Application of biofloc technology in recirculation A rtemia culture system*
Xuejiao LIANG , Chi ZHANG , Dongdong DU , Meirong GAO , Liying SUI ,**
1 Key Laboratory of Marine Resource Chemistry and Food Technology (TUST), Ministry of Education; College of Marine and Environmental Sciences, Tianjin University of Science and Technology, Tianjin 300457, China
2 Institute of Fisheries Science, Tibet Academy of Agricultural and Animal Husbandry Sciences, Lhasa 850000, China
Abstract Biofloc technology (BFT) improves water quality, and productivity of the farmed species through converting ammonium nitrogen to microbial protein, stabilizing microbial community, and reducing the production cost. In this study, a small-scale biofloc development unit was designed in combination of recirculation system (RAS) for Artemia culture. Artemia growth, water quality, and microbial composition of bioflocs in RAS were studied in comparison with in-situ batch culture (Glu). Glucose was added in RAS and Glu at C/N ratio of 10. The cultures without glucose addition, but with 50% daily water renewal(WRe) and without water renewal (NWRe) were considered as the controls. Arte mia were cultured at 25 ° C and salinity 30 for 24 days and fed formulated feed. The results showed that compared to the controls,Glu significantly improved the Artemia biomass, increased the biofloc volume, and reduced the content of total ammonia nitrogen (TAN), nitrite nitrogen (NO 2-N) and nitrate nitrogen (NO 3-N) in water column( P <0.05). In addition, RAS had similar results with Glu. High throughput sequencing analysis on biofloc microbial composition demonstrated that glucose supplement shaped the microbial community structure,and increased proportion of potential probiotic bacteria and suppressed pathogenic bacteria growth.Furthermore, we analyzed the relationship between the microbial composition of biofloc and environmental factors. Canonical correspondence analysis (CCA) indicated that inorganic nitrogen in culture water had great impact on biofloc microbial composition in NWRe and WRe, whilst the dissolved organic carbon(DOC) modified the microbial community in Glu and RAS. This study shows the advantages of BFT in Artemia culture and provides practical information for applying BFT-RAS in indoor Artemia culture.
Keyword: biofloc technology; Artemia; water quality; microbial community structure
1 INTRODUCTION
Biofloc technology (BFT) has been successfully applied in nursery and grow-out of several fish and shrimp species (Fischer et al., 2020; Sandoval-Vargas et al., 2020; Xu et al., 2021). BFT can stabilize microbial community and improve water quality, and thus benefit productivity of farmed species by transforming ammonium nitrogen into the microbial protein (De Schryver et al., 2008; Crab et al., 2010).The application of BFT in aquaculture is usually in situ, where the development of bioflocs is promoted within farming system through adding extra organic carbon into water column or in the feeds. However,uncontrolled in-situ biofloc development leads to lower dissolved oxygen and undesired bacterial proliferation, which consequently resulted in some negative impacts on the farmed animals. The alternatives can be achieved through developing bioflocs in a separate unit that is connected to the culture facilities, to ensure better water quality as well as to provide extra food to the farmed species by screening the floc biomass from the biofloc development unit (Kuhn et al., 2010; Anand et al.,2014; Ekasari et al., 2015; Eryalcin, 2018).
The brine shrimpArtemiais highly required live food for marine fish and shrimp larviculture due to its easy availability, higher protein and lipid content, and relatively higher n-3 polyunsaturated fatty acids(PUFA) and eicosapentaenoic acid (EPA) content(Sorgeloos et al., 2001; Anh et al., 2009). As a filterfeeding zooplanktonic crustacean,Artemiafeed on planktonic unicellular algae, small protozoa,microorganisms, and organic debris. Moreover, the nutritional composition ofArtemiais mainly depending on its food composition (Toi et al., 2013).With the rapid development of aquaculture, the demand forArtemiahas been increasing. Over the past decades, pond production has emerged in the southeastern Asian countries and China, as an alternative to cope with possibleArtemiacyst shortage and to provideArtemiabiomass for shrimp nursery and maturation (Le et al., 2018).Artemiatake up microorganisms and organic particles with size ofless than 50 μm (Chacur, 2001), therefore BFT have been applied successfully to the outdoorArtemiapond culture in condition of better controlling C/N ratio and the size of biofloc aggregation (Sui et al., 2013;Le et al., 2018).
