Potential of Ulva prolifera in phytoremediation of seawater polluted by cesium and cobalt: an experimental study on the biosorption and kinetics*
Xuemei WANG , Tifeng SHAN ,**, Shaojun PANG ,**
1 CAS and Shandong Province Key Laboratory of Experimental Marine Biology, Center for Ocean Mega-Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao),Qingdao 266237, China
Abstract Radionuclides accidentally released from nuclear power plants, such as 137 Cs and 60 Co, can lead to severe contamination of marine ecosystems. Living macroalgae are effi cient in absorbing metal elements from seawater. A 10-day simulation was conducted to assess the potential of scavenging cesium(Cs) and cobalt (Co) with live U lva prolifera that was cultured in seawater medium containing Cs and Co in diff erent concentrations (0 (the control), 10, 20, 50, and 100 mg/L). In the experiment, 5 kg (fresh weight)of U. prolifera was cultured in natural seawater in 90-L tanks. Results showed that after the experiment,the average bioconcentration factors (BCFs) of the control group were 247.2 and 1 126.4 for Cs and Co,respectively. The absolute absorption quantity of U. prolifera increased and the BCFs decreased with the increase in Cs and Co concentrations. The biosorption of the two metals on the first day well fit the pseudosecond-order equation ( R 2 >0.95), indicating that adsorption is the rate-limiting step in the total biosorption process. Concentrations of both metal ions declined significantly in the first hour and decreased by 25.2%and 15.5% in 48 h, respectively. Therefore, live U. prolifera is effi cient at scavenging Cs and Co in seawater,providing potential applications for the phytoremediation of radionuclides contaminated seawaters.
Keyword: radionuclide pollution; phytoremediation; green tide; Ulva prolifera; biosorption kinetics* Supported by the State’s Key Project of Research and Development Plan,China (No. 2016YFC1402507), the China Agriculture Research System of Ministry of Finance, and the Ministry of Agriculture (No. CARS-50)** Corresponding authors: shantifeng@qdio.ac.cn; sjpang@qdio.ac.cn
1 INTRO DUCTION
With rapid worldwide development in the construction of coastal nuclear power plants (NPPs),the safety of NPPs posed a major risk to the public,particularly after the Fukushima 1 accident in 2011.The radionuclides released after the accident could cause serious pollution in coastal waters (Fukuda et al., 2014; Zhuang et al., 2018). After the Fukushima 1 accident, the radiation level near the NPP was 15 000-30 000 times higher over the normal level (Xiao et al.,2011). One of the major radionuclides released was137Cs, which has a relatively long half-life of 30 years of radioactivity (Wang et al., 2018a).60Co was also present in wastewater from NPPs and has a half-life of 5 years (Lingamdinne et al., 2016). Although Co is a source of vitamin B12, an essential trace element for life, it would become toxic at a high concentration,and on the other hand, cause low blood pressure,paralysis, and bone defects, as well as damage in the lungs, heart, and liver in humans (Ahmadpour et al.,2009; Mosoarca et al., 2018).
The removal of radionuclides from polluted seawater should be in high priority because of their negative eff ects on the marine environment. Several methods—e.g., ion exchange, adsorption, chemical precipitation, and membrane technology—have been proposed for removing radionuclides from seawater(Hu et al., 2019). Biosorbents have been testified to have higher removal effi ciency, and they are more abundant, less expensive, and importantly, more environmentally friendly, than both physical and chemical adsorbents (Bailey et al., 1999; Alluri et al.,2007). Therefore, the potential value ofliving macroalgae as biosorbents has been assessed for the removal oflead, copper, chromium, strontium, and cobalt (Doshi et al., 2008; Southichak et al., 2008;Mosoarca et al., 2018; Wang et al., 2018b). The consumption of algal biomass would be huge once nuclide pollution occurs. The ability to obtain a large biomass in a short period is a major technical bottleneck for using macroalgae in marine nuclide pollution remediation.
