Reduced salinity interacts with ultraviolet radiation to alter photosystem II function in diatom Skeletonema costatum*
Shasha ZANG , Fang YAN , Daode YU , Jingjing SONG , Lei WANG ,Zhiguang XU ,**, Hongyan WU ,**
1 College of Life Science, Ludong University, Yantai 264025, China
2 Marine Science Research Institute of Shandong Province, Qingdao 266104, China
3 Key Laboratory of Marine Biotechnology in Universities of Shandong (Ludong University), Yantai 264025, China
Abstract To investigate the eff ect of reduced salinity on diatoms’ capacity to cope with changing ultraviolet radiation (UVR) and photosynthetically active radiation (PAR), Skeletonema costatum was grown in a range of salinity (15, 25, and 35). The photosystem II (PSII) function was analyzed by increasing PAR and UVR to mimic a mixing event in turbulent waters. The results show that high UVR exposure significantly reduced PSII activity, especially in cells grown at low salinity. UVR, but not salinity, stimulated the ‘removal’ rate of PSII protein PsbA. Salinity alone, in the range of 15 to 35, did not regulate PSII acceptor region; however,the low salinity+UVR treatment decreased the energy flux for electron transport per PSII reaction center in S. costatum. It showed that low salinity exacerbated the damaging eff ect of UVR on PSII function in S.costatum by suppressing PsbA protein synthesis and modifying the photochemistry of PSII. Although higher catalase (CAT) activity and NPQs were induced, they were unable to prevent the combined damage eff ect oflow salinity+UVR. Our findings indicate that reduced salinity and increased UVR potentially aff ect the abundance and distribution of S. costatum with the escalation of climate disturbances.
Keyword: diatom; ultraviolet radiation (UVR); photoinactivation; photosystem II
1 INTRODUCTION
Diatoms (Bacillariophyceae) are essential microalgae that make up a major portion of the phytoplankton population and, as such, account for~20% of the global primary productivity (Falkowski,2012; Young and Morel, 2015). They are most often found in turbulent coastal waters where rapid vertical mixing leads to vastly irregular light exposure(MacIntyre et al., 2000). Exposure to a sudden bright light can produce oxidative stress which destroys the photosynthetic apparatus (Nishiyama et al., 2006;Nishiyama and Murata, 2014), leading to net photoinactivation. To protect this system, diatoms oppose PSII photoinactivation via proteolysis of photodamaged proteins from the thylakoid membrane(Silva et al., 2003; Nixon et al., 2010) prior to reinsertion with newly formed protein (Nixon et al.,2010; Komenda et al., 2012; Barnett et al., 2015).Another important strategy of diatoms to cope with excitation of photosystems is to activate the nonphotochemical quenching (NPQ), which, in turn,triggers the trans-thylakoid proton gradient and activates the xanthophyll cycle (Lavaud and Lepetit,2013; Lavaud and Goss, 2014). In addition, excessive light exposure induced the formation of reactive oxygen species (ROS) viz. superoxide anion (O2ˉ) and peroxides. Superoxide dismutase (SOD) is the first enzyme of the enzymatic antioxidative pathway to convert superoxide radical into H2O2, which is subsequently neutralized to H2O catalase (CAT).These antioxidant enzymes not only control the steady state level of the reactive oxygen species but also allow them to perform important functions at specific sites under a variety of environmental conditions. The global mean atmospheric temperature is projected to rise up to 6 °C by the year 2100 (Intergovernmental Panel on Climate Change, 2013; Gattuso et al., 2015).A rise in temperature may increase the moistureholding capacity of the atmosphere (Trenberth and Jones, 2007), enhance freshwater run-offinto coastal zones (Rückamp et al., 2011; Pawlowicz, 2015), and intensify seasonal ice melt (Screen and Simmonds,2010; Bintanja et al., 2013; Thorpe and Andrews,2014). This would, subsequently, lead to reduced salinity, particularly in regions of mid/high latitudes(Intergovernmental Panel on Climate Change, 2013).Evidently, a steep decrease in salinity was reported in the Norwegian Sea coastline since the early 1970s,and worse yet, some regions exceeded 0.5 practical salinity units (Blindheim et al., 2000). A reduction in the average coastal salinity by approximately 5 was reported in the western coastal area of Greenland(Kamenos et al., 2012), and in China, large variations in salinity in the coastal waters of Jiangsu province due to freshwater inputs from high rain fall was reported (Kang et al., 2016; Gao et al., 2019). Salinity fluctuations could cause osmotic stress and change the cellular ionic composition ofSynechococcussp.(Allakhverdiev et al., 2000); therefore aff ecting a wide variety of metabolic pathways (Balzano et al.,2011; Kirrolia et al., 2011). For instance, growth rate,photosynthetic activity, and carbon fixation rate in the diatomThalassiosiraweissflogiiwas shown to be reduced with a decrease in salinity from 35 to 15(Radchenko and Il’yash, 2006). The opposite phenomenon was observed inEmilianiahuxleyi, with growth rate, cell size, and photosynthetic performance increasing with the reduction in salinity from 35 to 25(Xu et al., 2020). In addition, there are species that show a strong capacity to adapt to a large fluctuation in salinity levels: for example, the optimal growth ofTetraselmissuecicaremained constant in salinity from 20 to 60, however, both growth and photosynthetic activity were inhibited at a salinity of 10 (Pugkaew et al., 2019).
Due to climate change and stratospheric ozone depletion, the level of solar ultraviolet radiation(UVR) exposure has increased dramatically (Bais et al., 2019). Eff orts have been made to assess the harmful consequence of UVR on the phytoplankton population (Häder et al., 2015; Williamson et al.,2019). UVR was shown to damage DNA and protein molecules, suppress utilization of carbon, nitrogen,and other nutrients, stimulate ROS generation, and cause cell death (Häder et al., 2007, 2015). The growth and photosynthesis of phytoplankton are inhibited by UVR, and thus, the primary productivity is reduced (Harrison and Smith, 2009; Häder and Gao, 2017; Neale and Thomas, 2017). Alongside studies on the sole eff ects of salinity or UVR on marine microalgae, their synergistic eff ects have also been reported. Rijstenbil (2005) demonstrated that exposure to UVR alone elevated ROS generation and a combined exposure of UVR and high salinity (60)further increased ROS production by ~50% inCylindrothecaClosterium. Similarly, Wu et al. (2017)reported that UVR and high salinity cooperatively decreased the photochemical activities of PSII in the benthic diatomNitzschiasp. However, the eff ects oflow salinity and UVR on the physiological activities of diatoms remain unclear and further studies need to be performed to identify the pathway(s) involved in the synergistic eff ect of UVR and low salinity on PSII function.
The diatomSkeletonemacostatumis one of the most abundant harmful algal bloom causing organisms, found in coastal waters globally (Kooistra et al., 2008; Balzano et al., 2011; Boller et al., 2015).S.costatumhas a wide salinity tolerance: it can sustain its growth well in salinities between 10 and 35(Balzano et al., 2011), with maximum growth rate reported at salinities between 18 and 35, depending on growth conditions like temperature or irradiances(Yan et al., 2002; Rai and Rajashekhar, 2014).BecauseS.costatumare prevalent in turbulent waters,they experience frequent fluctuations in UVR and photosynthetically active radiation (PAR) levels. In this study,S.costatumwas cultured under three salinity conditions (15, 25, and 35) and alterations in PsbA, photoinactivation, NPQs, CAT, and SOD activity, in response to PAR and PAR+UVR exposure,were examined. Our purpose was to examine how coastal salinity fluctuations regulate the ability of diatoms to adapt to fluctuating light.