IndoorArtemiaculture is considered ecologically friendly approach for aquaculture effl uent water treatment and provide tailor-made size ofArtemiawith better biosafety. Previous studies have shown that BFT benefited to the survival and growth ofArtemia, as well as reducing the nitrogen content in the culture column in indoor batch culture (Gao et al.,2017; Wang et al., 2019). In this study, a lab-scale biofloc development unit were designed in combination of recirculation system (RAS) forArtemiaculture.Artemiagrowth, water quality, and microbial composition in BFT-RAS were compared to in-situ BFT batch culture. The information obtained in this study will support the application of BFT-RAS in indoorArtemiaculture.
2 MATERIAL AND METHOD
2.1 Experimental design
Four treatments were included in the study:1) NWRe: no water renewal and no glucose addition;2) WRe: 50% water renewal every other day and no glucose addition; 3) Glu: no water renewal, but with glucose addition in situ; 4) RAS: recirculation aquaculture system connecting with biofloc development tank and with glucose addition. The treatment NWRe, WRe, and Glu contained three replicates with unit culture volume of 8 L.
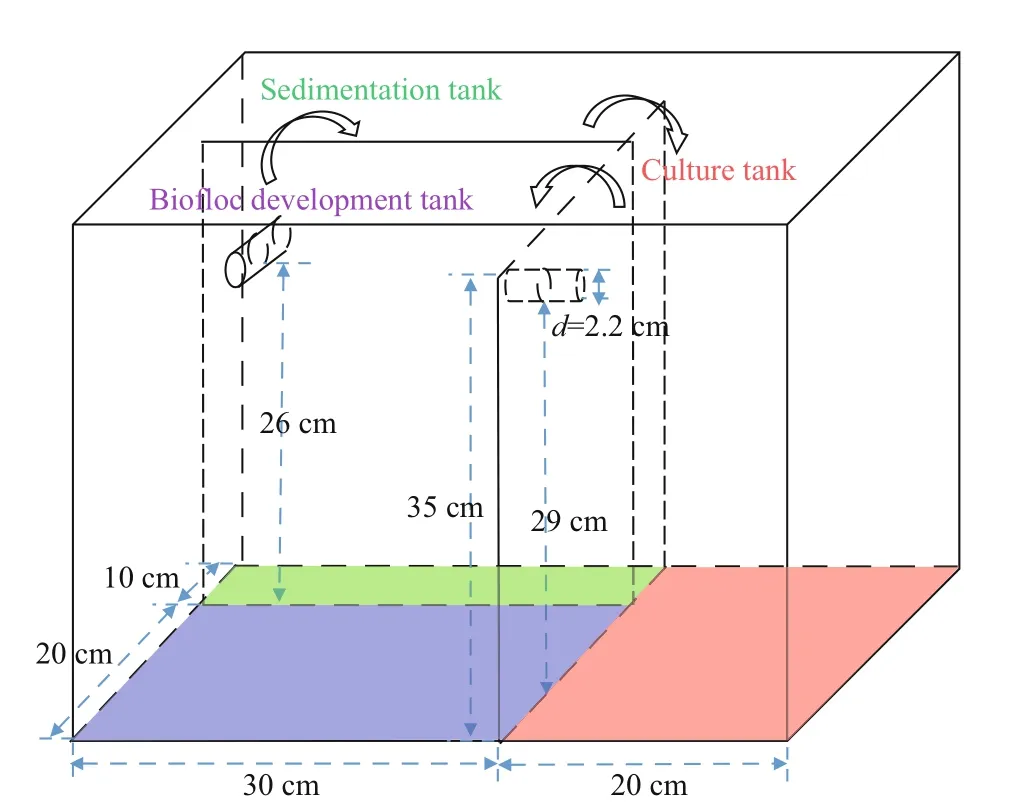
Fig.1 Design of RAS Artemia culture system
The RAS system consisted of three connected tanks, namely culture tank (18 L), biofloc development tank (16 L), and sedimentation tank (8 L) (Fig.1).Two openings connecting with tubes were made in between the tanks. The submerged pump was placed in the sedimentation tank to lift water into the culture tank. The height diff erence between two openings allowed water flowing from culture tank to the connected tanks. The tubes were covered with mesh net to preventArtemiaentering the biofloc development tank. The mesh size ranged from 500 to 1 000 μm depending on the developmental stage ofArtemia. The sedimentation tank allowed the bigger biofloc aggregates settling. Continuous aeration was provided in the biofloc development tank andArtemiaculture tank. Water was circulated partially from 10 a.m. to 10 p.m.
