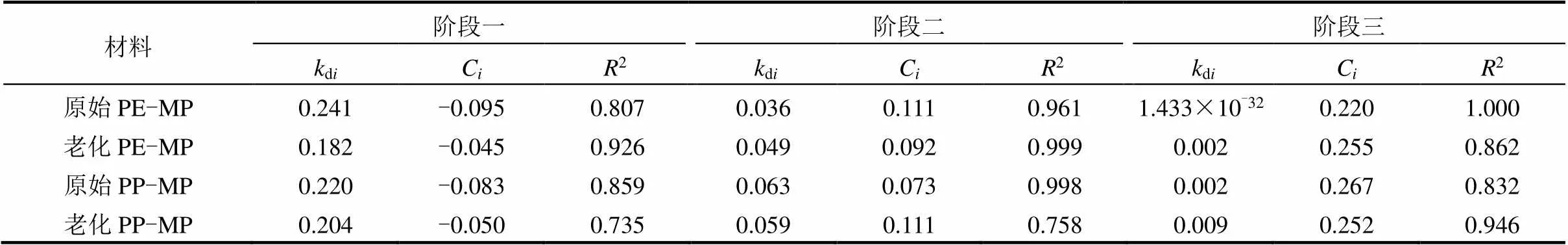
表2 原始和老化PE-MP和PP-MP吸附Zn(II)的颗粒扩散模型参数
注:di是颗粒内扩散模型的吸附速率常数,g/(mg·h0.5);C为一个与边界层厚度有关的常数.

图8 原始和老化PE-MP和PP-MP的颗粒内扩散模型拟合曲线
2.3 吸附等温线和吸附热力学
如图9和表3所示,随着Zn(II)浓度的增加,原始和老化PE-MP和PP-MP的吸附容量也增加.当不同浓度的Zn(II)溶液达到吸附平衡时,老化的PE-MP和PP-MP的吸附容量高于原始的PE-MP和PP-MP,证实了放电等离子体老化后提高了PE-MP和PP- MP对Zn(II)的吸附容量,这可能是老化处理后微塑料粗糙的颗粒表面产生更大的比表面积和更多的污染物吸附位点[39].
Langmuir(0.9702<2<0.9910)等温吸附模型比Freundlich(0.9314<2<0.9594)等温吸附模型具有更高的拟合度.结果表明,等离子体老化前后PE-MP和PP-MP对Zn(II)的吸附机理均为单层吸附[40].同时,老化后微塑料的2有一定的增加,这表明老化可能会使微塑料表面的吸附位点更加均匀.一些学者研究了Cu2+,Pb2+等金属在不同类型微塑料上的吸附,也发现了相似的吸附机理[29,41].与原始PE-MP和PP-MP相比,老化PE-MP和PP-MP的m值分别从0.223和0.275增加到0.274和0.330,这与本研究结果趋势相同.可能是由于老化后微塑料表面更加粗糙,增加了Zn(II)的吸附位点,微塑料表面的含氧官能团及结晶度的提高也可能是影响微塑料对Zn(II)吸附性能的因素.
在Freundlich模型表达式中,1/可以用来描述吸附剂和吸附物之间的结合能力.当值为1时,吸附为线性等温线;当1/>1或1/<1时,表明吸附过程分别与化学过程或有利的物理过程有关[42-44].从表3可以看出,Freundlich模型的参数1/均小于1,表明吸附是容易发生的.同时发现,老化后PE-MP和PP-MP的1/值均有所增加,表明老化后PE-MP和PP-MP对Zn(II)的吸附过程也更加复杂.
