Characterization and comparison of two C-type lectins with novel key motifs from mud crab Scylla paramamosain*
Boxin ZENG , Taiwei DONG , Yanting XIA , Shun YANG ,2, Mengmeng HUANG ,2,**,Hui FEI ,2,**
1 College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou 310018, China
2 Zhejiang Provincial Key Laboratory of Silkworm Bioreactor and Biomedicine, Zhejiang Sci-Tech University, Hangzhou 310018,China
Abstract As an important pattern recognition receptor (PRR) in the innate immune system, C-type lectin plays an important role in the innate immune process ofinvertebrates. Two C-type lectins Sp CTL-C and Sp CTL-D were characterized from mud crab ( Scylla paramamosain). The predicted Sp CTL-C and Sp CTL-D proteins both contain a single carbohydrate-recognition domain (CRD) with key motif Gln-Pro-Ala (QPA) and Met-Pro-Ala (MPA), respectively. Sp CTL-C and Sp CTL-D transcripts distributed in all examined tissues, and the expression level was the highest in hepatopancreas. As PRR, the purified recombinant proteins r Sp CTL-C and r Sp CTL-D have high affi nity for three kinds of pathogen-associated molecular patterns (PAMPs): β-glucan, lipopolysaccharide, and peptidoglycan. Besides, r Sp CTL-D can bind to all nine microorganisms tested, while r Sp CTL-C can bind to seven microorganisms except for Staphylococcus aureus and Micrococcus luteus. Both r Sp CTL-C and r Sp CTL-D showed agglutination activity towards fungi Pichia pastoris and Saccharomyces cerevisiae. However, r Sp CTL-C and r Sp CTL-D exhibited diff erent antimicrobial activities: r Sp CTL-D has a certain inhibitory eff ect on the growth of Vibrio fluvialis and M. luteus, while r Sp CTL-C has no obvious inhibitory activity. The results show that r Sp CTL-C and r Sp CTL-D had better phagocytosis-promoting eff ect on M. luteus than the negative control. Meanwhile,both r Sp CTL-C and r Sp CTL-D had certain encapsulation-promoting activity. Collectively, two C-type lectins with novel key motifs make an important impact as PRR in immune response towards pathogens. At the same time, they play diff erent functions in the innate immunity of mud crab S. paramamosain.
Keyword: Scylla paramamosain; immune response; innate immunity; C-type lectin
1 INTRODUCTION
Invertebrate defense against pathogens depends on innate immunity because of the lack of acquired immunity (Loker et al., 2004; Rowley and Powell,2007). The innate immune system recognizes and responds to diff erent types of pathogens, depending on the common structural and functional characteristics of microorganisms. As the first line of defense, the innate immune system keeps the host from infection and its defense function enables the host to eff ectively resist bacteria, fungi, and viral pathogens (Zhang et al., 2019; Tran et al., 2020). The conserved determinants of the origin of microorganisms and viruses are detected by the receptors of the innate immune system, initiate the activation of the signal cascade, which leads to the transcriptional regulation ofinflammatory mediators that play a role in pathogen control and clearance, and ultimately leads to an eff ective immune response (Brubaker et al., 2015;Tran et al., 2020).
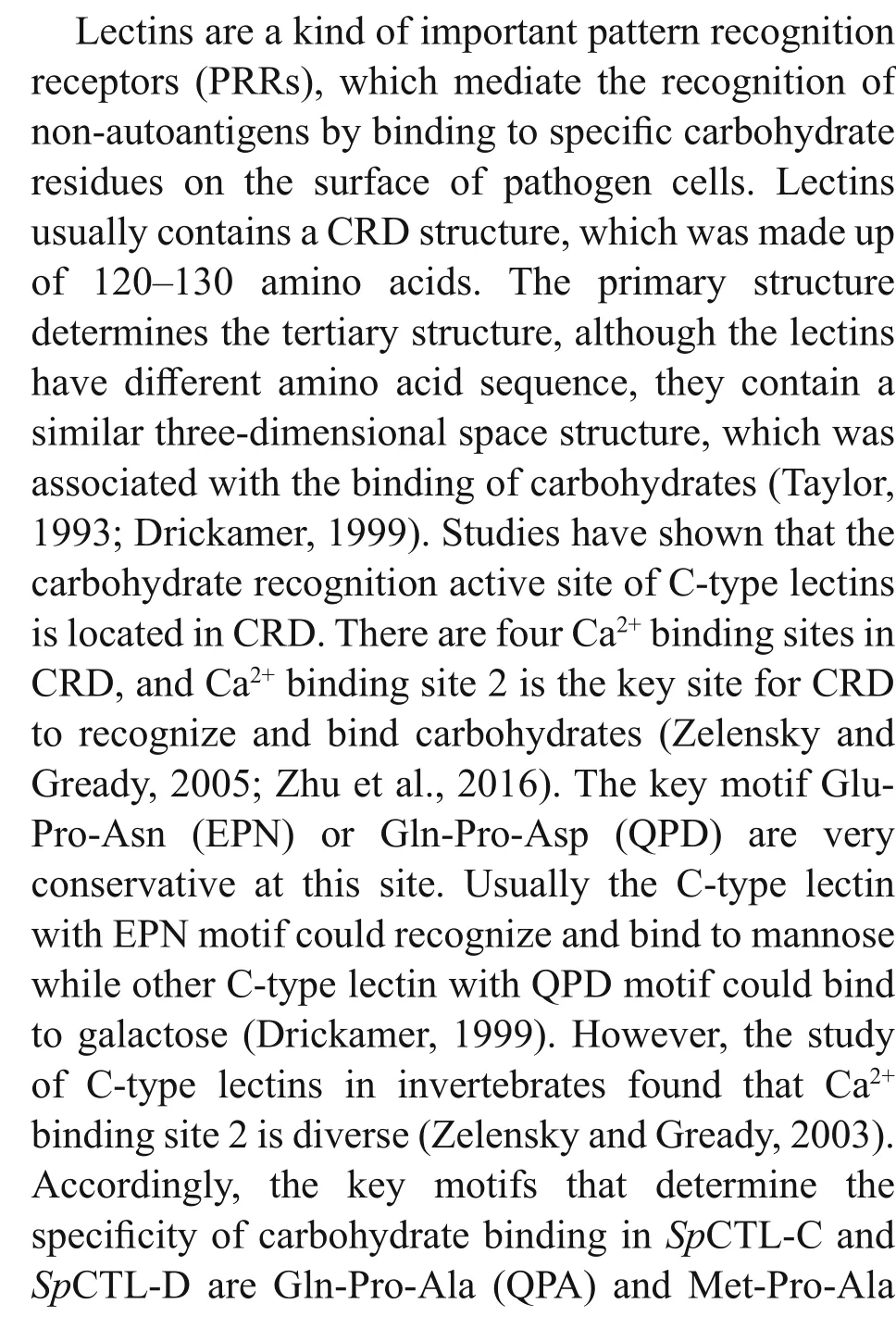
Based on the diff erence of molecular structure,animal lectins can be mainly classified into C-type lectins (CTL), P-type lectins, I-type lectins, S-type lectins, pentamer proteins, and so on (Drickamer and Taylor, 1993). In the lectin superfamily, C-type lectins play an important role in identifying and clearing foreign invasions and have attracted more and more attention in recent years (Christophides et al., 2002).C-type lectins are a kind of protein superfamily that needs Ca2+to participate in selective carbohydrate binding (Weis et al., 1998). They are classified into 17 groups according to their structure and functions.Collectins mainly serve as pattern recognition receptor (PRR) participating in discrimination of pathogens (Cambi et al., 2005), while another group selectins mediate cellular adhesion to active opsonization (Patel et al., 2002). CTL has at least one characteristic carbohydrate recognition domain(CRD), which are composed of 115-130 amino acids(Drickamer, 1993; Zelensky and Gready, 2003). Glu-Pro-Asn (EPN)/Gln-Pro-Asp (QPD) and Trp-Asn-Asp (WND) are two highly conserved amino acid motifs in this domain, which are related to the specific recognition of carbohydrates (Weis et al., 1998).C-type lectins play an important role in invertebrate innate immune response, including activating prophenoloxidase (Christophides et al., 2002),microbial agglutination (Wang et al., 2009), inhibiting bacterial growth, and promoting blood cell phagocytosis. For example,ScyllaparamamosainSpCTL-B can bind to a variety of Gram-positive bacteria, Gram-negative bacteria, and fungi (Wei et al., 2018).