Artemiacysts (from Great Salt Lakes, INVE,Belgium) were hatched under standard conditions for 24 h (Lavens and Sorgeloos, 1996). The newly hatchedArtemianauplii were collected and transferred to a 50-L conical tank for adaptation culture at 25 ° C and salinity 30. The density ofArtemiawas 5 inds./mL.Artemiawere fed commercial feed (Selco-rotifer,INVE, Belgium). Three days laterArtemiawere randomly transferred into the experimental facilities and were cultured at 25 ° C and salinity 30 for 24 days.The initial density was 1 ind./mL.Artemiawere fed twice a day (9:00 a.m. and 9:00 p.m.). Distilled water was added every morning to keep the salinity constant.All treatments were fed 9.0-mg/(L·d) feed. Glucose was added every other day in the culture from day 7 onwards at C/N ratio of 10 (0.06 g/L on day 7-11,0.11 g/L afterwards). The circulation of RAS started on day 7. The amount of glucose supplement was calculated according to the formula proposed by Avnimelech (1999).
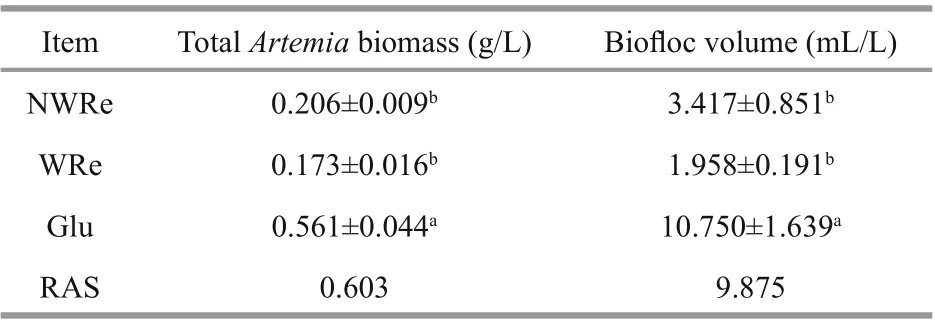
Table 1 Total Artemia biomass and biofloc volume in diff erent treatments
2.2 Parameter measurement
On day 24,Artemiain each group were collected and washed with deionized water, and the exterior water was absorbed with filter paper. The wet weight ofArtemiabiomass was then determined. The biofloc volume (BFV) was determined using Imhoff tubes after settling the culture water for 24 h.
On days 7, 14, 19, and 24 and before adding glucose, 50-mL water in each tank was filtered through a 0.22-μm microporous membrane. The content of total ammonia nitrogen (TAN), nitrite nitrogen (NO2-N), and nitrate nitrogen (NO3-N) were determined with photo-spectrometer using sodium hypochlorite oxidation, diazo-azo, and zinc cadmium reduction method (GB/T 12763.4), respectively. The content of dissolved organic carbon (DOC) was measured with total organic carbon (TOC) analyzer(Shimadzu, Japan).
2.3 Microbial diversity analysis
On day 24, the bioflocs was collected from each tank and stored at -20 °C. The TIANamp Genomic DNA kit (Tiangen Biotech, China) was used to extract the total genomic DNA, and the microbial diversity was analyzed by high-throughput sequencing(Novogene, Tianjin, China). The V4 fragments of 16S rRNA gene were PCR amplified with the primer 515F(5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R(5′-GGACTACHVGGGTWTCTAAT-3′) (Caporaso et al., 2011). OTUs clustering analysis was performed using UParse software with 97% similarity (Edgar,2013). Phylogenetic trees were constructed using MUSCLE 9 (version 3.8.31) software (Edgar, 2004).Canonical correspondence analysis (CCA) was carried out in combination with inorganic nitrogen content (TAN, NO2-N, and NO3-N) and DOC content.Shannon index analysis (Lozupone et al., 2011),weighted Unifrac distances and UPGMA analysis(Lozupone et al., 2007) were based on the homogeneous data.