2 MATERIAL AND METHOD
2.1 Strains and growth circumstances
Skeletonemacostatum(Greville) Cleve (strain 2042) was obtained from the Institute of Oceanography,Chinese Academy of Sciences. For culture medium,natural seawater with a salinity of 35 obtained from the coastal water was filter-sterilized through a 0.22-μm polycarbonate membrane filter (Corning,NY, USA) and enriched with f/2 medium solutions(Guillard and Ryther, 1962; Vartanian et al., 2009).The medium with a salinity of 35 was considered as a control for comparison of salinity-induced eff ects.The low and medium salinity seawater (15 and 25)was obtained by diluting the filtered seawater with distilled water and was amended with f/2 nutrients to the same levels as the control. Salinity was checked using a salinity meter (LS10T, Guangzhou, China).
Skeletonemacostatumcultures were grown under 100- μ mol/(m2·s) PAR using white cool tubes of fluorescent with a 12-h∶12-h light∶dark cycle at 18 °C.TheS.costatumunderwent ~7 transfers of semicontinuous dilution to ensure uniform growth. The specific growth rates ofS.costatumgrown at 15, 25,and 35 were 0.25±0.02, 0.48±0.03, and 0.53±0.02 per day, respectively. Cells with steady growth rates were harvested for the following upward light exposure experiment. Each treatment was repeated four times.
2.2 Upward light shift and recovery assessment
Skeletonemacostatumculture replicates with diff erent salinity level were split into two UVtransparent quartz tubes, with 50-μg/mL lincomycin added to one quartz tube to block chloroplast protein synthesis (Bachmann et al., 2004; Gao et al., 2018),thereby inhibiting PSII repair (Tyystjärvi and Aro,1996). Next, the tubes with and without lincomycin were placed in darkness for 10 min to allow the lincomycin to exert its eff ect and then exposed to simulated solar radiation at UVR 140 W/m2and PAR 26 W/m2, which were set based on the averaged daytime solar irradiances for UVR and PAR measured locally in May whenS.costatumred tide bursts out with high frequency. Two radiation treatments were applied: 1) cultures receiving PAR+UVR (295-700 nm): quartz tubes covered with Ultraphan film 295 (UV Opak, Digefra, Munich, Germany); 2)cultures receiving only PAR (400-700 nm): quartz tubes covered with Ultraphan film 395 (UV Opak,Digefra, Munich, Germany). TheS.costatumcultures were maintained in a water bath at 18±1 °C.
The cultures received the solar radiation treatment for 120 min. Samples were collected at times 0 (before the onset of treatment), 15, 30, 60, 90, and 120 min after the onset of treatment and to measure chlorophyll fluorescence and protein analyses. After high light exposure, theS.costatumcultures were placed back under growth light (100- μ mol/(m2·s) PAR) for half hour to allow to recuperate. Samples were collected for the final analyses.
A solar simulator (Sol 1200, Dr. Hönle GmbH,Germany) equipped with a 1 000-W xenon arc lamp was used for the experiment. The output irradiance of UVR and PAR were measured with a broadband filter radiometer (ELDONET; Real Time Computer,Germany).
2.3 Chlorophyll fluorescence measurement and parameterization
To determine chlorophyll fluorescence parameters, a portable pulse amplitude modulated fluorometer(WATER-PAM, Walz, Germany) was used. The variable to maximal chlorophyll-afluorescence ratio (Fv/Fm) of dark-adapted samples was used to represent the photochemical effi ciency of PSII. TheFv/Fmratio was calculated utilizing the minimal (F0) and maximal (Fm)fluorescence yield of 5-min dark-adapted cells and calculated as:Fv=(Fm-F0). For measuringF0andFm, the measuring and pulse of saturating light were maintained at <0.1 μmol/(m2·s) and 4 000 μmol/(m2·s), respectively.
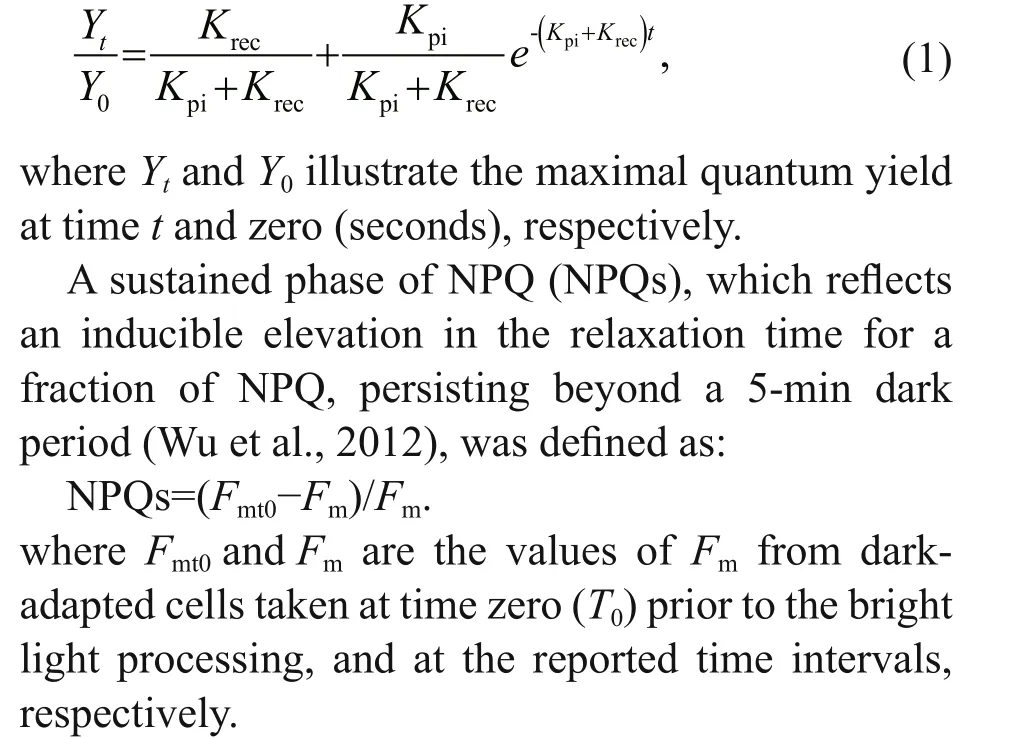
The rate constant for photoinactivation (Kpi, /s),and the rate constant for PSII repair (Krec, /s) were evaluated.Kpiwas obtained from lincomycin-treated specimens via plotting a single-phase exponential decay through a plot ofFv/Fmversus the cumulative photons implemented during the bright light processing.Krecwas obtained from non-lincomycintreated samples. Both measurements were performed conforming to the Kok equation (Kok, 1956;Campbell and Tyystjärvi, 2012):
2.4 Polyphasic rise of Chl-a fluorescence transients and the JIP-test
After 120-min high light exposure, 5-mL samples were collected from each independent culture (n=4).The polyphasic nature of fluorescence transients due to Chlawas analyzed using a plant effi ciency analyzer (Hansatech Instruments Ltd., UK). To begin, the samples were placed in absolute darkness for 5 min. The fluorescence signals were recorded within a time scan from 10 μs to 2 s, and labeled as steps O, J, I, and P. The JIP-test identified the energy flux in the membranes of the photosynthetic apparatus (Strasser et al., 2000). According to the energy flux model in JIP-test, photons are absorbed by the antennae pigments (ABS, absorption flux)which are excited. A part of this excitation energy,the trapping flux (TR), is transferred to the reaction center (RC) while the remaining part is dissipated either as fluorescence or as heat. In the RCs, the TR is converted to redox energy by reducing QAto QA- ,which is then reoxidized to QA, thus leading to an electron transport flux (ET) and consequently to photochemistry. The relative variable fluorescence at phases J (VJ), and the initial slope at the beginning of the variable fluorescence transients (theoretically at time zero) (M0), the possibility of the electron transport beyond QA(ψ0), and the energy fluxes for ABS, dissipation (DI), TR and ET per PSII reaction center can be shown in the following equations based on Strasser et al. (2000):
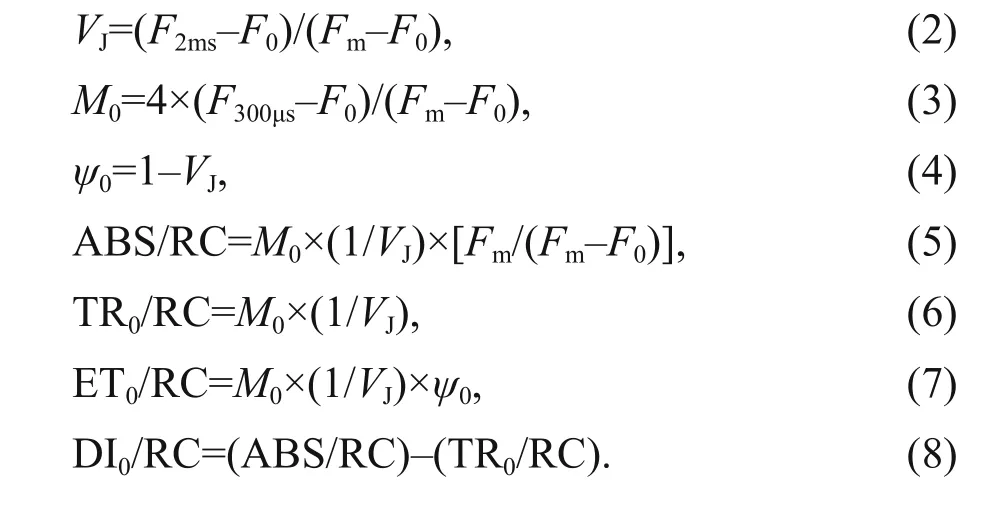
2.5 Quantitative immunoblotting
Culture samples (20 mL) were vacuum-filtered onto glass fiber filters (25-mm diameter, Whatman),and instantly flash frozen in liquid nitrogen before storing at -80 °C until further analysis. The whole available protein was measured utilizing a Lowry protein assay kit (Bio-Rad DC assay) followed by western blots for PSII PsbA subunit. Standard curve generation: total protein (2 μg per lane) was run alongside a range of known controls (15.625-, 31.25-,62.5-, and 125-fmol PsbA) per lane (AS01 016S;Agrisera, www.agrisera.se). The PsbA protein levels were quantified against the standard curves of protein(Brown et al., 2008; Wu et al., 2011).