Scyllaparamamosainis an important mariculture crab in China, with high nutritional value, medicinal value, and economic value (Cao et al., 2013).However, in the process of cultivation, it is easy to be aff ected by water quality, bacteria, and virus infections, which leads to the low survival rate and causes huge losses to aquaculture ofS.paramamosain.For example, bacterial infections such asVibrioparahaemolyticusandAeromonashydrophilain 2010 led to a nearly 60% drop in the production of crabs in Shantou, China. Although there have been many coping strategies such as immune enhancers, how to resist increasingly serious diseases by improving the immunity of mud crabs has become an urgent problem in the crab farming industry.
In this study, we have identified and verified the functions ofSpCTL-C andSpCTL-D derived fromS.paramamosain. The pathogen-associated molecular patterns (PAMPs) binding activity, antibacterial activity, bacterial binding activity, blood coagulation activity, opsonization enhancement activity of blood cells, and the tissue distribution of the two lectins were studied systematically using molecular biology and immunological techniques, and preliminary exploration ofits molecular recognition mechanism.
2 MATERIAL AND METHOD
2.1 Material
Escherichiacoli,Micrococcusluteus, andStaphylococcusaureuswere purchased from the Microbial Culture Collection Center (Beijing, China).Vibriofluvialis,V.parahaemolyticus, andSaccharomyces cerevisiaewere obtained from the Global Bioresource Center (USA).Pichiapastoriswas from Invitrogen.Aeromonassobria,Edwardsiellatarda, andA.hydrophilawere isolated by our laboratory.Vibrioalginolyticuswere obtained from Minnan Normal University.
Scyllaparamamosainwere collected from a seafood market in Hangzhou, Zhejiang Province.Filtered seawater was used to acclimate the crabs for 7 days before experiments.
2.2 Method
2.2.1 Bioinformatics analysis
The cDNA and deduced amino acid sequences ofSpCTL-C andSpCTL-D were analyzed using BLAST(http://www.ncbi.nlm.nih.gov/blast) and the Expert Protein Analysis System (http://www.expasy.org).ExPASy (http://web.expasy.org/computepi/) was used to predict the molecular weight and isoelectric point (pI) ofSpCTL-C andSpCTL-D. The ClustalW Multiple Alignment program (http://www.ebi.ac.uk/clustalw/) was used to create a multiple sequence alignment. The signal peptide was predicted by the SignalP 3.0 server (http://www.cbs.dtu.dk/services/SignalP-3.0/). MEGA 7.0 was used to produce the phylogenetic tree, and the neighbor-joining (NJ)method was used for phylogenetic analysis.
2.2.2 Tissue distribution ofSpCTL-C,SpCTL-D, and RNA extraction and cDNA synthesis ofS.paramamosain
Total RNA was extracted from various tissues(muscle, gill, hemocytes, gonad, and hepatopancreas)of four randomly sampled individuals. Total RNA was isolated using TRIzol reagent (Sangon Biotech).The first strand synthesis was carried out based on Promega M-MLV RT usage information using the DNase I-treated total RNA as template and oligo(dT)-adaptor primer. The reactions were incubated at 42 °C for 1 h, terminated by heating at 95 °C for 5 min.The amplified cDNA was diluted and stored at -80 °C.
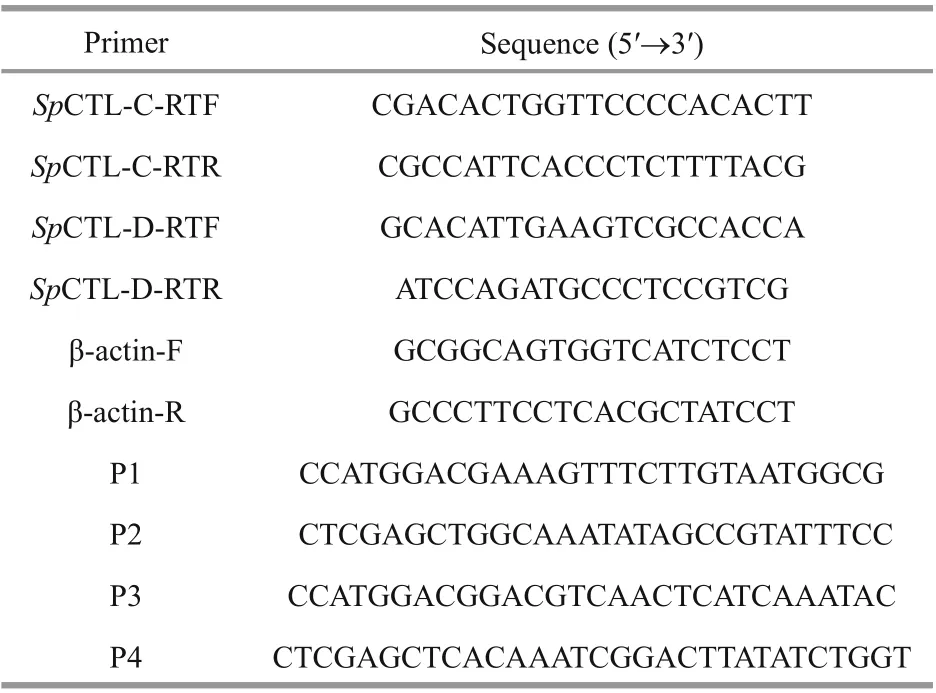
Table 1 Primers used in the study
The tissue distribution and temporal expression ofSpCTL-C andSpCTL-D transcript in diff erent tissues were determined by quantitative real-time PCR(Q-PCR) as previously described (Huang et al., 2017).Specific primers ofSpCTL-C,SpCTL-D, and internal reference gene β-actin were designed (Table 1). The reaction system was configured according to the instructions of the kit. The procedure was as follows:95 °C for 1 min, followed by 40 cycles (95 °C for 5 s,60 °C for 30 s). All samples were analyzed in four duplications and the data obtained from Q-PCR analysis were subjected to one-way analysis of variance (one-way ANOVA) followed by an unpaired,two-tailedt-test. A probability ofP<0.05 was considered statistically significant.