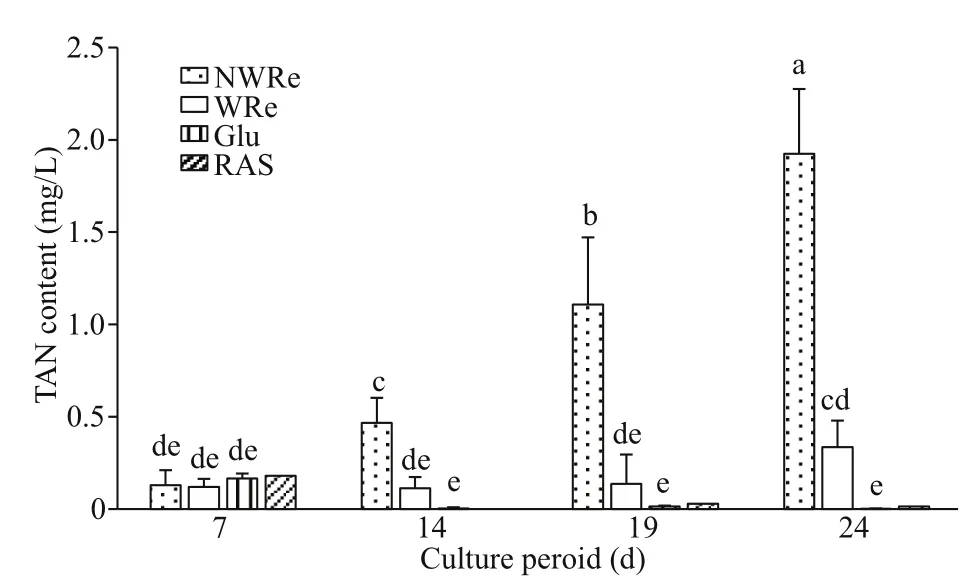
Fig.2 TAN content in the culture water
2.4 Statistical analysis
The data were presented as mean±standard deviation. Except for RAS, all values were performed with three technical triplicates. Data ofinorganic nitrogen parameters (TAN, NO2-N, and NO3-N),biofloc volume, and totalArtemiabiomass were analyzed using one-way ANOVA after Levene’s test for homogeneity of variance. Duncan’s multiple range comparison was conducted to analyze the significant diff erences among the groups atP<0.05 (SPSS Statistics, Version 19).
3 RESULT
3.1 Total Artemia biomass and biofloc volume
TotalArtemiabiomass in Glu was significantly higher than WRe and NWRe (P<0.05), while RAS had similar level with Glu (Table 1). There was no significant diff erence on BFV between NWRe and WRe (P>0.05), but BFV in Glu was significantly higher than NWRe and WRe (P<0.05). RAS had similar value with Glu.
3.2 Water quality parameters
On day 7, TAN contents were lower and no significant diff erence was observed among the treatments (P>0.05) (Fig.2). Along with culture period, TAN content in NWRe significantly increased(P<0.05), while WRe and Glu had much lower values.TAN content in RAS remained trace level from day 14 onwards.