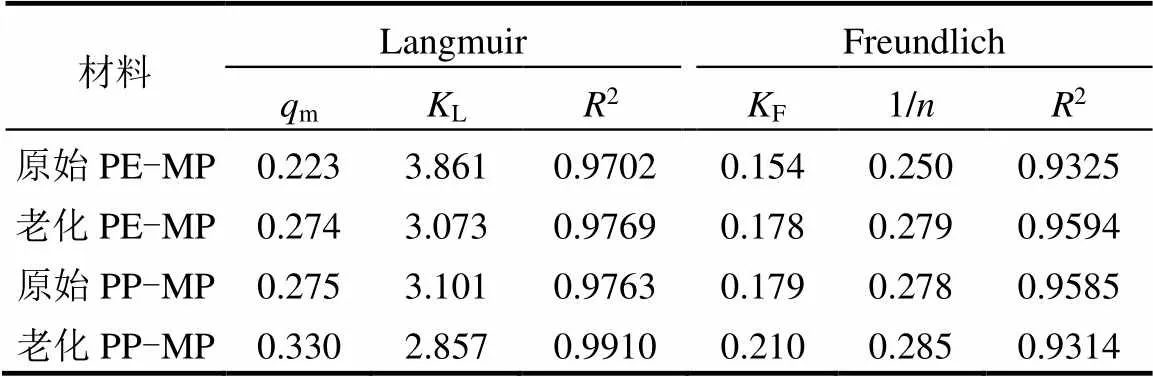
表3 老化前后PE-MP和PP-MP等温线模型参数
注:m为微塑料对Zn(II)的吸附容量,mg/g;L为Langmuir常数,L/mg;F为Freundlich常数,mg1-1/nL1/ng-1;为非均质系数,下同.
如表4所示,在288~308K的条件下,Δ0>0,表明微塑料吸附Zn(II)的过程是吸热反应;老化PE-MP的焓变大于原始PE-MP,而老化PP-MP的焓变小于原始PP-MP,这表明老化使Zn(II)与PE-MP表面的相互作用更强,而Zn(II)与PP-MP表面的相互作用减弱[45].Δ0值也>0,表明Zn(II)的自由度和Zn(II)在固液界面的浓度升高.而Δ0<0,表明微塑料吸附Zn(II)是自发反应过程[15],且Δ0的绝对值随着温度的升高而增大,表明温度升高有利于吸附的自发反应程度增强.另一方面,微塑料老化后Δ0的绝对值略有减小,该结果表明老化会使微塑料吸附Zn(II)的自发反应程度减弱,而老化后微塑料吸附Zn(II)能力增强,这可能是因为老化后微塑料吸附位点增多能够抵消自发反应程度的减弱.
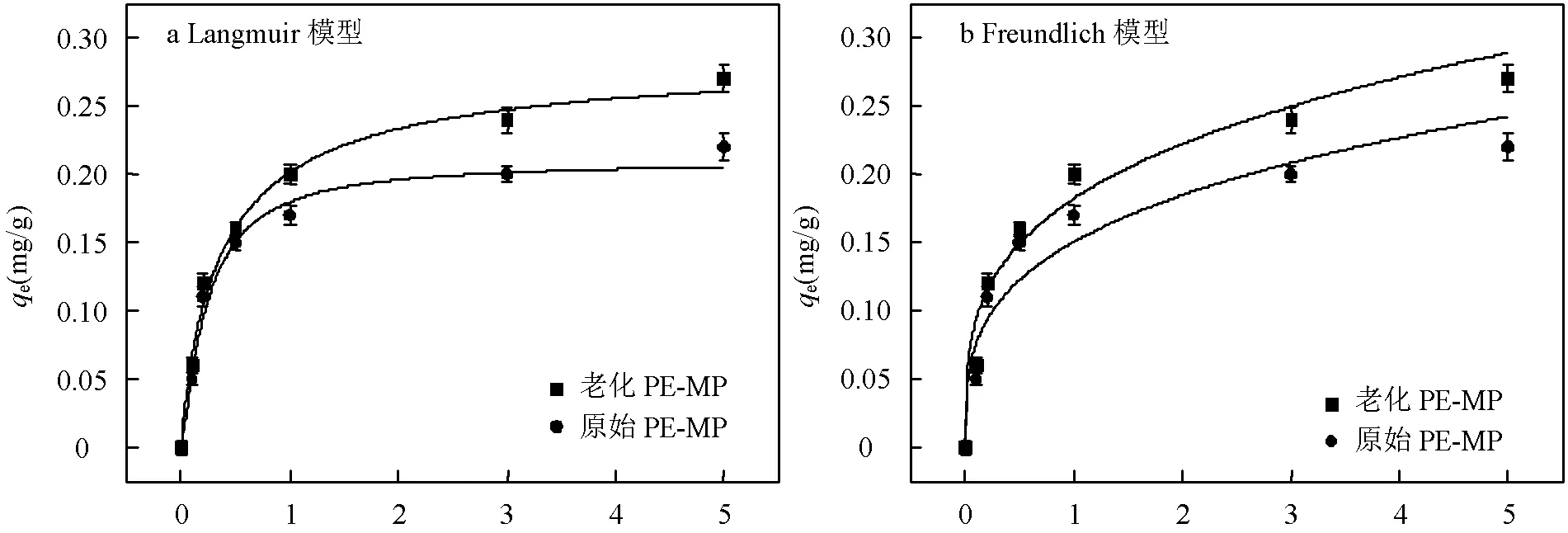
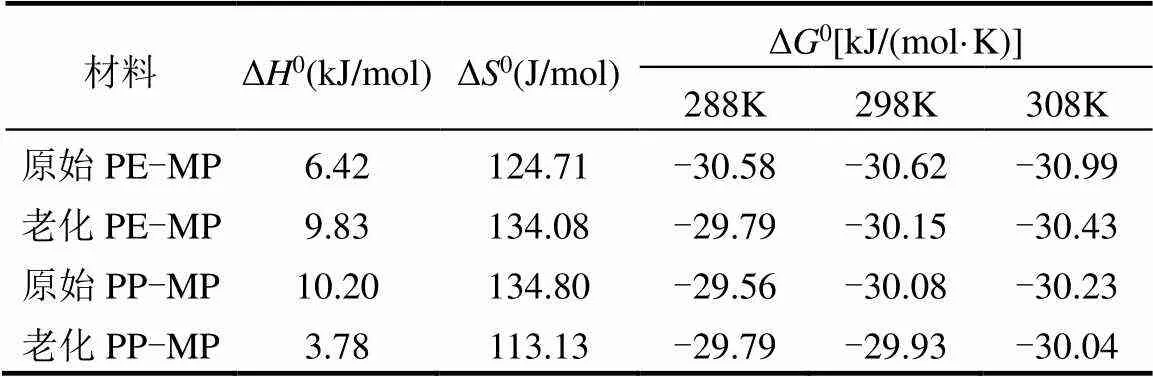
表4 老化前后PE-MP和PP-MP热力学参数