2.2.3 Prokaryotic expression and purification of recombinantSpCTL-C andSpCTL-D
The cDNA fragments ofSpCTL-C,SpCTL-D were amplified with primers P1-P4 (Table 1) with two restriction enzyme cleavage sites at 5′-ends (NcoI andXhoI). Target genes were digested with restriction enzymes and ligated to expression vector pET-32a.The recombinant plasmids (pET-32a-SpCTL-C and pET-32a-SpCTL-D) were transformed into antibiotic competent cells inE.coliDH5α. TheSpCTL-C andSpCTL-D positive transformants were inoculated into LB medium containing a final concentration of 100-μg/mL ampicillin, and incubated at 37 °C with a shaker rotating 220 r/min until the absorbance at 600 nm (OD600) reached 0.4-0.6. Isopropyl-beta-Dthiogalactopyranoside (IPTG) was added to the LB medium to a final concentration of 0.01 mol/L, and cultured in a shaker at 18 and 30 °C converted to 220 r/min for 20 h and 4 h, respectively. After the cells were obtained by centrifugation, they were ultrasonically processed and centrifuged to obtain the soluble recombinant protein in the supernatant.Recombinant rSpCTL-C and rSpCTL-D were purified by Ni2+chelating agarose column and eluted with 100 and 250-mol/L imidazole in tris buff ered saline (TBS)buff er, respectively. After dialysis to TBS buff er,recombinant proteins were concentrated with PEG-20000, a certain concentration of recombinant protein was obtained. At the same time, recombinant thioredoxin (rTrx) was purified according to the same method. The obtained rSpCTL-C, rSpCTL-D, and rTrx were stored at -80 °C for the subsequent use in the experiments.
2.2.4 PAMPS binding experiments of rSpCTL-C and rSpCTL-D based on enzyme-linked immuno sorbent assay (ELISA)
PAMPs binding assays were performed as previously described with some modifications (Yu et al., 2007). An amount of 10-μg peptidoglycan (PGN),glucan (Glucan), and lipopolysaccharide (LPS) in 100-μL carbonate-bicarbonate buff er (pH=9.6) were used to coat a 96-well microliter plate (Costar) and incubated at 4 °C overnight, respectively. The incubated PAMPs were poured out and washed with phosphate buff ered saline-Tween (PBS-T) (pH 7.4)for three times. Nonspecific binding to the wells was prevented by addition 3% bovine serine albumin(BSA) at 37 °C for 1 h. Then the plate was washed by PBS-T for three times, and 100-μL rSpCTL-C or rSpCTL-D were added to each well using two-fold dilution method (Ca2+concentration 10 mol/L). The same concentration of rTrx was set as a negative control. Both of them were incubated at 18 °C for 3 h.After three times washing with PBS-T, 100-μL horseradish peroxidase (HRP)-labelled rabbit anti-6 ×His-tag polyclonal antibody (Abcam, diluted 1∶4 000 in PBS-T) was added to each well and incubated at 37 °C for 1 h. After three times washing, detection was performed following the instructions of the El-TMB Chromogenic Reagent kit (Sangon). The absorbance under OD450for detection was measured.The filled wells with 100-μL TBS (pH=8.0) were used as blanks. Three replicates were set for each group.
2.2.5 Microbial binding experiments of rSpCTL-C and rSpCTL-D
The experimental bacteria (M.luteus,S.aureus,V.fluvialis,V.parahaemolyticus,S.cerevisiae,A.sobria,V.alginolyticus,E.tarda, andA.hydrophila)were cultured and resuspended to a final concentration at 1×108CFU. Then they were used to coat a 96-well microliter plate (Costar) and incubated at 4 °C overnight. After rinsing the plates, 200-μL BSA solution was added to each well and sealed at 37 °C for 2 h. An aliquot of 100-μL recombinant protein(Ca2+concentration 10 mol/L) was added to each well and incubated at 18 °C for 3 h after three times rinse.After washing the plates, 100-μL HRP labeled rabbit anti-His tag antibody was added to each well (diluted with 1∶3 000), and incubated at 37 °C for 1 h. After rinsing the board for three times, color development was carried out according to the instructions of ELTMB kit, and absorbance was measured under 450 nm(OD450).
2.2.6 rSpCTL-C and rSpCTL-D microbial agglutination experiments
The experimental bacteria (S.aureus,M.luteus,V.fluvialis,A.sobria,P.pastoris, andS.cerevisiae)were cultured to the logarithmic growth stage, and then washed three times with 0.1-mol/L NaHCO3buff er (pH=9.0) after centrifugation. The bacteria were mixed with 0.1-mg/mL FITC dye and incubated for 2 h at room temperature for staining. After cleaning excess dye, FITC-labeled microbes were resuspended to TBS buff er (50-mol/L TBS, 10-mol/L CaCl2) with concentration of 1×1010cell/mL. An aliquot of 25-μL rSpCTL-C and rSpCTL-D were incubated with 10-μL bacterial suspension respectively in dark for 1 h at room temperature. The agglutination activity of rSpCTL-C and rSpCTL-D were observed under the fluorescence microscope. rTrx of the same concentration was used as negative control.