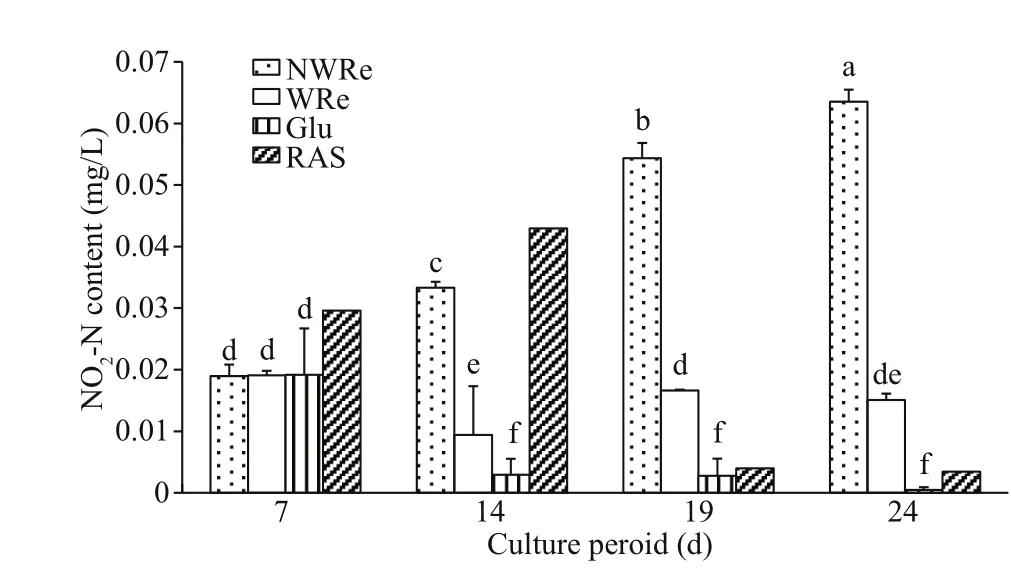
Fig.3 Nitrite nitrogen content in the culture water
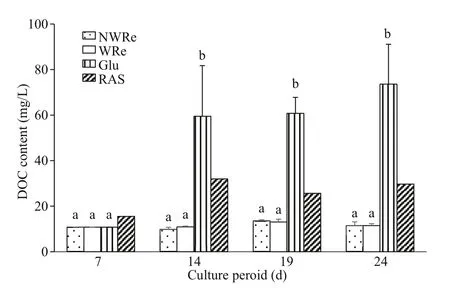
Fig.5 Content of dissolved organic carbon in the culture water
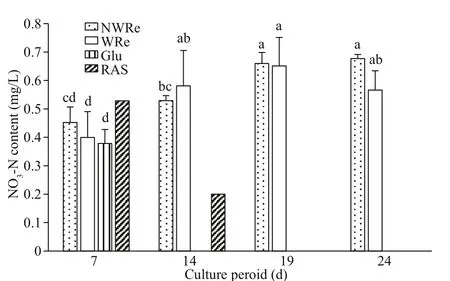
Fig.4 Nitrate nitrogen content in the culture water
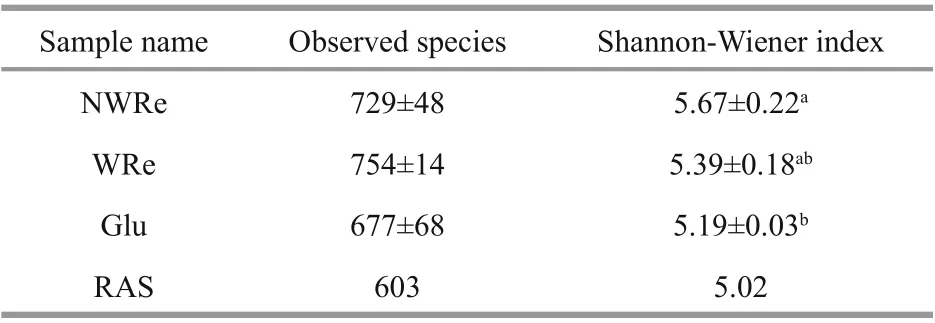
Table 2 Microbial diversity and richness of the bioflocs in the treatments
On day 7, NO2-N content in RAS was slightly higher than other three treatments, and no significant diff erence was observed among three treatments(P>0.05) (Fig.3). On day 14, NWRe had significant higher value than WRe and Glu (P<0.05), and the values in RAS remained at a higher level. On day 19 and day 24, NO2-N content in NWRe and WRe increased and remained the same trend as day 14,whilst the values in Glu and RAS were remarkably reduced.
On day 7, there was no significant diff erence on NO3-N content among NWRe, WRe, and Glu(P>0.05) (Fig.4). From day 14 onwards, NO3-N content in NWRe and WRe were at high level, while those in Glu remained un-detectable. Values in RAS were at low level on day 14, and were un-detectable on days 19 and 24.
When no glucose addition, the DOC content in NWRe and WRe remained at low level for entire culture period (Fig.5). From day 14 onwards, glucose addition increased DOC content in Glu and RAS, and remained at higher level towards the end of the experiment.