注: Δ0为自由能变化量,J/mol; Δ0和Δ0分别为焓(J/mol)和熵(J/(mol·K),下同.
2.4 环境因素的影响
如图10a所示,在pH值3~6范围内,微塑料对Zn(II)的吸附能力随pH值的增加而增加.在较低pH值条件下,H+与Zn(II)竞争吸附位置,导致pH值降低时对Zn(II)的吸附容量降低[38].同时,随着pH值升高,去质子化作用增强及静电排斥力作用减弱使得微塑料上基团更易与Zn(II)结合,从而提高其吸附能力.
Ca2+是水体环境中重要的阳离子,会影响微塑料对Zn(II)的吸附.如图10b所示,随Ca2+浓度的增加,老化前后的微塑料对Zn(II)的吸附能力降低.加入8mg/L Ca2+后,原始PE-MP、老化PE-MP、原始PP-MP和老化PP-MP对Zn(II)的吸附容量分别降至0.10,0.13,0.12和0.15mg/g.说明离子强度对微塑料吸附Zn(II)的影响较大.Zn(II)吸附量随Ca2+浓度而降低,说明Ca2+与Zn(II)发生了竞争吸附作用,Ca2+抢占了微塑料表面的吸附位点,使微塑料对Zn(II)的吸附容量降低.
如图10c所示,随着腐殖酸浓度的增加,老化前后的微塑料对Zn(II)的吸附能力逐渐降低.这可能是因为腐殖酸与Zn(II)的络合力大于微塑料对Zn(II)的吸附力,导致Zn(II)会优先与腐殖酸络合,微塑料的吸附量减少,腐殖酸的存在抑制了微塑料对Zn(II)的吸附[46].另一方面,腐殖酸会占据微塑料表面的吸附位点,削弱了微塑料对Zn(II)的吸附.