2.2.7 Encapsulation experiment of rSpCTL-C and rSpCTL-D
Ni-NTA agarose particles were equilibrated in 5-mol/L TBS-Ca2+buff er, incubated with rSpCTL-C,rSpCTL-D, and rTrx, respectively, shaken overnight at 4 °C. After melting with 1% agarose, the 48-well cell culture plate was coated, and the extracted hemocytes of carb were added to each well separately,and the cells were settled for 10 min. Then three protein-coated agarose particles (120-150) were added into each well, and three groups of each protein were set in parallel, incubated at 18 °C for 6 h, and the encapsulation was observed under the optical microscope. The encapsulation rate = (the number of encapsulated beads/all beads tested)×100%. The data are expressed in means±SE (n=3).
2.2.8 Phagocytosis-promoting experiment of rSpCTL-C and rSpCTL-D
The experimental bacteria (M.luteus,E.coli) were cultured to logarithmic growth phase and after centrifugation were washed for three times with 0.1-mol/L NaHCO3(pH=9.0). The bacteria were stained with 0.1-mg/mL FITC and incubated at room temperature for 2 h. After washing the excess stain,bacteria were resuspended with TBS-Ca2+to a bacterial concentration of 1×1010cells/mL. The blood cells were re-suspended in TBS group, negative control rTrx group, and rSpCTL-C, rSpCTL-D group according to the final concentration of 5×107/mL.M.luteusandE.coliwith the final concentration of 1×1010/mL was added and incubated at room temperature for 1 h. Flow cytometry (Accuri™ C6 Plus, BD biosciences, USA) was used to detect the phagocytosis rate of blood cells and the phagocytosis percentage was defined as (the hemocytes ingesting bacteria/all hemocytes tested) ×100%. All Assay was performed in triplicate. The data are expressed as means ± SE (n=3).
2.2.9 Antibacterial activity assay of rSpCTL-C and rSpCTL-D
Various microbes (V.parahaemolyticus,V.fluvialis,P.pastoris,S.cerevisiae,M.luteus, andV.alginolyticus) were cultured to the logarithmic growth phase and were washed 3 times with TBSCa2+and resuscitated to 104CFU. 50 μL the same concentration of rSpCTL-C and rSpCTL-D were mixed with bacterial suspension at 1∶1 and incubated at room temperature for 1 h. 50-μL TBS plus bacterial solution was used as blank control, and 50-μL rTrx was used as negative control. Take 20-μL mixture to 96-well cell culture plate, add 200-μL corresponding culture medium, incubate in a constant temperature shaker for 12-16 h until reaching the platform stage and read OD600every half an hour. The microbial growth curve was analyzed and drawn by Origin 8.0 software and Excel.
2.2.10 Statistical analysis
All experimental data were analyzed using oneway analysis of variance (one-way analysis of variance, ANOVA). The expression level was analyzed using the software GraphPad Prims 8.0. The results are expressed as mean±SE.P<0.05 means significant diff erence, andP<0.01 means extremely significant diff erence.

Fig.1 Multiple sequence alignment of CRD in Sp CTL-C, Sp CTL-D, and other CTLs
3 RESULT
3.1 cDNA cloning and sequence analysis of Sp CTL-C and Sp CTL-D
The cDNA sequences ofSpCTL-C andSpCTL-D were screened from GenBank with accession numbers KC757381.1 and HQ325748.1, respectively. The open reading frame (ORF) ofSpCTL-C encodes 158 amino acids. The ORF ofSpCTL-D encodes 176 amino acids. Both of them contains a single CRD.The predicted theoretical isoelectric point and molecular weight ofSpCTL-C are 5.29 and 17.775 kDa, whileSpCTL-D is 4.89 and 19.698 kDa,respectively. According to multiple sequence alignments,SpCTL-C andSpCTL-D both contain CRDs which have two pairs of disulfide bonds formed by four highly conserved cysteines maintaining the stability of the structure. The key motif determining the carbohydrate binding specificity was QPA and MPA inSpCTL-C andSpCTL-D respectively.Multiple sequence alignment analysis ofSpCTL-C andSpCTL-D against the CTLs from various species show that they diff er in amino acid composition(Fig.1). Figure 1 shows that some amino acid residues,such as cysteine and tryptophan residues, are highly conserved in the CTLs of diff erent species. A phylogenetic tree was constructed by using NJ algorithm to analyze the sequence alignment based on the alignment of C-type lectin amino acid sequences fromS.paramamosain,Eriocheirsinensis,Penaeus japonicus,Penaeusvannamei,Portunus trituberculatus,Penaeusmonodon,Homosapiens,Gallusgallus,Salmosalar,Musmusculus, and two distinct groups were separated in the phylogenetic tree (Fig.2).
3.2 Tissue expression pattern of Sp CTL-C and Sp CTL-D
The mRNA transcripts ofSpCTL-C andSpCTL-D were detected in gill, muscle, gonad, and hepatopancreas (Fig.3). They were most abundant in hepatopancreas, moderately expressed in gonad and muscle, and relatively lower in gill and hemocytes.The expression level ofSpCTL-C in hepatopancreas,gonad, muscle, and gill were 266 119-, 108.29-,15 419-, and 10.13-fold relative to that in hemocytes,respectively. The expression level ofSpCTL-D in hepatopancreas, gonad, muscle and gill were 43.82,16.14-, 68.08-, and 2.89-fold relative to that in hemocytes, respectively.