Green tides are vast blooms of floating green macroalgae, mainly involvingUlva(orEnteromorpha),which are not distinct genera in taxonomy (Hayden et al., 2003; Nelson et al., 2008). Algal blooms in coastal waters initially are resulted from eutrophication, and they are often harmful to aquaculture and tourism (Sun et al., 2008). The Qingdao coast of the Yellow Sea in China first experienced a small-scale green tide in the summer of 2007. In the following years, massive green tides occurred each summer in the Yellow Sea (Liu et al., 2015; Zhao et al., 2015). Before the 2008 Olympic Regatta in Qingdao, the largest green tide in the world at that time occurred along the Qingdao coast,measuring 3 849 km2and having an approximate harvested biomass of one million tons (fresh weight)(Pang et al., 2010; Keesing et al., 2011). Through morphological and molecular analyses, the dominant species was determined filamentousUlvaprolifera(Müller) J. Agardh (Sun et al., 2008). The main treatment method for green tides at present is salvage by boat, yet disposing of massive amounts ofUlvabiomass remains a major challenge. We may find a novel application for making use of this immense biomass “endowed” by nature if the green algae can be used as a sorbent for nuclide pollution.
Diff erent macroalgal species have diff erent adsorption capacities for nuclides. In general, the green algae have a higher adsorption capacity than red algae, but lower than brown algae, due to the diff erent composition of the cell wall (Wang et al.,2018b). Considering the huge biomass requirement of bioremediation and the immense biomass of algal bloom, we explored the removal potential for nuclides ofSargassumhornerifrom golden tide (Wang et al.,2021). The content of the corresponding heavy metal inU.proliferacould reach 44.67 and 503.63 mg/kg,and the bio-concentration factors (BCFs) could be 625.89 and 9 119.79 after being cultured for 12 d in the seawater containing 80-μg/L Cd2+and Pb2+,respectively (Liu et al., 2014). However, there was no literature about the scavenging ability ofU.proliferafor important nuclides such as137Cs and60Co commonly found in polluted water after nuclear accidents. To potentially exploit the waste products of green tides for remediation purposes, we investigated the scavenging ability ofU.proliferafor Cs and Co from seawater under simulation conditions and analyzed the biosorption characteristics, kinetics, and models. Based on our results, we discuss the potential ofU.proliferain the remediation of seawater polluted by Cs and Co.
2 MATERIAL AND METHOD
2.1 Biosorption experiment
Ulvaproliferawas collected from the coastal region of Qingdao, China, during a green tide in July 2017. The algae were acclimatized for 2 d in indoor seawater circulation tanks with constantly aerated sand-filtered seawater.U.proliferawas then acclimatized in an intelligent light incubator (Jiangnan Co. Ltd., Ningbo, China) in 3-L beakers (200-g (fresh weight) algea per beaker) at 15 °C and a photoperiod of 12 h∶12 h (light∶dark) using fluorescent white light at 100 μmol photons/(m2·s). The culture medium was sterilized seawater enriched with 70-mg/L NaNO3and 10-mg/L NaH2PO4. Chloride salt of Cs or Co was used in the present investigation.U.proliferawas then cultured under the same conditions in the culture medium at four diff erent concentrations of Cs or Co(10, 20, 50, and 100 mg/L). Culture media without Co or Cs were set as controls. The background concentrations of Cs and Co were detected to be 0.023 7 μg/L and 0.004 5 μg/L using an Agilent 7500ce inductively coupled plasma mass spectrometry(ICP-MS) with serial standard solutions under default parameters. All culture media were constantly aerated and renewed daily. The Cs or Co content of algae was measured at 10 diff erent time points. All sampling and cultures were performed in triplicate.
In the tank simulation experiment, 5 kg (fresh weight) ofU.proliferawas placed into each 90-L tank. It was cultured in the above-mentioned sandfiltered natural seawater under ambient conditions.Chloride salt of Cs or Co was added to each tank, and the final concentration of Cs or Co was 100 mg/L(calculated value). The Cs content of the algae and Co concentration of the culture medium were measured at diff erent time points.