The number of observed species was highest in WRe and the lowest in RAS, no significant diff erences was observed among NWRe, WRe, and Glu (P>0.05)(Table 2). The Shannon-Wiener index (H′) of bioflocs was the highest in NWRe and the lowest in RAS(P<0.05).
Proteobacteria and Bacteroidetes were the most abundant phyla in each treatment (Fig.6). Phylogenic analysis showed that the bacterial composition within the replicates clustered together, except for one replicate in NWRe, which clustered closer to WRe.
The total proportions of top10 bacteria family in Glu and RAS were about 60%, mainly belonging to Rhodobacteraceae, Alteromonadaceae, and Saprospiraceae; while they were less than 50% in WRe and NWRe with majority of Rhodobacteraceae,Alteromonadaceae, and Saprospiraceae (Fig.7).
The abundance of the top 35 bacteria genera is shown in Fig.8. They were clustered into two branches based on evolutionary relationships. The major bacteria genus in Glu and RAS showed a higher abundance in branch I, while those of NWRe and WRe mainly located in branch II. The dominant bacteria genus in RAS also diff ered from Glu, mainly consisted of genusMarinobacter,Acinetobacter,Reinekea, andAlternomonas. On the other hand, the proportion ofVibriowas the highest in NWRe, but the lower in WRe and RAS.
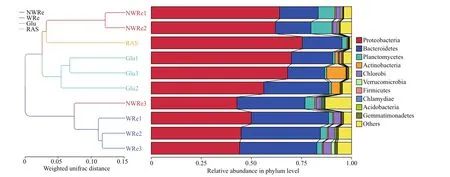
Fig.6 UPGMA clustering tree based on weighted Unifrac distance at phylum level
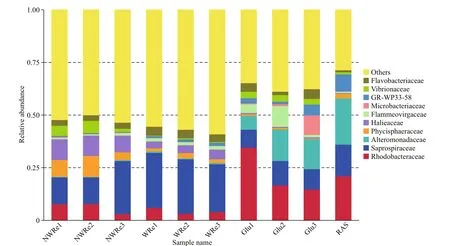
Fig.7 Relative abundance of the ten dominant families
The first and second canonical axis explained 63.03% and 20.16% of the variation, respectively(Fig.9). The contents of TAN, NO2-N, and NO3-N negatively correlated with the first and second canonical axis. The DOC content positively correlated with the first canonical axis, but negatively correlated with the second canonical axis. There were significant correlations between TAN, NO2-N,NO3-N, and DOC content, and the microbial composition of the bioflocs (P<0.05). The inorganic nitrogen in culture water had a great impact on the microbial composition in NWRe and WRe, whilst the DOC content reparably modified the microbial community in Glu and RAS.
4 DISCUSSION
4.1 BFT improves Artemia growth performance
Bioflocs can be applied in aquaculture as feed additives or directly consumed by culture species.Microorganisms in bioflocs provide a variety of extra nutrients and energy for aquatic species, such as proteins, amino acids, lipids, and fatty acids(Wasielesky et al., 2006; Crab et al., 2010). Moreover,exogenous enzymes produced by a variety of bacteria can decompose large food particles, making them easier to be ingested (Moriarty, 1998). It is assumed that the bacterial enzyme contributes as well to digestion of microalgae cells byArtemia(Toi et al.,2013).
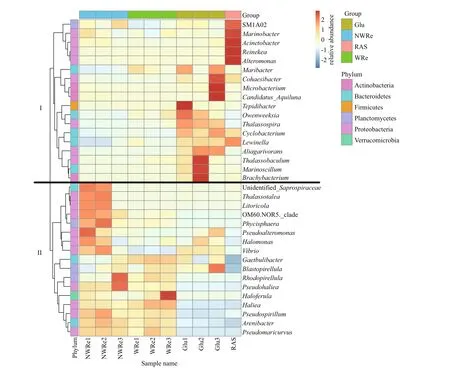
Fig.8 Abundance cluster heatmap of the top 35 bacterial genera
Artemiacan take up a wide range of food particles with size ranging from 4-8 μ m (Makridis and Vadstein, 1999) to 50 μ m (Lavens and Sorgeloos,1996) from early developmental stage to adult. On the other hand, individual bacterial cells are generally thought to be taken up with low effi ciency byArtemiadue to their small size (Nevejan et al., 2018).Therefore, biofloc aggregates with suitable size is ofimportance for filter feeders.