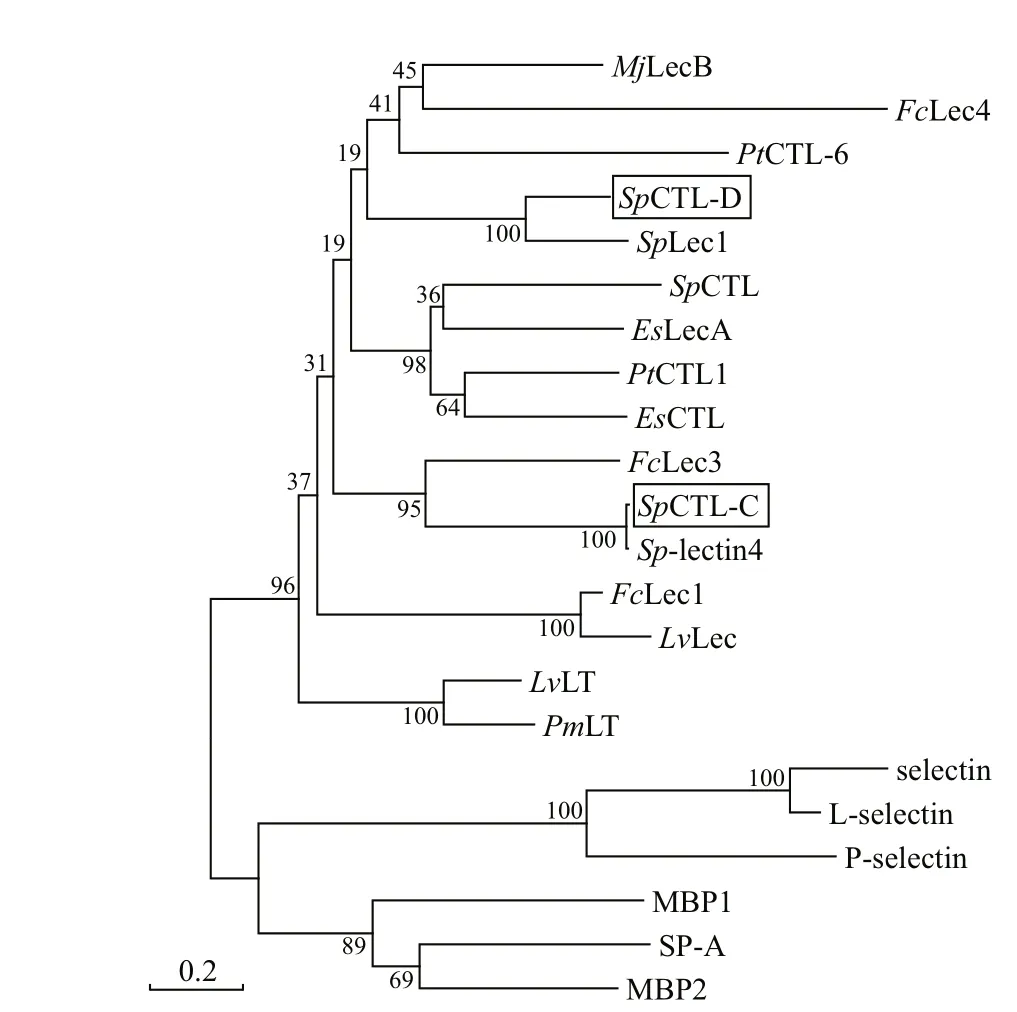
Fig.2 Phylogenetic analysis of Sp CTL-C and Sp CTL-D
3.3 Prokaryotic expression of r Sp CTL-C and r Sp CTL-D
Prokaryotic expression recombinant plasmids pET-32a (+)-SpCTL-C and pET-32a (+)-SpCTL-D were transformed intoE.coliBL21 respectively. After induction of expression by IPTG, their expression was detected using SDS-PAGE (Fig.4). The results showed that rSpCTL-C was soluble expressed at 16 °C and the final induction concentration of IPTG was 0.01 mol/L, while rSpCTL-D was soluble expressed at 30 °C and the final induction concentration of IPTG was 0.01 mol/L.
3.4 PAMPS-binding activity of r Sp CTL-C and r Sp CTL-D
Both rSpCTL-C and rSpCTL-D had binding affi nity to LPS, PGN, and Glucan. Meanwhile,rSpCTL-D had a higher binding affi nity than that of rSpCTL-C, and no binding activity was detected in the negative control (Fig.5).
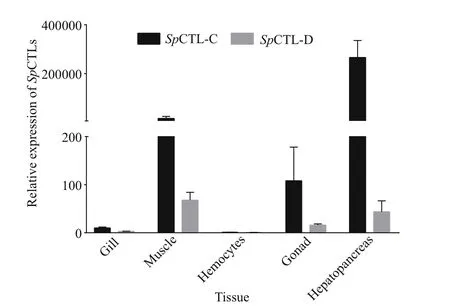
Fig.3 Real time expression of Sp CTL-C and Sp CTL-D
3.5 Analysis of antimicrobial activity of r Sp CTL-C and r Sp CTL-D
Comparing with the negative control and the blank control, rSpCTL-D had a certain inhibitory eff ect on the growth ofV.fluvialisandM.luteus, while rSpCTL-C had no obvious inhibitory activity (Fig.6).
3.6 Agglutination activity of r Sp CTL-C and r Sp CTL-D