The PsbA protein clearance rate constant (KPsbA,/s) was determined through plotting fmol PsbA per μg total protein for cells exposed to bright light and lincomycin exposure. The PsbA plot was then compared against a single-phase exponential decay under bright light exposure graph (Wu et al., 2012)to reveal the PsbA protein clearance rate from the PSII pool.
2.6 Measurement of CAT and SOD activity
After 120-min high light exposure, 20-mL samples were collected onto a membrane of polycarbonate(0.22 μm, Whatman) and washed with pH 7.6 phosphate buff er. The enzyme was extracted in 0.6-mL phosphate buff er (pH 7.6) containing 1-mmol/L ethylenediaminetetraacetic acid (EDTA), 50-mmol/L KH2PO4, and 1% (w/v) polyvinylpolypyrrolidone.Centrifuge the homogenized extract at 12 000×gat 4 °C for 10 min. CAT and SOD assessment kits(Nanjing Jiancheng Biological Engineering Company,China) were used to detect CAT activity and SOD, in accordance to manufacturer’s protocols. One SOD activity unit equals to the enzyme quantity that promotes a 50% reduction in the nitro-blue tetrazolium depletion rate at 560 nm (Wang and Wang, 2010).CAT activity, on the other hand, was calculated by analyzing the initial disappearance rate of H2O2at 240 nm (Li et al., 2015).
2.7 Statistical analysis
The outcomes are shown as means of replicates±standard error. The statistics data were derived utilizing SPSS v.22. The outcomes conformed to a normal distribution (Shapiro-Wilk,P>0.05) and the variances were considered equal (Levene’s test,P>0.05). Two-way multivariate ANOVAs(MANOVAs) was employed to study the consequences of salinity, UVR and lincomycin onFv/Fm, NPQs,PsbA content, CAT activity, and SOD activity inS.costatumat various durations oflight exposure.Post hoc measurements were determined using a Tukey HSD. ANOVAs (RM-ANOVAs) were repeatedly measured to determine the influence oflight exposure duration on PsbA content,Fv/Fm, and NPQs. The corresponding post hoc measurements were determined using a Tukey HSD. One-way ANOVAs analyzed the mean diff erences in parameters obtained from the JIP test, photoinactivation rate,PsbA removal rate, and PSII repair rate constant.P<0.05 is defined statistically significant.
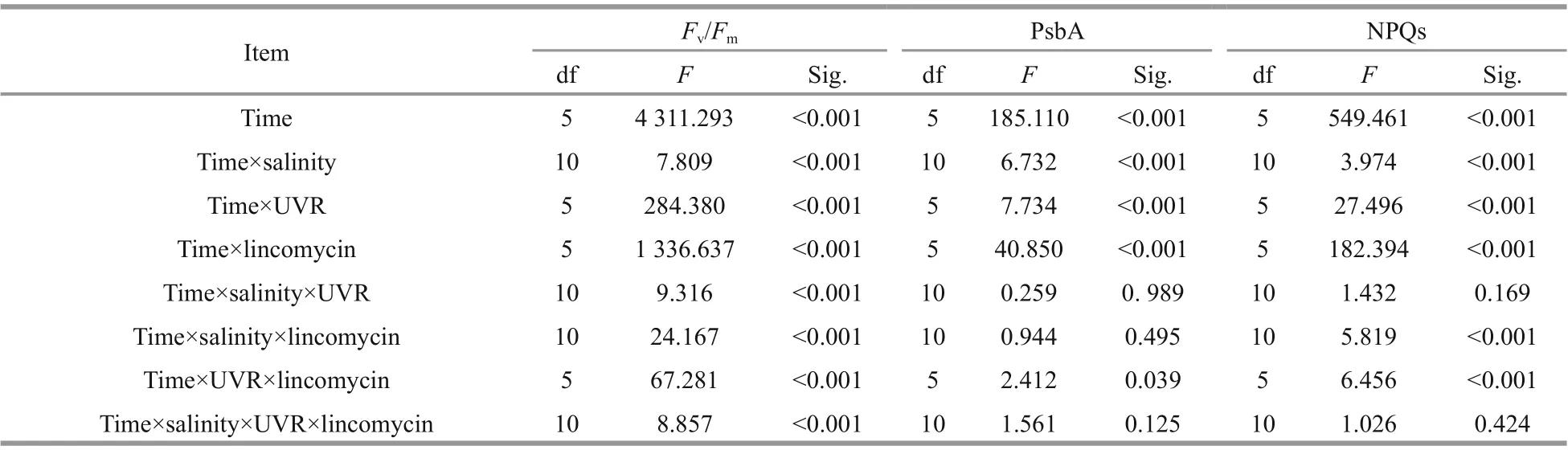
Table 1 Repeated analysis of variance examining the consequence oflight duration on F v/ F m, PsbA, and NPQs in multiple environments
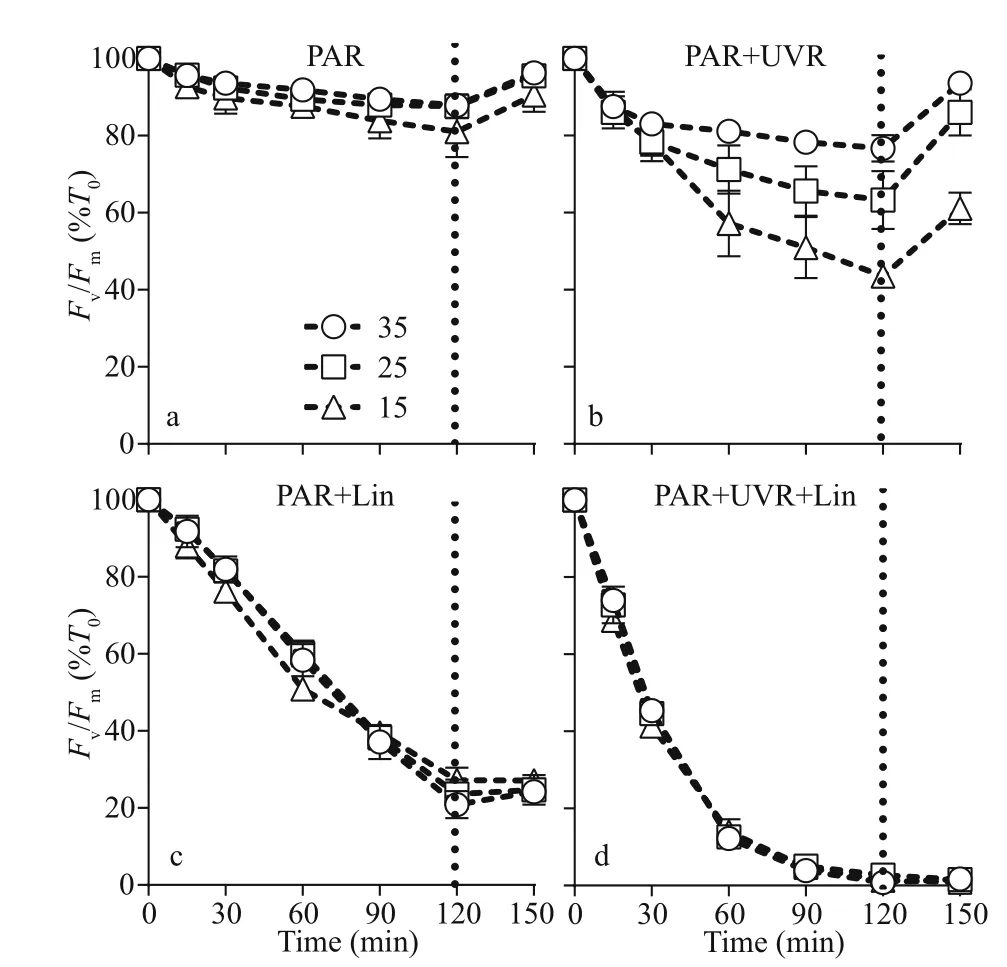
Fig.1 PSII maximum photochemical yield ( F v/ F m) versus high light duration in Skeletonema costatum cultures without (a, b) or with (c, d) lincomycin
3 RESULT
The maximal photochemical effi ciency (Fv/Fm)diminished with increasing bright light exposure inS.costatumcells cultured in all salinity (Fig.1 and Table 1). Cells receiving PAR+UVR showed a significant reduction inFv/Fm(F=439.953,P<0.001), as opposed to those receiving PAR: post bright light exposure,PAR+UVR inhibitedFv/Fmby 56.5% at 15, 36.7% at 25, and 23.4% at 35 (Fig.1b). The addition oflincomycin prompted a significant decline inFv/Fm(F=1 336.637,P<0.001) in bright light exposed cells,regardless of salinity, especially under UVR exposure(Fig.1d). In the absence oflincomycin, however,Fv/Fmincreased to 90.6%-96.3% of the original value when cells were shifted to growth light for 30 min (Fig.1a-b).As expected, cells exposed to lincomycin displayed zero recovery when placed under growth light(Fig.1c-d).