2.5 环境影响及老化工艺对比
微塑料老化是自然环境中常见的现象,老化引起的微塑料物理化学性质的变化会加速微塑料与环境中其他污染物的相互作用,对水环境和生物造成更大的危害.例如,单独的微塑料或Ag+对大型蚤没有毒性,而微塑料和Ag+的混合物可能对大型蚤有毒[47].本研究也发现微塑料的老化会增强对Zn(II)的吸附.微塑料在环境中的老化一直客观存在,与自然条件下相比,DBD等离子体能够显著缩短微塑料老化时间;同时DBD等离子体系统产生的紫外可见光、活性物质以及温度效应等,相比于其他实验室老化手段能更合理地模拟自然老化环境.周崇胜等[48]探究自然光老化微塑料的实验周期长达1年,Mao等[30]探究紫外光老化微塑料的试验周期也需3个月.而在本研究中,DBD等离子体放电10h左右已有明显的老化现象出现.另一方面,在本研究中,老化后PE-MP和PP-MP对Zn(II)的吸附量分别增加了0.05和0.04mg/g;张瑞昌等[49]研究酸、碱、氧化和高温-冻融4种老化方式对PE微塑料Zn(II)吸附行为的影响,发现老化后的PE-MP对Zn(II)的吸附量分别增加了0.023,0.03,0.02和0.015mg/g;王琼杰等[50]采用紫外光老化600h后,PE-MP和PP-MP对Zn(II)的吸附量分别提高了9.54和40.8 μg/g.这也体现了DBD等离子体老化微塑料对Zn(II)吸附的影响更大.以上结论表明DBD等离子体有助于在更短时间内探究微塑料的老化,也更便于探究老化前后微塑料与重金属等的相互作用,有助于探索微塑料吸附重金属对环境的影响.
3 结论
3.1 等离子体可明显缩短PE-MP和PP-MP的老化时间.
3.2 老化后,出现O-H键和C-H键等的伸缩振动,并在PE-MP表面形成含氧官能团. PE-MP和PP-MP的结晶度增加,表面起皱,出现裂纹或孔洞.
3.3 老化后PE-MP和PP-MP对Zn(II)的吸附能力有提高.微塑料对Zn(II)的吸附反应动力学符合准二级动力学模型,老化前后PE-MP和PP-MP对Zn(II)的吸附均以化学吸附为主.
3.4 颗粒内扩散模型的拟合具有良好的线性,吸附过程可分为快速吸附,慢速吸附和吸附平衡3个阶段.
3.5 吸附平衡数据符合Langmuir等温吸附模型,为单层吸附.热力学结果表明,微塑料对Zn(II)的吸附是自发的吸热过程,且随着温度的升高,吸附的自发反应程度增强.
3.6 pH值、离子强度和腐殖酸对微塑料吸附Zn(II)有明显的影响.
[1] Zhou X,Hao L,Wang H,et al. Cloud-point extraction combined with thermal degradation for nanoplastic analysis using pyrolysis gas chromatography-mass spectrometry [J]. Analytical Chemistry,2019,91(3):1785-1790.
[2] 孙 璇,俞安琪,王学松,等.富里酸在聚苯乙烯微塑料上的吸附行为[J]. 中国环境科学,2022,42(1):285-292.
Sun X,Yu A Q,Wang X S,et al. Adsorption behaviors of fulvic acid onto polystyrene microplastics [J]. China Environmental Science,2022,42(1):285-292.
[3] 范秀磊,甘 容,谢 雅,等.老化前后聚乳酸和聚乙烯微塑料对抗生素的吸附解吸行为[J]. 环境科学研究,2021,34(7):1747-1756.
Fan X L,Gan R,Xie Y,et al. Adsorption and Desorption Behavior of Antibiotics on Polylactic Acid and Polyethylene Microplastics Before and After Aging [J]. Research of Environmental Sciences,2021,34(7):1747-1756.
[4] Anthony L A. Microplastics in the marine environment [J]. Marine Pollution Bulletin,2011,62(8):1596-1605.
[5] Luís G A B,Barbara C G G. Microplastics in the marine environment: Current trends and future perspectives [J]. Marine Pollution Bulletin,2015,97(1/2):5-12.