By incubating various microbes with rSpCTL-C and rSpCTL-D, the agglutination activity of rSpCTL-C and rSpCTL-D were observed. Both rSpCTL-C and rSpCTL-D showed agglutination activity towards fungiP.pastorisandS.cerevisiaeand Gram-positive bacteriaS.aureus(Fig.7), but no agglutination was observed in other microorganisms(Fig.7), indicating that rSpCTL-C and rSpCTL-D can selectively bind and agglutinateS.cerevisiaeandP.pastoris.
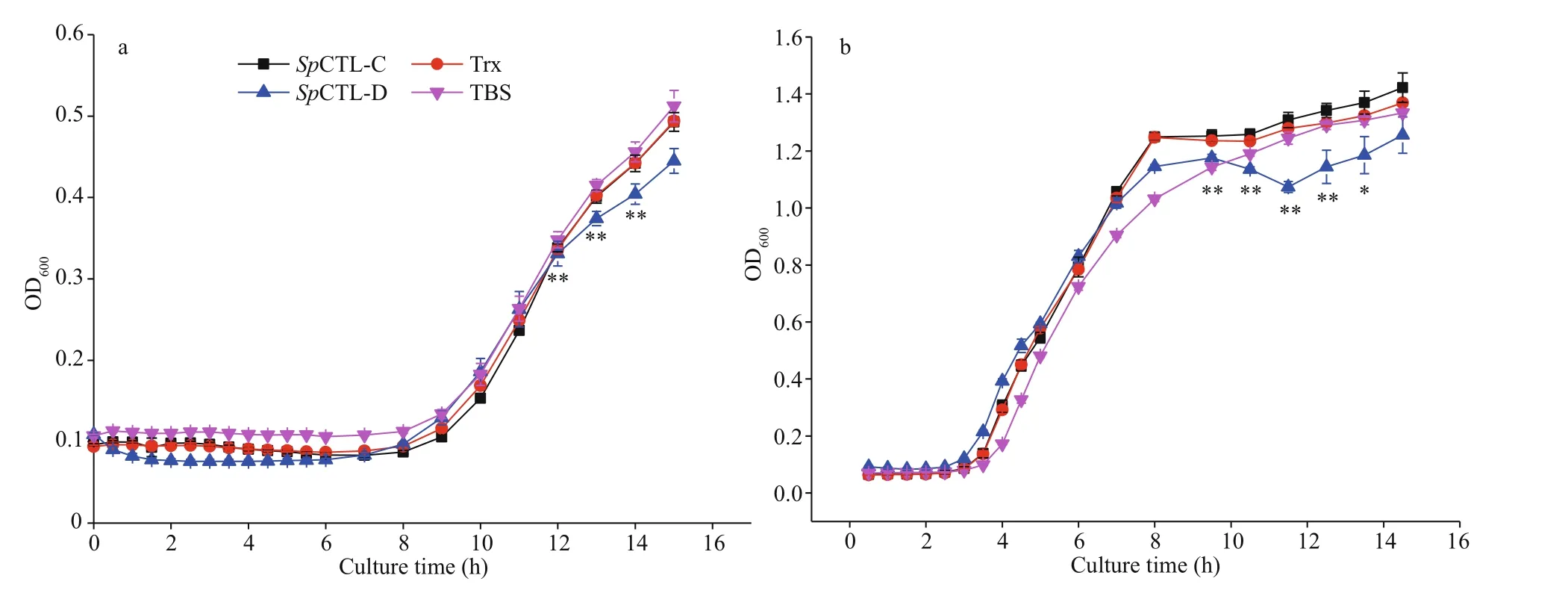
Fig.6 Antimicrobial activity analysis of r Sp CTL-C and r Sp CTL-D against V. fluvialis (a) and M. luteus (b)
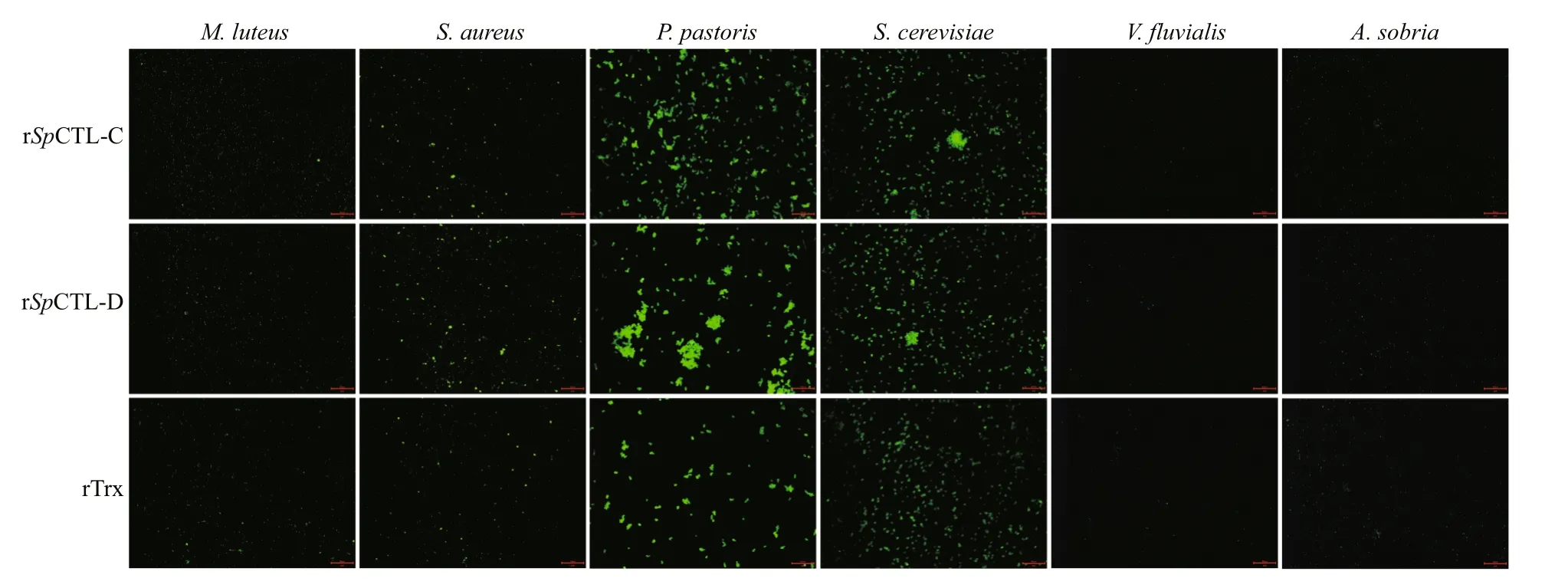
Fig.7 Bacteria agglutination of r Sp CTL-C and r Sp CTL-D on FITC-labeled V. fluvialis, A. sobria, S. aureus, M. luteus,S. cerevisiae, and P. pastoris
3.7 Analysis of microbial binding activity of r Sp CTL-C and r Sp CTL-D
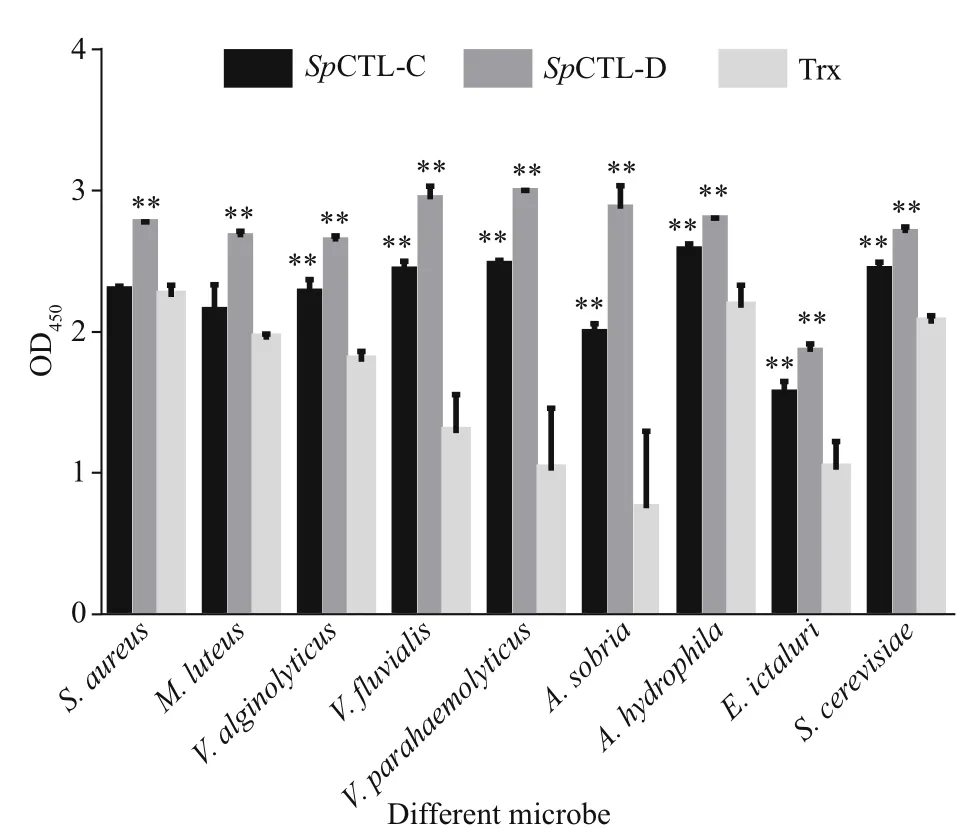
Fig.8 The microbe binding spectrum of r Sp CTL-C and r Sp CTL-D
The microbe-binding spectrum of rSpCTL-C and rSpCTL-D was detected by ELISA. Comparing with negative control, rSpCTL-D can bind to all nine tested microorganisms, while rSpCTL-C can bind to seven microorganisms except two Gram-positive bacteria(S.aureusandM.luteus). This result indicates that rSpCTL-C and rSpCTL-D have broad-spectrum “nonself” binding activity, and the two proteins have certain selectivity for microbial recognition and binding. The binding activity of rSpCTL-C and rSpCTL-D to various microorganisms was significantly higher than that of the negative control group (P<0.01) (Fig.8).