Salinity strongly regulated PsbA synthesis in theS.costatumcells: PsbA content decreased in low salinity. Before the high light treatment, the initial PsbA content was 37.81±7.23, 51.02±7.16, and 68.01±5.35 fmol μg/protein at 15, 25, and 35 salinity respectively (F=20.864,P=0.000). Under bright light,however, the level of PsbA in cells with undergoing PSII repair progressively decreased (Fig.2a-b;Table 1). Therefore, light duration, combined with UVR heavily influenced PsbA levels inS.costatumcells (F=7.734,P<0.001; Table 1): a 120-min bright light duration, in combination with UVR, decreased PsbA levels by 43.7% at 15, 36.9% at 25, and 29.1%at 35 (F=13.941,P<0.001;F=16.731,P<0.001;Fig.2b; Table 2). A further decrease in PsbA was observed in cells incubated with lincomycin during bright light exposure, irrespective of salinity treatment(F=40.850,P<0.001; Fig.2c-d). Post 30-min recovery under growth light, the PsbA levels in the PARexposed cells slight increased to 83.2%-87.4% of the original value (Fig.2a). However, no recovery was observed in cells treated with lincomycin (Fig.2c-d).
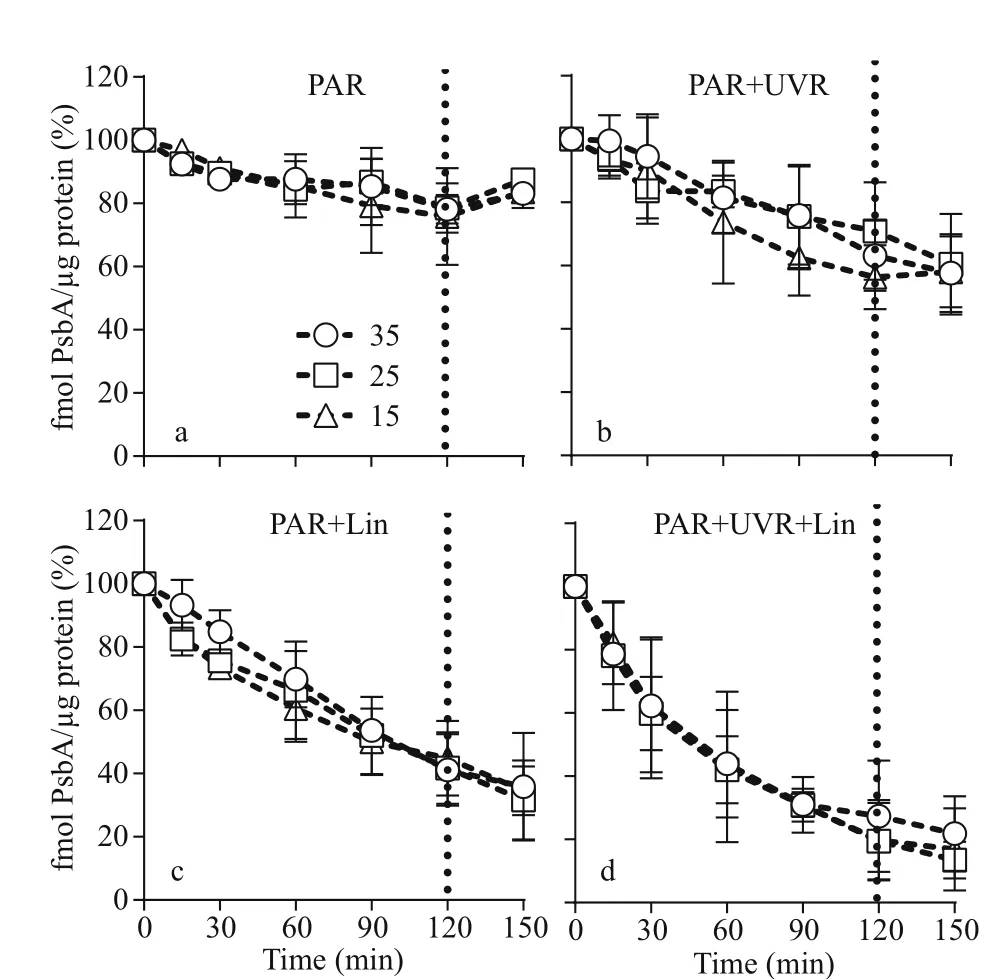
Fig.2 PsbA protein content changes in S. costatum cultures treated without (a and b) or with (c and d) lincomycin
To explore the role of salinity and UVR in regulating electron transport on the acceptor region of the PSII protein, alterations in the possibility of electron transfer beyond QA(ψ0) were measured(Fig.3a). Cells cultured in the three salinities conditions produced no significant alterations inψ0;however,ψ0in cells decreased significantly in response to bright light; the diff erence between PAR and PAR+UVR exposed cells at 15 salinity was highly significant (P=0.000; Fig.3a).VJincreased in both PAR and PAR+UVR exposed cells, regardless of salinity changes, especially in cells cultured at 15 exposed to UVR (Fig.3b). The effi ciency of conversion within the energy of excitation was determined by measuring the ABS (of antenna chlorophylls) per RC(ABS/RC), the dissipated energy flux per RC (DI0/RC), the TR (resulting in QAreduction) per RC (TR0/RC), and the ET (further than QA-) per RC (ET0/RC).The ABS/RC (P=0.000) and DI0/RC (P=0.000) only increased significantly under UV treatment at 15 salinity (Fig.3c-d). An increase in TR0/RC was observed in cells receiving high PAR and UVR exposure, particularly in low to medium salinity cultured cells under UVR exposure (Fig.3e).Compared to the original value, only ET0/RC (P=0.01)significantly decreased in UVR-exposed cells grown in 15 salinity; however, no marked changes occurred between PAR and PAR+UVR exposed cells cultured in all salinity levels (Fig.3f).