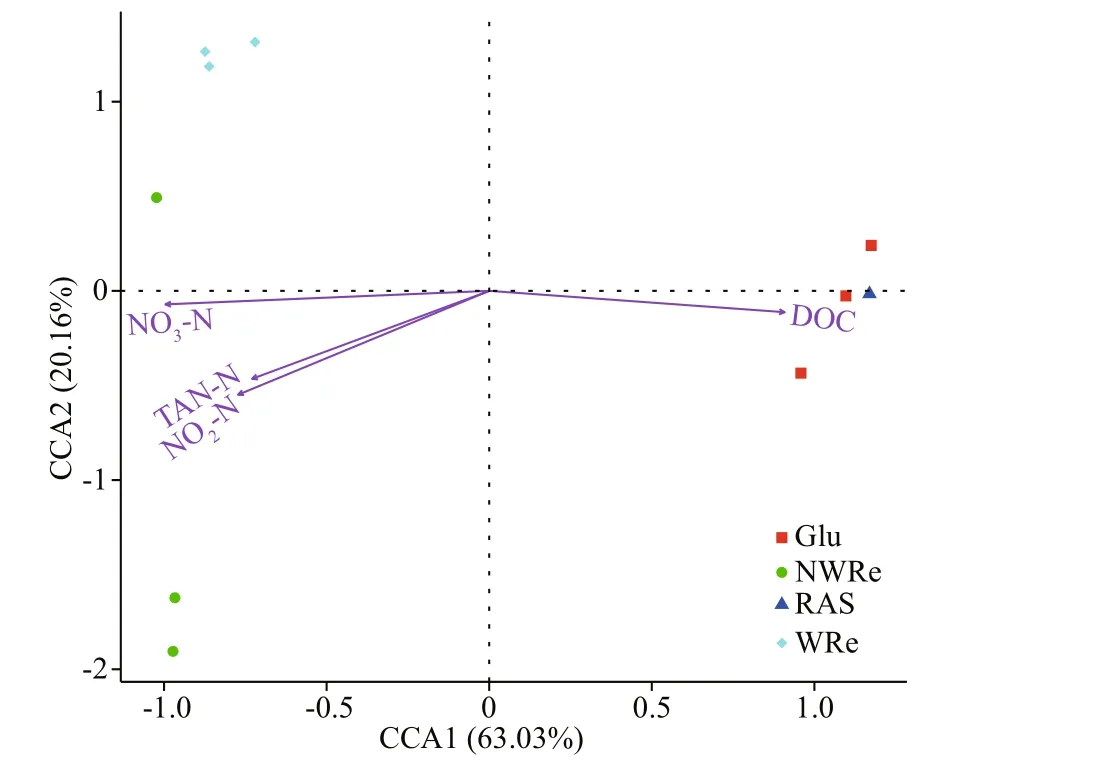
Fig.9 Canonical correspondence analysis (CCA) of bacterial family composition and water quality parameters
The totalArtemiabiomass obtained in the treatments supplemented with glucose (Glu and RAS)were significantly higher than that without adding glucose (WRe and NWRe). The beneficial eff ect of glucose supplementation should be contributed by the availability of extra nutrient source, as indicated by higher BFV values, and reduction of various nitrogen content in water column. In opposite, the inferior performance with no water renewal during experimental period (NWRe) resulted in accumulation of excessive inorganic nitrogen in the water and possible less food intake, whilst constant water renewal (WRe) may cause physical damage onArtemiaand unstable environmental conditions.
4.2 BFT benefits water quality
AlthoughArtemiaare able to tolerant harsh environment conditions, such as high salinity, low oxygen, and ultraviolet (UV) irradiation, etc.,continuous accumulation of nitrogenous waste in the culture column are detrimental toArtemia. In this study, we compared the inorganic nitrogen waste removal by using BFT and water renewal. The TAN,NO2-N, and NO3-N contents in non-water renewal treatment (NWRe) continued to rise and remained at a high level throughout the culture period. When 50%water was renewed every other day, TAN and NO2-N content in WRe remained lower level, but NO3-N content increased. Glucose addition significantly decreased the inorganic nitrogen content in Glu and RAS that remained at a very low level towards the end of culture period. This can be explained that glucose promoted the proliferation of heterotrophic microorganisms, and thus significantly reduce inorganic nitrogen in the water through converting the TAN into microbial protein as well as nitrifying TAN to NO2-N, and further to NO3-N (Hari et al., 2004;Schneider et al., 2005).