3.8 Encapsulation enhancement activity analysis of r Sp CTL-C and r Sp CTL-D
The encapsulation enhancement activity of rSpCTL-C and rSpCTL-D was carried out by incubating agarose gel beads with hemocytes. After microscopic examination and statistical analysis, the encapsulation percentage of rSpCTL-C and rSpCTL-D were 44.29% and 52.44% respectively (Fig.9a-b),which were significantly higher than 28% of rTrx(P<0.01). It indicated that both rSpCTL-C and rSpCTL-D had certain encapsulation-promoting activity, and rSpCTL-D had stronger encapsulationpromoting activity.
3.9 Analysis of promoting phagocytic activity of r Sp CTL-C and r Sp CTL-D
The phagocytosis rates of rSpCTL-C and rSpCTL-D proteins toM.luteuswere 50.5% and 53.4% respectively, which were higher than those of the negative control group. The phagocytosis rates toE.coliwere 16.4% and 17.7% respectively (Fig.10).The results showed that rSpCTL-C and rSpCTL-D had better phagocytosis-promoting eff ect onM.luteusthan the negative control, and had no obvious phagocytosis-promoting activity onE.coli.
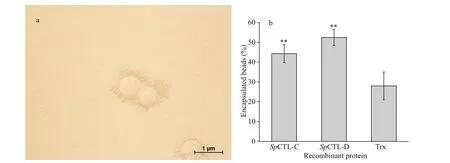
Fig.9 The enhancement of hemocytes encapsulation by r Sp CTL-C and r Sp CTL-D
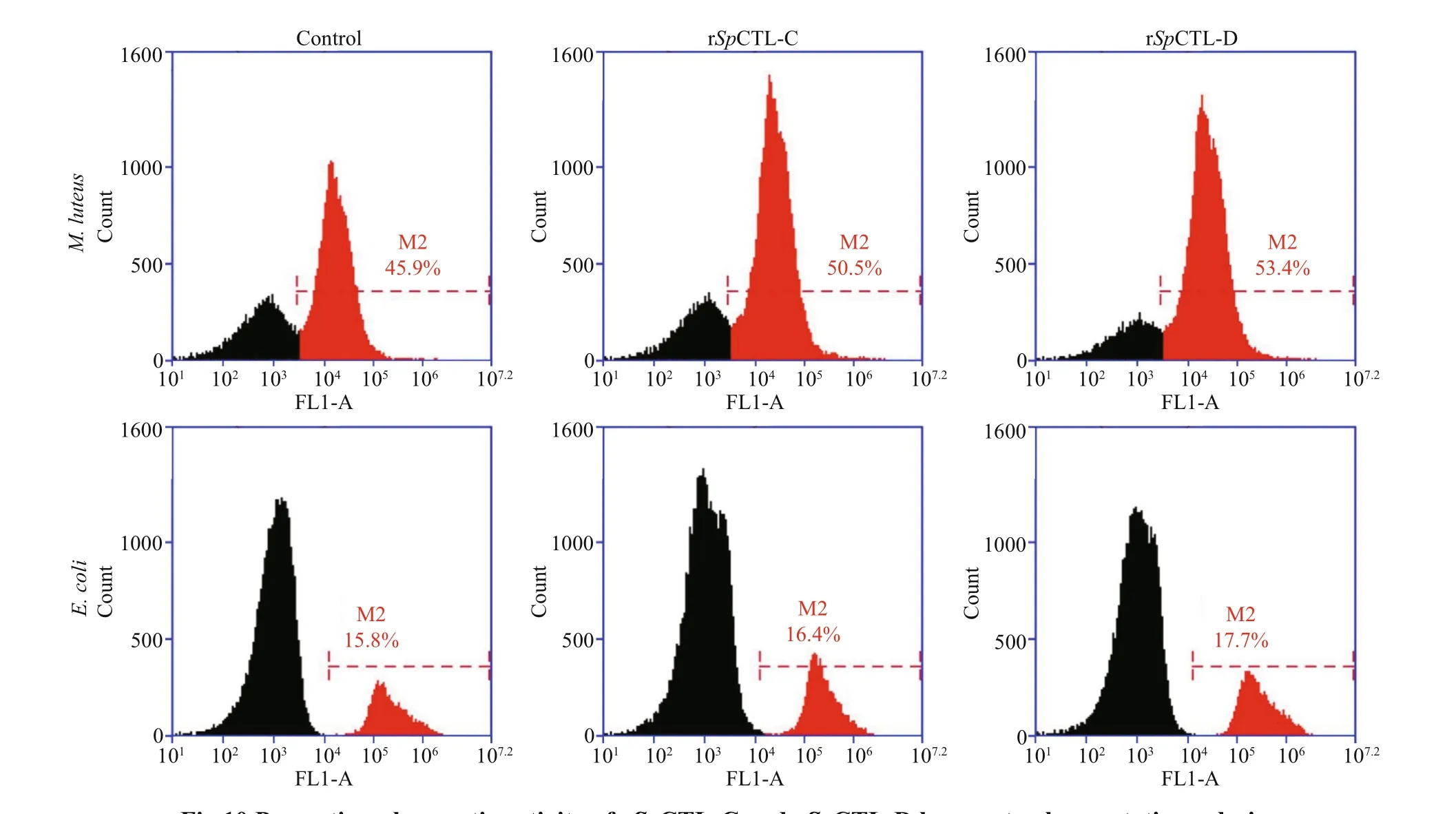
Fig.10 Promoting phagocytic activity of r Sp CTL-C and r Sp CTL-D hemocyte phagocytotic analysis
4 DISCUSSION
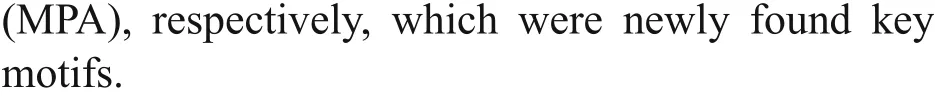
As the member of C-type lectin, both rSpCTL-C and rSpCTL-D have extensive PAMPs binding spectrum and bacterial binding spectrum, which can bind to PGN extracted from Gram-positive bacteria,LPS from Gram-negative bacteria, and β-glucan from yeast. At the same time, rSpCTL-D can bind to Grampositive bacteriaS.aureus,M.luteus; gram-negative bacteriaE.tarda,V.fluvialis,V.parahaemolyticus,A.sobria,A.hydrophila, andV.alginolyticus; fungusS.cerevisiae. However, rSpCTL-C can bind to all the tested microorganisms except two kinds of Grampositive bacteriaS.aureusandM.luteus. Some previous studies have shown that C-type lectin can bind PAMPs and have the ability to bind bacteria. For example, the C-type lectin, PtCTL-9 inP.trituberculatus, displayed a dose-dependent binding ability for above PAMPs (Kang et al., 2021).WhileSpCTL-B could bindS.aureus,β-hemolytic Streptococcus,E.coli,V.alginolyticus, andA.hydrophila, but it had a weaker affi nity for other tested microorganisms (V.parahemolyticusandS.cerevisiae) (Wei et al., 2018). Accordingly, diff erent C-type lectins can bind to diff erent bacteria, indicating that binding is selective, and diff erent lectins have diff erent functions.