[6] Wang W,Anne W N,Zhen L,et al. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan,China [J]. Science of the Total Environment,2017,575:1369-1374.
[7] Xiong X,Chenxi W,James J E,et al. Occurrence and fate of microplastic debris in middle and lower reaches of the Yangtze River – From inland to the sea [J]. Science of the Total Environment,2019,659:66-73.
[8] Zhang Y,Gao T,Kang S,et al. Microplastics in glaciers of the Tibetan Plateau: Evidence for the long-range transport of microplastics [J]. Science of the Total Environment,2021,758(prepublish).
[9] Chen G,Feng Q,Wang J. Mini-review of microplastics in the atmosphere and their risks to humans [J]. Science of the Total Environment,2020,703:135504.
[10] Tunali M,Uzoefuna E N,Tunali M M,et al. Effect of microplastics and microplastic-metal combinations on growth and chlorophyll a concentration of Chlorella vulgaris [J]. Science of the Total Environment,2020,743:140479.
[11] Meng J,Xu B,Liu F,et al. Effects of chemical and natural ageing on the release of potentially toxic metal additives in commercial PVC microplastics [J]. Chemosphere,2021,283:131274.
[12] 褚献献,郑 波,何 楠,等.微塑料与污染物相互作用的研究进展[J]. 环境化学,2021,40(2):427-435.
Chu X X,Zheng B,He N,et al. Progress on the interaction between microplastics and contaminants [J]. Environmental Chemistry,2021,40(2):427-435.
[13] Liu P,Wu X,Huang H,et al. Simulation of natural aging property of microplastics in Yangtze River water samples via a rooftop exposure protocol [J]. Science of the Total Environment,2021,785:147265.
[14] Lin W,Kuo J,Lo S. Effect of light irradiation on heavy metal adsorption onto microplastics [J]. Chemosphere,2021,285:131457.
[15] Fan X,Gan R,Liu J,et al. Adsorption and desorption behaviors of antibiotics by tire wear particles and polyethylene microplastics with or without aging processes [J]. Science of the Total Environment,2021,771:145451.
[16] Lang M,Yu X,Liu J,et al. Fenton aging significantly affects the heavy metal adsorption capacity of polystyrene microplastics [J]. Science of the Total Environment,2020,722:137762.
[17] Xu Z,Xue X,Hu S,et al. Degradation effect and mechanism of gas-liquid phase dielectric barrier discharge on norfloxacin combined with H2O2or Fe2+[J]. Separation and Purification Technology,2020,230:115862.
[18] Huang Q,Fang C. Degradation of 3,3′,4,4′-tetrachlorobiphenyl (PCB77) by dielectric barrier discharge (DBD) non-thermal plasma: Degradation mechanism and toxicity evaluation [J]. Science of the Total Environment,2020,739:139926.
[19] Galmiz O,Pavliňák D,Zemánek M,et al. Hydrophilization of outer and inner surfaces of Poly (vinyl chloride) tubes using surface dielectric barrier discharges generated in ambient air plasma [J]. Plasma Processes and Polymers,2017,14(9).
[20] 王忠凯,季军荣,汤 睿,等.双有机改性磁性膨润土对Cu(II)和Zn(II)的吸附研究[J]. 高校化学工程学报,2022,36(2):276-286.
Wang Z K,Ji J R,Tang R,et al. Dually organic modified magnetic bentonite for adsorption of Cu(II) and Zn(II) [J]. Journal of Chemical Engineering of Chinese Universities,2022,36(2):276-286.
[21] Calderon B,Fullana A. Heavy metal release due to aging effect during zero valent iron nanoparticles remediation [J]. Water Research,2015,83:1-9.
[22] Sang W,Lu W,Mei L,et al. Research on different oxidants synergy with dielectric barrier discharge plasma in degradation of Orange G: Efficiency and mechanism [J]. Separation and Purification Technology,2021,277:119473.
[23] Frere L,Paul-Pont I,Moreau J,et al. A semi-automated Raman micro-spectroscopy method for morphological and chemical characterizations of microplastic litter [J]. Marine Pollution Bulletin,2016,113(1/2):461-468.