On the other hand, the fact that the DOC content in Glu and RAS remained constant from 14thday onwards indicated that the added glucose could be consumed effi ciently by microorganisms. The continuously increased BFV in Glu and RAS also confirmed that the carbon and nitrogen were assimilated and converted into microbial biomass.
4.3 BFT shapes microbial community structure
High-throughput sequencing analysis was performed in this study, to compare the microbial diversity and community structure of the bioflocs in diff erent treatments. The low Shannon index in Glu and RAS indicated that glucose supplement facilitated the growth of certain heterotrophs, and thus reduced the biofloc diversity.
Proteobacteria and Bacteroidetes are the dominant bacteria phyla usually found in aquaculture water and also formed large proportion in bioflocs (Cardona et al., 2016; Wei et al., 2016). Proteobacteria are also considered symbiotic bacteria in aquaculture (Sakami et al., 2008), which can eff ectively remove organic matter and inorganic nitrogen from waste water(Wagner et al., 1993; Rosenberger et al., 2002; Miura et al., 2007). The relative abundance of Proteobacteria in Glu and RAS was higher than NWRe and WRe,indicating that extra glucose favored the growth of Proteobacteria.
The glucose addition not only diversified the microbial community, but also facilitated the growth of potential probiotic bacteria and suppressed the pathogenic bacteria in the water. In this study, the proportion of family Rhodobacteraceae in Glu and RAS was remarkably higher than the others’.Rhodobacteraceae is considered surface-attached bacteria (Hjelm et al., 2004; Cytryn et al., 2005),which limits the survival of pathogenic bacteria in bioflocs (Cardona et al., 2016).Rhodobacterhas been associated with enhancing growth performance of aquatic animal via immune regulation (Chiu and Liu,2014). On the other hand, the proportion ofVibrioin RAS were lower than NWRe, and RAS had fewerFlavobacterium. Most of bacteria belonging toVibriohave been characterized as pathogens for aquatic animals (Karunasagar et al., 1994). AndFlavobacteriumcolumnarelead to the rotten gill disease of Anguillidae, Cyprinidae, and Ictaluridae fish. Therefore, the BFT applied in this study improved biosecurity condition ofArtemiaculture system,which may further benefit to fish and crustacean aquaculture through providing high qualityArtemiabiomass.
5 CONCLUSION
Our study provides data supporting the application of BFT in indoorArtemiaculture with recirculation system. The results indicate that glucose addition to batch culture significantly improved theArtemiagrowth, increased the biofloc volume, and reduced the content of TAN, NO2-N, and NO3-N in water column, as well as shaped the microbial community structure of bioflocs and led to increased proportion of potential probiotic bacteria and suppression of pathogenic bacteria. Moreover, similar beneficial results to the in-situ BFT batch culture were obtained in our designation through connecting a biofloc development unit to the recirculationArtemiaculture system. In the future, the RAS-BFT parameters can be further optimized in order to facilitate large-scale application in the production of bio-secured live feedArtemiabiomass.
6 DATA AVAILABILITY STATEMENT
The data presented in this paper can be shared upon request.
 Journal of Oceanology and Limnology2022年4期
Journal of Oceanology and Limnology2022年4期
- Journal of Oceanology and Limnology的其它文章
- Validation and error analysis of wave-modified ocean surface currents in the northwestern Pacific Ocean*
- The energy conversion rates from eddies and mean flow into internal lee waves in the global ocean*
- Observation of physical oceanography at the Y3 seamount(Yap Arc) in winter 2014*
- Decadal variation and trend of the upper layer salinity in the South China Sea from 1960 to 2010*
- Observations of turbulent mixing and vertical diff usive salt flux in the Changjiang Diluted Water*
- Experimental research on oil film thickness and its microwave scattering during emulsification*