The light duration heavily influenced NPQs, and worked in cohort with salinity, UVR, and lincomycin(P<0.001; Fig.4; Table 1): NPQs increased following treatment with bright light, UVR, and lincomycin.
This was associated with significant decreases inFv/Fm(Fig.1) and a loss of the PsbA protein (Fig.2).Low salinity (15), in combination with UVR and lincomycin, decreased NPQs (Fig.4d), and salinity worked in concert with lincomycin to regulate the level of NPQs (P<0.001; Table 3). In the 30-min recovery phase under growth light, NPQs reduced to almost zero in cells cultured at 35 (Fig.4a-b), but maintained high values in cells with lincomycin (Fig.4c-d).
To identify potential interrelations amongst thermal dissipation, photoinactivation, and clearance of PsbA,the correlation between NPQs induction andKpi/KPsbAwas analyzed in all aforementioned growth conditions(Fig.5). Unlike the PAR treatment, diatoms under the PAR+UVR treatment, especially at 25 and 35 salinity,produced elevated NPQs with greaterKpitoKPsbAratio(Fig.5a). Figure 5b shows the plot ofKPsbAversusKpi,for lincomycin-exposed cells wherein photoinhibited PSII repair was eff ectively prevented. Although UVR exposure greatly improvedKPsbA, it still lagged behindKpi. All salinity conditions displayed no change inKPsbA(P=0.998) orKpi(P=0.445). The plot ofKrecversusKPsbA(Fig.5c) demonstrated that the functional recovery of PSII far surpassedKPsbA.Krecwas highest at 35 and decreased significantly with decreasing salinity (P=0.000). UVR treatment significantly reducedKrec, the PAR+UVR induced degradation of PsbA did not measure up to the apparentKrec(Fig.5b-c).
Furthermore, upon transfer to bright light, the CAT activity inS.costatumdid not change significantly at 25 and 35 salinity, while the presence of UVR significantly increased CAT activity at 15 (P=0.000;Fig.6a). There existed no noticeable influence of salinity concentration or UV treatment on SOD activity (Fig.6b; Table 4).
4 DISCUSSION
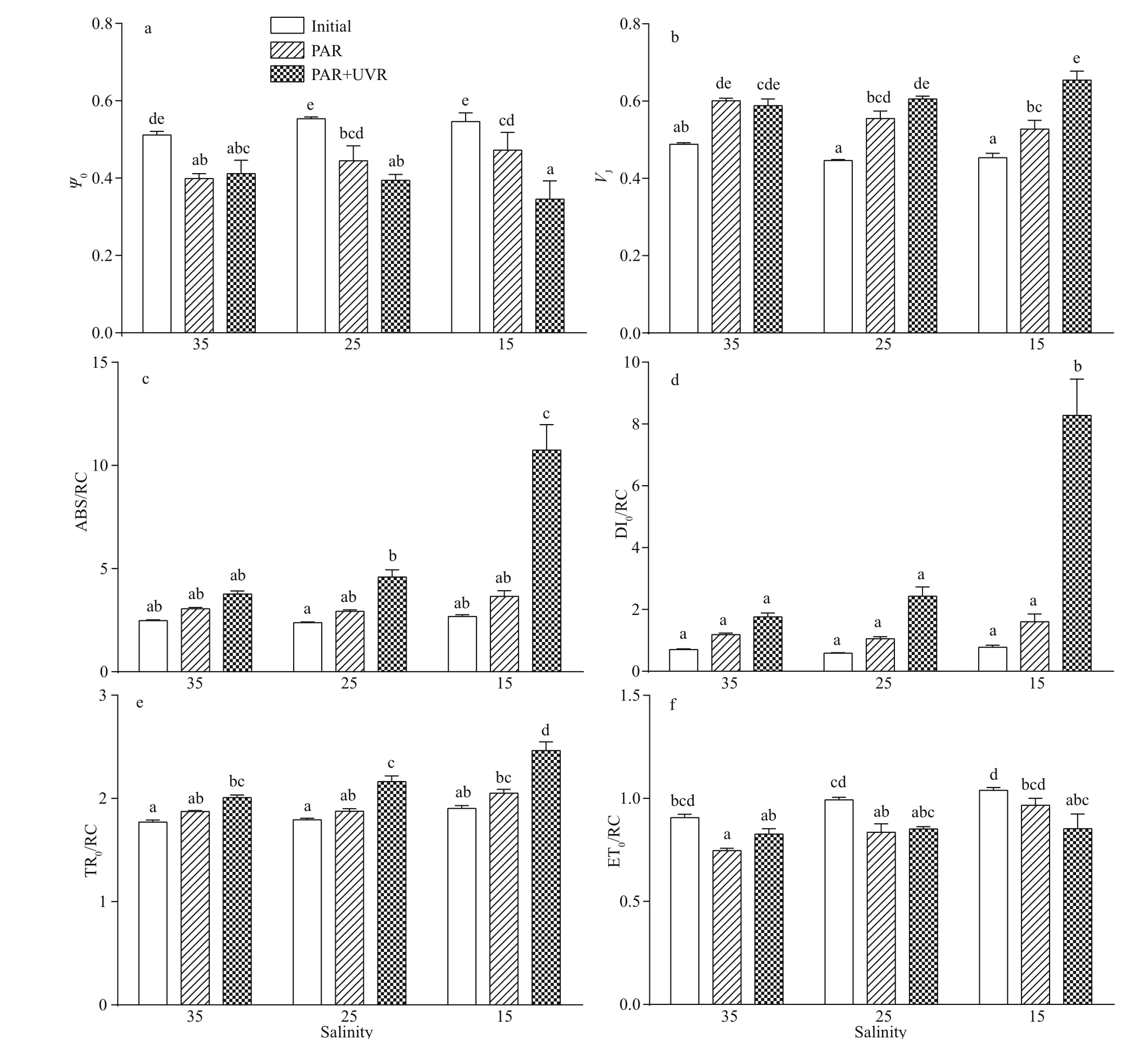
Fig.3 Salinity impact on each parameter in cells of S. costatum grown at 15, 25, and 35 salinity before (initial) and after 120-min exposure to PAR or PAR+UVR
WhenS.costatumcells grown at diff erent salinities went from growth light to high light, the maximum PSII quantum yield (Fv/Fm) diminished with exposure time, suggesting a highly suppressive consequence of high PAR and PAR+UVR treatment on photosynthesis,particularly for UVR exposure. It has been extensively demonstrated that photosynthetic organisms are highly susceptible to UVR damage, even with very low dose of UVR (Gao et al., 2009; Li et al., 2015;Williamson et al., 2019). In recent years, the combination eff ects of UVR and other environmental factors have been received much more consideration(Häder et al., 2015). Higher temperatures was reported to mitigate the inhibitory eff ects of UVR (Halac et al.,2010; Helbling et al., 2011), while increased CO2and high salinity could exacerbate the stress of UVR on photosynthetic performance in diatoms (Wu et al.,2017; Gao et al., 2018). In this study, we showed that PSII activity inS.costatumwas significantly reduced during PAR+UVR exposure and accelerated in cells cultured in low salinity. This may be due to UVRinduced degradation of PsbA and the suppression of new PsbA protein synthesis caused by low salinity.Turnover of the PsbA is essential for PSII repair and activity restoration after photoinactivation (Edelman and Mattoo, 2008; Komenda et al., 2012). When lincomycin—an inhibitor for chloroplast protein synthesis—was added, a significant decrease was observed in bothFv/Fmand in the level of PsbA,indicating the importance of PsbA generation in repair and preservation of active PSII. In the present study,salinity, had no eff ect on PsbA removal rate (KPsbA),whereas UVR accelerated it. The UVR-induced degradation of PsbA was also demonstrated in the diatomThalassiosiraweissflogii(Gao et al., 2018).For the eff ective repair of PsbA following photodamage, the damaged PsbA protein from the thylakoid membrane must be replaced with a newly formed intact protein (Komenda et al., 2012; Eaton-Rye and Sobotka, 2017). In our study, the PSII reaction center complex assembly remained faulty in low salinity conditions as it lacked the newly synthesized PsbA protein for reinsertion. Therefore,although UVR increased PsbA removal, the PSII photochemical activity was likely not restored in low salinity, as shown in Fig.1. UVR-stimulatedKPsbAfailed to counter the photoinactivation of PSII (Kpi),thereby, producing a large quantity ofinactivated PSII in UVR-exposed cells.