It has been found that C-type lectins of marine organisms are mainly distributed in immune tissue organs, such as hepatopancreas, hemolymph and some tissues or organs directly contacted with the outside world. Previous studies have shown thatScCTL-1 was expressed in almost all tissues inSinonovaculaconstricta, with the highest expression level in the hepatopancreas (Lan et al., 2020). InMarsupenaeusjaponicus, CTLD was mainly expressed in the hepatopancreas (Wang et al., 2017). The results of Northern hybridization showed that C-type lectin(Fclectin) gene in Chinese shrimpFenneropenaeuschinensishad obvious hybridization bands only in the blood cells (Liu et al., 2007). In this study,SpCTL-C andSpCTL-D were mainly expressed in the hepatopancreas and muscle, and the expression level in hepatopancreas was higher than that in the muscle,which provedSpCTL-C andSpCTL-D might be connected with immune defense inS.paramamosain.
Studies have shown that some C-type lectins in invertebrates can inhibit the growth of microorganisms and even kill them. In this study, rSpCTL-D had a certain inhibitory eff ect onV.fluvialisandM.luteus,while rSpCTL-C had no inhibitory eff ect on the growth of the tested microorganisms.M.japonicusMjHeCL maintains the expression of antimicrobial peptides to inhibit the proliferation of microflora in hemolymph (Wang et al., 2014).ArgopectenirradiansAiCTL-7 can inhibit the growth ofE.coliat the concentration of 100 μg/mL. Therefore, diff erent kinds of microorganisms can be inhibited by diff erent C-type lectins, and further studies are required to understand the molecular mechanisms behind this process.
The most important feature of the C-type lectin family is that it can bind to a variety of cell surfaces by recognizing carbohydrate molecules on the cell surface and agglutinate cells with the participation of Ca2+(Weis et al., 1998). rSpCTL-C and rSpCTL-D have specific agglutination eff ects onS.aureus,S.cerevisiae, andP.pastoris. Both of them could not agglutinate the tested Gram-negative bacteria. The studies on C-type lectins of other crustaceans showed that they all had diff erent agglutination activities. In the presence of Ca2+,RimicarisexoculaterReCTL-1 can agglutinateS.aureusandS.cerevisiae, and rReCTL-2 can agglutinateM.luteus,Vibriovulnificus,andV.parahaemolyticus(Wang et al., 2020).S.paramamosainSpCTL-B has extensive microbial agglutination activity against Gram-positive / Gramnegative bacteria (Wei et al., 2018). The results show that C-type lectin, as a PRR, recognizes foreign microorganisms by carbohydrate domain and mediates cell-to-cell agglutination. CRD with diff erent key motifs has specific agglutination activity.
Phagocytosis is an evolutionarily conserved immune mechanism for the clearance of pathogens and apoptosis. The whole process is completed by a single blood cell and can be roughly divided into four stages: chemotaxis, adhesion, endocytosis, and killing digestion (Coteur et al., 2002; Soares-Da-Silva et al.,2002). Previous studies have shown that C-type lectin can not only recognize and bind PAMPs and pathogenic microorganisms as PRR, but also promote the phagocytosis or encapsulation of blood cells to foreign invaders, which is the main way to remove the foreign microorganisms and plays an important role in innate immunity (Ling and Yu, 2006; Wang et al.,2017). In the present study, both recombinant proteins rSpCTL-C and rSpCTL-D can promote the phagocytosis ofM.luteusby blood cells. At the same time, both rSpCTL-C and rSpCTL-D could promote the encapsulation activity of blood cells to agarose particles, and the encapsulation activity of rSpCTL-D was stronger. Similar results have been found in studies of other species; PtCTL-9 from swimming crabP.trituberculatuscan significantly improve the encapsulation activity of blood cells to large invasive substances (Kang et al., 2021).MjGCTL fromM.japonicuswas observed to recruit hemocytes to encapsulate agarose particles (Alenton et al., 2017).In summary,SpCTL-C andSpCTL-D not only participates in the recognition and binding of the immune process, but also acts as an opsonin to promote the phagocytosis and encapsulation of foreign substances, suggesting they can act as collectin and selectin.
5 CONCLUSION
In conclusion, this study shows that rSpCTL-C and rSpCTL-D can combine with PAMPs and a variety of pathogenic microorganisms, and remove some microorganisms by agglutination, and they can also promote blood cell phagocytosis and encapsulation to exert immune function. Therefore, rSpCTL-C and rSpCTL-D play an important role in the innate immunity ofS.paramamosain.
6 DATA AVAILABILITY STATEMENT
Data that support the findings of this study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2022年4期
Journal of Oceanology and Limnology2022年4期
- Journal of Oceanology and Limnology的其它文章
- Validation and error analysis of wave-modified ocean surface currents in the northwestern Pacific Ocean*
- The energy conversion rates from eddies and mean flow into internal lee waves in the global ocean*
- Observation of physical oceanography at the Y3 seamount(Yap Arc) in winter 2014*
- Decadal variation and trend of the upper layer salinity in the South China Sea from 1960 to 2010*
- Observations of turbulent mixing and vertical diff usive salt flux in the Changjiang Diluted Water*
- Experimental research on oil film thickness and its microwave scattering during emulsification*