[24] Liu J,Zhang T,Tian L,et al. Aging significantly affects mobility and contaminant-mobilizing ability of nanoplastics in saturated loamy sand [J]. Environmental Science & Technology,2019,53(10):5805- 5815.
[25] Gao F,Li J,Sun C,et al. Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment [J]. Marine Pollution Bulletin,2019,144:61-67.
[26] Ge X,Shen H,Su C,et al. Pullulanase modification of granular sweet potato starch: Assistant effect of dielectric barrier discharge plasma on multi-scale structure,physicochemical properties [J]. Carbohydrate Polymers,2021,272:118481.
[27] Ding L,Mao R,Ma S,et al. High temperature depended on the ageing mechanism of microplastics under different environmental conditions and its effect on the distribution of organic pollutants [J]. Water Research,2020,174:115634.
[28] Liu P,Shi Y,Wu X,et al. Review of the artificially-accelerated aging technology and ecological risk of microplastics [J]. Science of the Total Environment,2021,768:144969.
[29] Fu Q,Tan X,Ye S,et al. Mechanism analysis of heavy metal lead captured by natural-aged microplastics [J]. Chemosphere,2021,270: 128624.
[30] Mao R,Lang M,Yu X,et al. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals [J]. Journal of Hazardous Materials,2020,393:122515.
[31] Anne A,Stuart B,Katherine D,et al. An overview of degradable and biodegradable polyolefins [J]. Progress in Polymer Science,2010,36(8):1015-1049.
[32] Dong Y,Gao M,Song Z,et al. As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism [J]. Chemosphere,2020,239:124792.
[33] Yong M,Zhang Y,Sun S,et al. Properties of polyvinyl chloride (PVC) ultrafiltration membrane improved by lignin: Hydrophilicity and antifouling [J]. Journal of Membrane Science,2019,575:50-59.
[34] Liu G,Zhu Z,Yang Y,et al. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater [J]. Environmental Pollution,2019,246:26- 33.
[35] Wu X,Liu P,Huang H,et al. Adsorption of triclosan onto different aged polypropylene microplastics: Critical effect of cations [J]. Science of the Total Environment,2020,717:137033.
[36] Piperopoulos E,Calabrese L,Mastronardo E,et al. Assessment of sorption kinetics of carbon nanotube‐based composite foams for oil recovery application [J]. Journal of Applied Polymer Science,2019,136(14):47374.
[37] Wang W,Wang J. Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics [J]. Chemosphere,2018,193: 567-573.
[38] Dutta D P,Venugopalan R,Chopade S. Manipulating Carbon Nanotubes for Efficient Removal of Both Cationic and Anionic Dyes from Wastewater [J]. Chemistry Select (Weinheim),2017,2(13):3878- 3888.
[39] Zhang H,Wang J,Zhou B,et al. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics,isotherms and influencing factors [J]. Environmental Pollution,2018,243:1550-1557.
[40] Liu P,Qian L,Wang H,et al. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes [J]. Environmental Science & Technology,2019,53(7):3579-3588.
[41] Yang J,Cang L,Sun Q,et al. Effects of soil environmental factors and UV aging on Cu2+adsorption on microplastics [J]. Environmental Science and Pollution Research,2019,26(22):23027-23036.
[42] Akram M,Xu X,Gao B,et al. Adsorptive removal of phosphate by the bimetallic hydroxide nanocomposites embedded in pomegranate peel [J]. Journal of Environmental Sciences,2020,91:189-198.
[43] Sahoo S,Uma,Banerjee S,et al. Application of natural clay as a potential adsorbent for the removal of a toxic dye from aqueous solutions [J]. Desalination and Water Treatment,2014,52(34-36): 6703-6711.
[44] Yang Z,Zhu T,Xiong M,et al. Tuning adsorption capacity of metal–organic frameworks with Al3+for phosphorus removal: Kinetics,isotherm and regeneration [J]. Inorganic Chemistry Communications,2021,132:108804.
[45] Wang F,Pan Y,Cai P,et al. Single and binary adsorption of heavy metal ions from aqueous solutions using sugarcane cellulose-based adsorbent [J]. Bioresource Technology,2017,241:482-490.