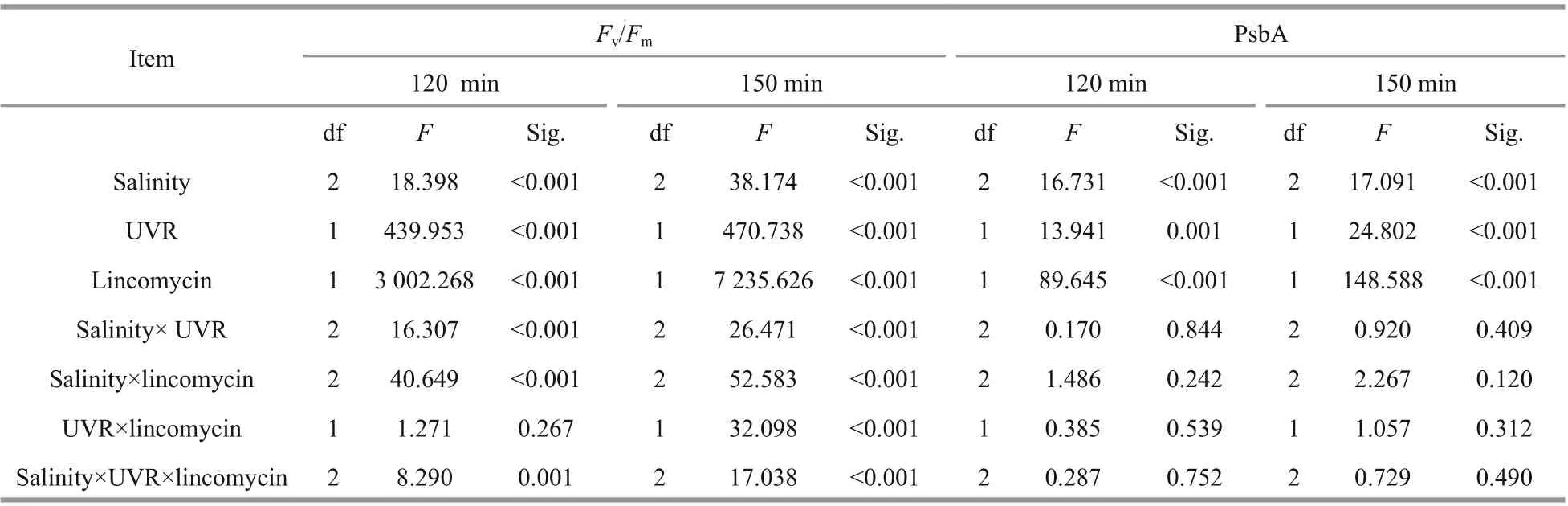
Table 2 Multivariate analysis of variance measuring the consequences of salinity, UVR, and lincomycin on F v/ F m and PsbA levels at varying time intervals
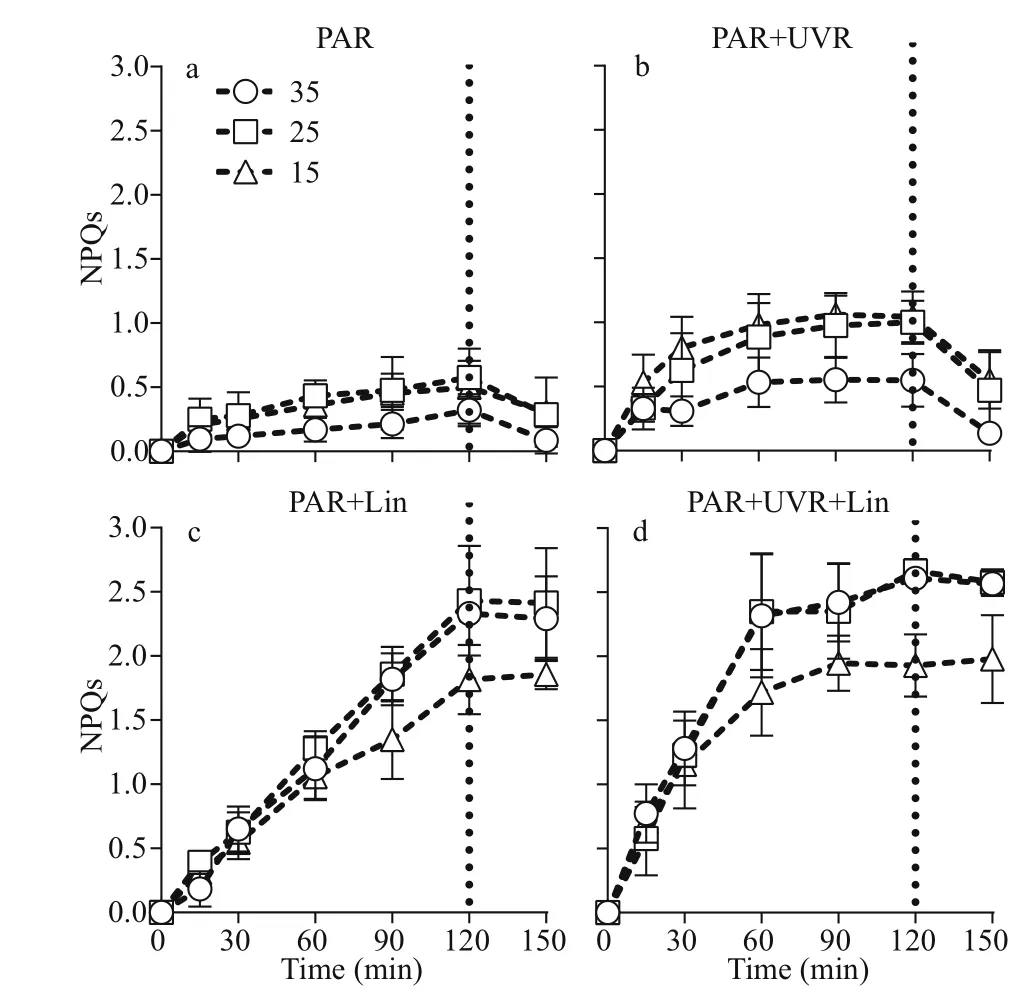
Fig.4 Sustained NPQ (NPQs) responses versus exposure time of high light in S. costatum cultures treated without (a, b) or with (c, d) lincomycin
Multiple studies have demonstrated that UVR promotes the functional loss of QAand QBquinone electron acceptors and causes damage to the donor regions (Vass et al., 1999; Jiang and Qiu, 2005). In this study, the possibility of the electron transfers beyond QA(Ψ0) reduced significantly upon exposure to high PAR and PAR+UVR, particularly in cells cultured at 15. This indicates that high PAR, in the presence of UVR, damages the acceptor region of the PSII protein inS.costatum. Low salinity exacerbates the damaging eff ect of UVR. However, salinity alone,in the range of 15 to 35, did not regulate PSII acceptor region. Similarly, a significant increase ofVJin cells cultured in 15 under UVR exposure indicated elevated closed PSII reaction centers levels, and consequently,in the level of reduced QAat phase J. TR0/RC is an estimation of electron flow only from the PSII donor region to QA, and the expression of TR0/RC refers only to active PSII reaction centers. Our results indicate that UVR inactivated some PSII reaction centers, and low salinity decreased the PsbA levels.As a result, the dysregulation of the small pool of PSII reaction centers promoted an increase in TR0/RC,ABS/RC, and DI0/RC. The ET0/RC only decreased significantly under UVR exposure in low salinity,with changes inΨ0(Fig.3). Hence, theS.costatumcells were unable to maintain PSII activity owing to the synergistic eff ect oflower salinity and UVR.