2.2 Nuclides quantification
Cs or Co content in algae and seawater was measured using an inductively coupled plasma optical emission spectrometer (ICP-OES). Algal samples were dried and digested according to methods described in Wang et al. (2018b). Following digestion,the samples were diluted with deionized water, and Cs or Co concentrations were detected with the Agilent 5110 ICP-OES in the presence of serial standard solutions under default parameters. The absorption wavelengths (λ) used to determine the analyzed nuclides were 697.327 nm for Cs and 228.615 nm for Co.
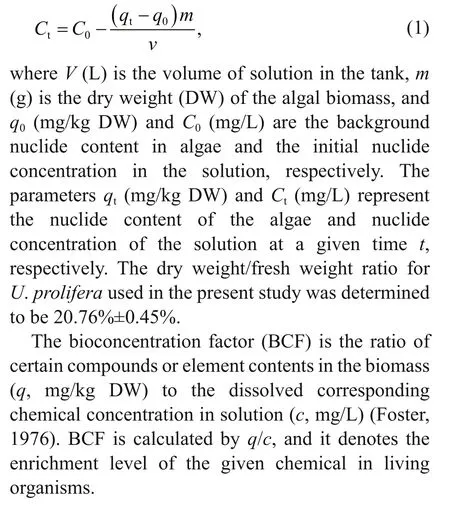
For the tank experiment, the Cs concentration of the culture medium was calculated via the content in algae because certain seawater components may interfere with Cs detection. The formula is as follows:
2.3 Quality control and statistical analysis
All chemicals used in the present research were analytical reagents for quality control. The biosorption test was conducted in an incubator with accurately controlled environmental conditions for 10 d. Each concentration group was set up and all measurements were conducted in triplicate. Results are represented as the mean±standard deviation (SD). The tank experiment data were analyzed using a one-way analysis of variance (ANOVA) with SPSS Ver. 22.Homogeneity of variance was checked, and data were transformed if necessary before analysis. The Tukey’s multiple comparison method was employed when significant diff erences (P<0.05) were observed.
2.4 Kinetics model
Pseudo-first-order (Lagergren, 1898) and pseudosecond-order equations (Ho and McKay, 1999) were employed to evaluate the dominating mechanism of the Cs or Co biosorption process byU.proliferabiomass. Linear regression analysis was used to calculate the correlation coeffi cients (R2). The equations oflinear forms are as follows:

where the nuclide contents in algae at time t and under equilibrium were designatedqtandqe(mg/kg DW),respectively. The parameterk1(/h) is a pseudo-firstorder rate constant, andk2(g/(mg·h)) is a pseudosecond-order rate constant.
3 RESULT
3.1 Biosorption process
The Cs contents of theU.proliferabiomass in diff erent Cs concentration groups were determined after diff erent culture times ranging from 2 h to 10 d(Table 1). The Cs contents ofU.proliferain the control group were almost the same at diff erent times,except for 96 h, a little higher than other time points.During the experiment, the biosorption capacity increased significantly with increased Cs concentration in the medium. The Cs contents ofU.proliferaincreased rapidly in the first day, especially in the first 2 h, and then much more slowly over the following 9 d. The Co contents of theU.proliferabiomass in the diff erent Co concentration groups were also determined at diff erent times, from 2 h to 10 d (Table 2). Co contents ofU.proliferain the control group were statistically significant diff erences (P<0.05) at some time points. According to our results, the biosorption process of Cs byU.proliferawas similar to that of Co.
For Cs, the BCF ofU.proliferawas 136.0-368.7 in the control group and 11.9-44.8 in the experimental groups (Table 3). On the tenth day, the mean BCFs ofU.proliferaat Cs concentrations of 10, 20, 50, and 100 mg/L were 44.8, 34.8, 35.5, and 33.5, respectively.For Co, the BCF was 447.9-2631.4 in the control group and 10.2-318.3 in the experimental groups(Table 4). On the tenth day, the mean BCFs ofU.proliferaat diff erent Co concentrations (10, 20, 50,and 100 mg/L) were 318.3, 267.2, 137.9, and 77.3,respectively. We conclude that the increase in the initial Cs or Co concentration reduces the BCFs ofU.proliferafor Cs or Co, although the absolute biosorption quantity increases substantially.