[46] 杜晓丽,尹子杰,陈梦瑶,等.径流溶解性有机物对生物滞留介质去除Cu2+和Pb2+的影响[J]. 中国环境科学,2021,41(9):4142-4148.
Du X L,Yin Z J,Chen M Y,et al. Effect of dissolved organic matter in runoff on the removal of Cu2+and Pb2+by bioretention medium [J]. China Environmental Science,2021,41(9):4142-4148.
[47] Abdolahpur Monikh F,Vijver M G,Guo Z,et al. Metal sorption onto nanoscale plastic debris and trojan horse effects in Daphnia magna: Role of dissolved organic matter [J]. Water Research,2020,186: 116410.
[48] 周崇胜,范铭煜,丁云浩,等.常见微塑料的自然光解老化 [J]. 环境化学,2021,40(6):1741-1748.
Zhou C S,Fan M Y,Ding Y H,et al. Insights into natural photo-aging of common-used microplastics [J]. Environmental Chemistry,2021,40 (6):1741-1748.
[49] 张瑞昌,李泽林,魏学锋,等.模拟环境老化对PE微塑料吸附Zn(II)的影响[J]. 中国环境科学,2020,40(7):3135-3142.
Zhang R C,Li Z L,Wei X F,et al. Effects of simulated environmental aging on the adsorption of Zn(II) onto PE microplastics [J]. China Environmental Science,2020,40(7):3135-3142.
[50] 王琼杰,张 勇,张阳阳,等.老化微塑料对水体中重金属铜和锌的吸附行为研究[J]. 环境科学学报,2021,41(7):2712-2726.
Wang Q J,Zhang Y,Zhang Y Y,et al. Adsorption of heavy metal ions Cu2+and Zn2+onto UV-aged microplastics in aquatic system [J]. Acta Scientiae Circumstantiae,2021,41(7):2712-2726.
Dielectric barrier discharge plasma aging of microplastics and its effect on Zn(II) adsorption.
LU Wei1,SANG Wen-jiao1*,LI Min2,ZHANG Wen-bin1,JIA Dan-ni1,ZHAN Cheng1,HE Yong-jian1,CHEN Cui-zhen2,XIANG Xue-lian3
(1.School of Civil Engineering and Architecture,Wuhan University of Technology,Wuhan 430070,China;2.Wuhan Water Science Research Institute,Wuhan 430014,China;3.Yichang Institute of Urban Planning & Design Co.,Ltd,Yichang 443001,China).,2022,42(8):3744~3754
In order to shorten the aging time of microplastics and mimic the natural aging conditions in the experiment,dielectric barrier discharge (DBD) plasma was used in the aging experiments of polyethylene microplastics (PE-MP) and polypropylene microplastics (PP-MP). And the adsorption process and mechanism of Zn(II) on PE-MP and PP-MP before and after aging was investigated. With the extension of discharge time and the elevation of input voltage,tiny cracks or holes appeared on the surface of microplastics and oxygen-containing functional groups were formed. The Zn(II) adsorption capacity of aged PE-MP and PP-MP was increased by 22.7% and 14.8%,respectively. The adsorption of Zn(II) on microplastics before and after aging conformed to the pseudo-second-order kinetic model. The intra-particle diffusion model showed that the adsorption process of Zn(II) on microplastics could be involved three processes: rapid adsorption,slow adsorption and adsorption equilibrium. In addition,the adsorption of Zn(II) on microplastics before and after aging conformed by Langmuir model. The thermodynamic results indicated that the adsorption of Zn(II) on microplastics was a spontaneous endothermic process. Ca2+,humic acid and low pH were not conducive to the adsorption of Zn(II) by microplastics.
microplastics;aging;plasma;adsorption;Zn(II)
X52
A
1000-6923(2022)08-3744-11
2022-01-10
国家自然科学基金资助项目(51108360,51208397)
* 责任作者,副教授,whlgdxswj@126.com
卢 伟(1996-),男,江西宜春人,武汉理工大学硕士研究生,主要从事高级氧化方面研究.发表论文2篇.