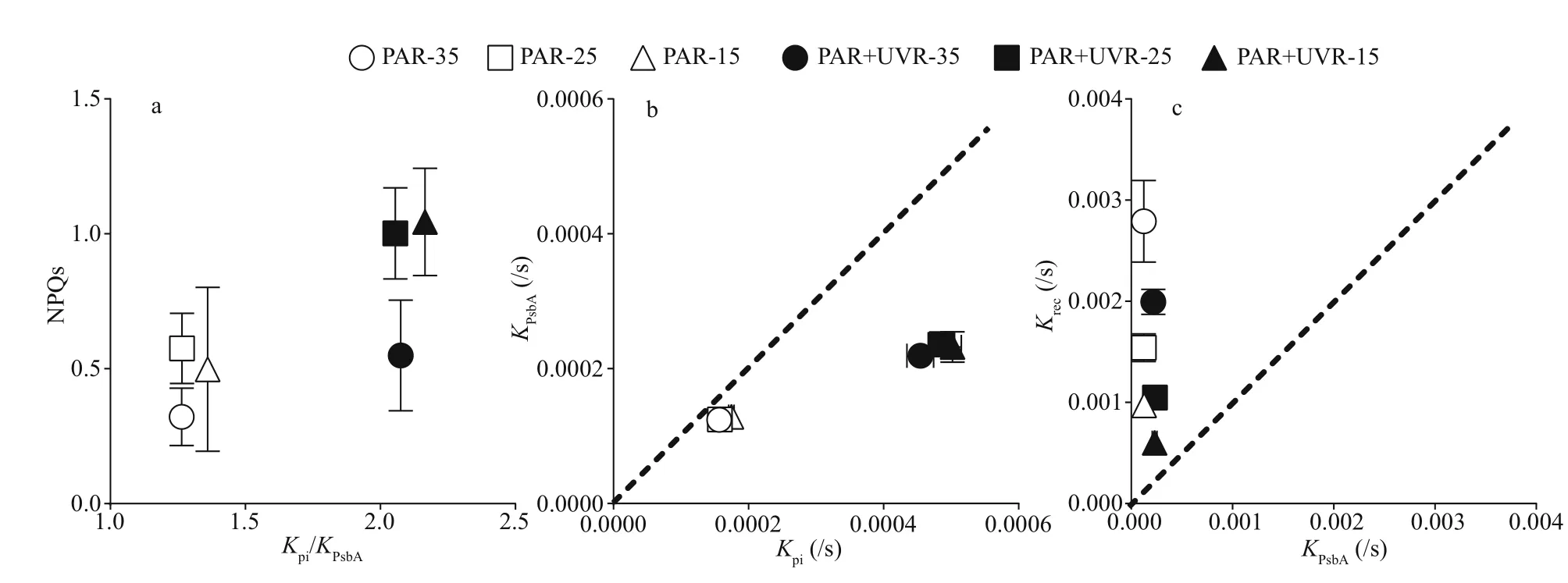
Fig.5 The interrelationship among NPQs, photoinactivation, and PsbA loss in S. costatum cultures exposed to PAR or PAR+UVR at varying salinity
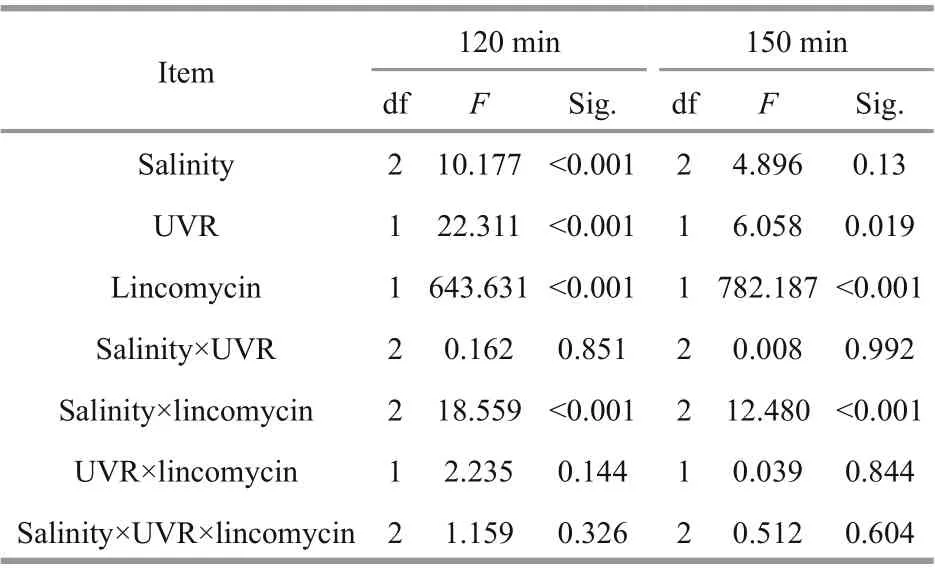
Table 3 Multivariate analysis of variance measuring the consequences of salinity, UVR, and lincomycin on NPQs at varying time intervals
Diatoms activate NPQs once photoinactivation overcomes removal of PsbA protein (Wu et al., 2012).NPQ is an essential photo-rescue pathway that releases excess excitation energy as heat (Goss and Jakob, 2010). This study demonstrated that UVR exposure increased NPQs activity inS.costatum;which is consistent with the UVR and NPQ studies done onT.weissflogii(Gao et al., 2018) andGephyrocapsaoceanica(Jin et al., 2013). In diatoms,NPQ is regulated via the trans-thylakoid proton gradient and the xanthophyll cycle (Lavaud et al.,2004). UVR can stimulate the de-epoxidation of xanthophylls and NPQ (Goss et al., 1999; Sobrino et al., 2005). Our study demonstrated a two-phased response ofS.costatumto a light challenge, PSIIrepair is initially accelerated followed by the activation of NPQs. Cells grown in salinity of 15 exhibited more dependence on NPQs to counter the failure of PSII repair, as opposed to those grown in salinity of 35 (Fig.4). A similar response was discovered in another marine diatom,Thalassiosirapseudonana, when exposed to sub- or super-optimal growth temperatures under bright light exposure(Yuan et al., 2018). The NPQs were highest in the presence oflincomycin, suggesting a strong role of NPQs in the maintenance of PSII activity or in suppressing photoinactivation when PSII repair is blocked. It is worth noting that NPQ is mostly related to xanthophyll cycle pigments, which is unlikely to be aff ected by lincomycin theoretically. Meanwhile, the apparently high NPQs values in the presence oflincomycin may be due to the mixup of sustained NPQ with photoinhibitory damage that was elevated by lincomycin. However, the cells grown in salinity of 15, in the presence oflincomycin and irreparable PSII damage, displayed relatively smaller increase in NPQs, as compared to cells grown in salinity of 35.This might be due to the low amount of xanthophylls caused by reduced salinity as those have been found in diatom speciesPseudo-nitzschiaaustralis(Ayache et al., 2020) andT.weissflogii(Bussard et al., 2017).Xanthophylls production is also shown as an antioxidative mechanism deployed to prevent ROS formation (Pelah et al., 2004; Markina and Aizdaicher,2016). Therefore, although additional studies are required to demonstrate the underlying mechanisms of NPQs induction, we speculated that the xanthophyll reduction in conditions oflow salinity might be associated with the dissipation of excess energy, and might contribute to the enhancement of UVR-induced oxidative stress and regulation of the antioxidant defence system (Shiu and Lee, 2005), as evidenced by the significant increase in CAT activity in cells cultured in 15 salinity when exposed to UVR.
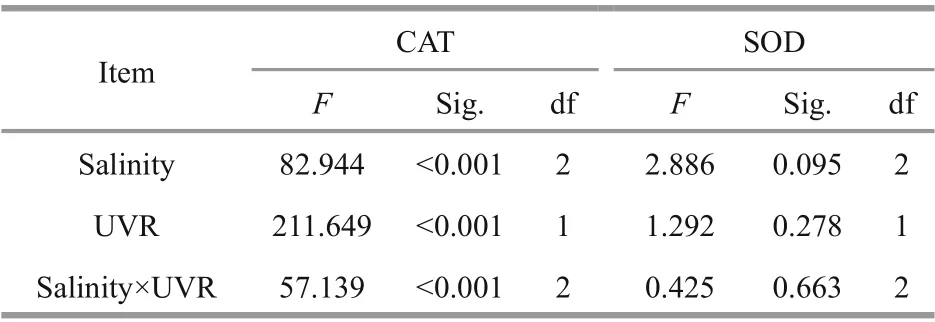
Table 4 Multivariate analysis of variance measuring the consequences of salinity and UVR on CAT and SOD activity after 120-min exposure
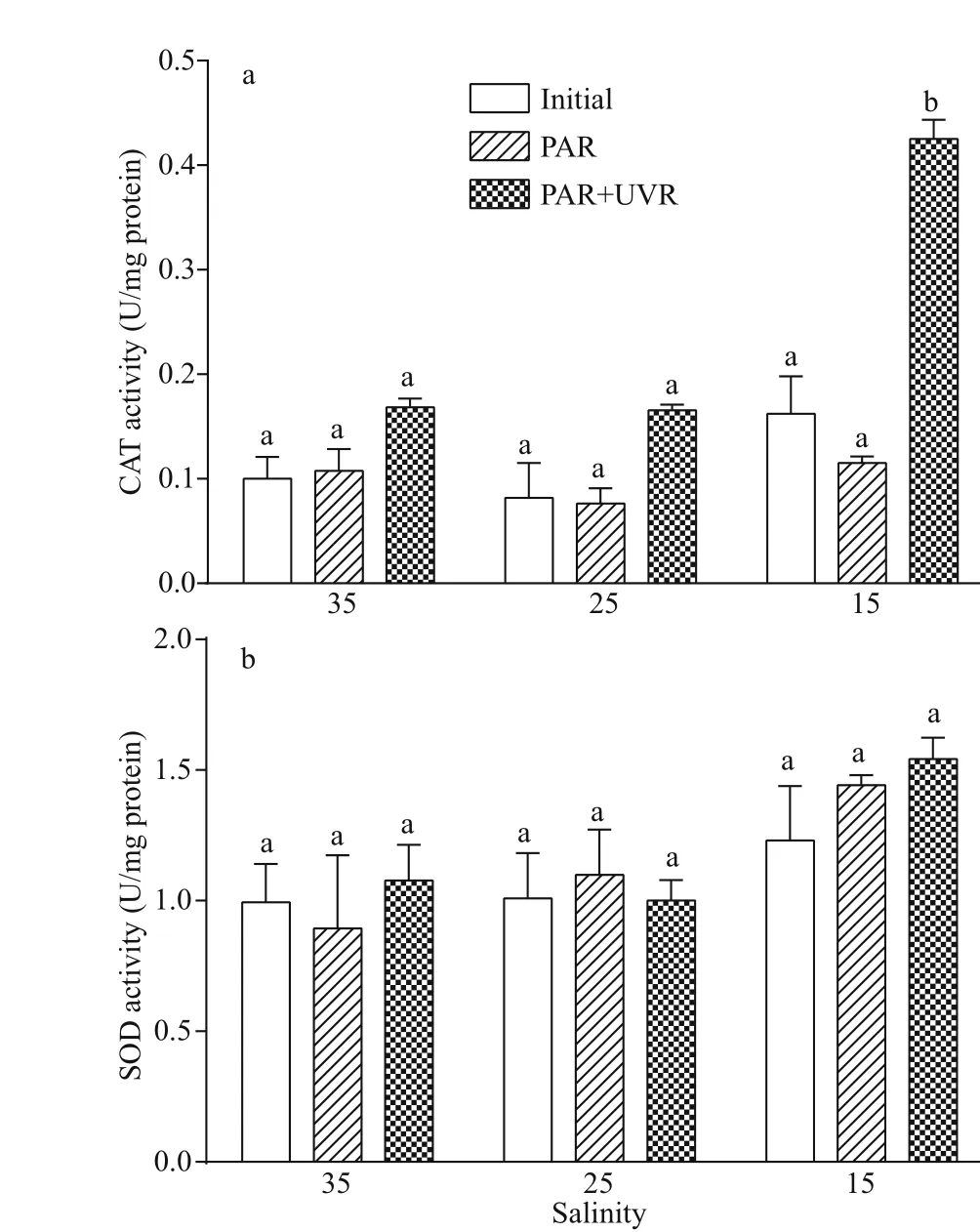
Fig.6 Alterations in CAT (a) and SOD (b) activity in cells of S. costatum grown at 15, 25, and 35 salinity before(Initial) and after 120-min exposure to PAR or PAR+UVR
5 CONCLUSION
This study was the first to demonstrate the cooperative interaction of UVR and low salinity on PSII function in the marine diatomS.costatum. Low salinity reduced PsbA synthesis and worked synergistically with UVR to modify the photochemistry of PSII and, thus, intensified the UVR-induced photoinactivation. Although higher CAT activity and NPQs were induced, they were unable to prevent the damaging combined eff ect oflow salinity and UVR. This study suggests that reduced salinity and increased UVR could potentially aff ect the abundance and distribution ofS.costatum,and might aff ect the formation ofS.costatumdominated harmful blooms with the accentuation of climate disturbances.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
 Journal of Oceanology and Limnology2022年4期
Journal of Oceanology and Limnology2022年4期
- Journal of Oceanology and Limnology的其它文章
- Validation and error analysis of wave-modified ocean surface currents in the northwestern Pacific Ocean*
- The energy conversion rates from eddies and mean flow into internal lee waves in the global ocean*
- Observation of physical oceanography at the Y3 seamount(Yap Arc) in winter 2014*
- Decadal variation and trend of the upper layer salinity in the South China Sea from 1960 to 2010*
- Observations of turbulent mixing and vertical diff usive salt flux in the Changjiang Diluted Water*
- Experimental research on oil film thickness and its microwave scattering during emulsification*