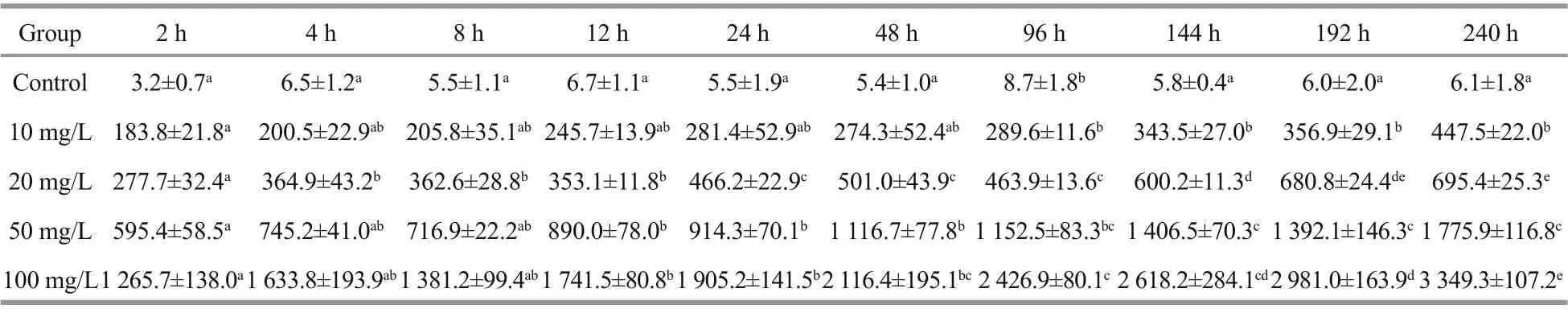
Table 1 Mean Cs content (mg/kg DW) in Ulva prolifera at diff erent times after exposure to four concentrations of Cscontaining culture medium
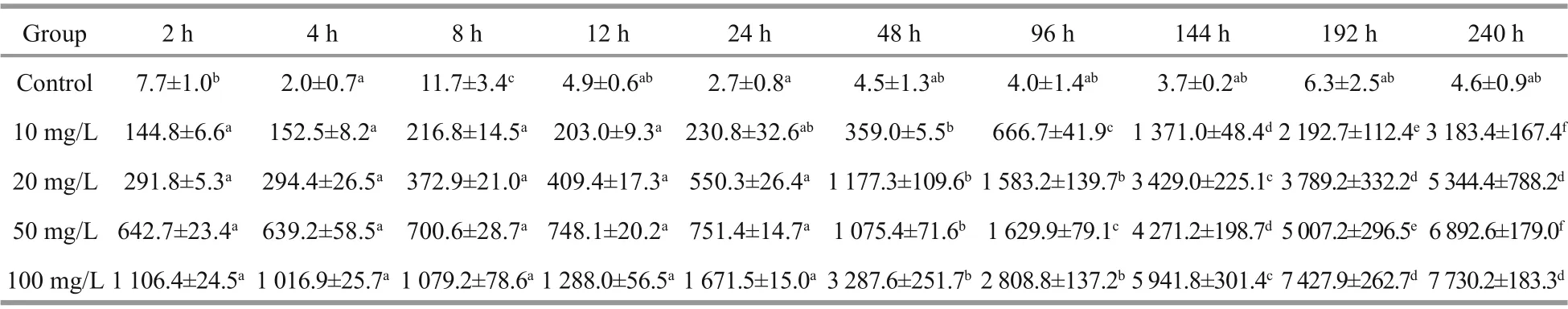
Table 2 Mean Co content (mg/kg DW) in Ulva prolifera at diff erent times after exposure to four concentrations of Cocontaining culture medium
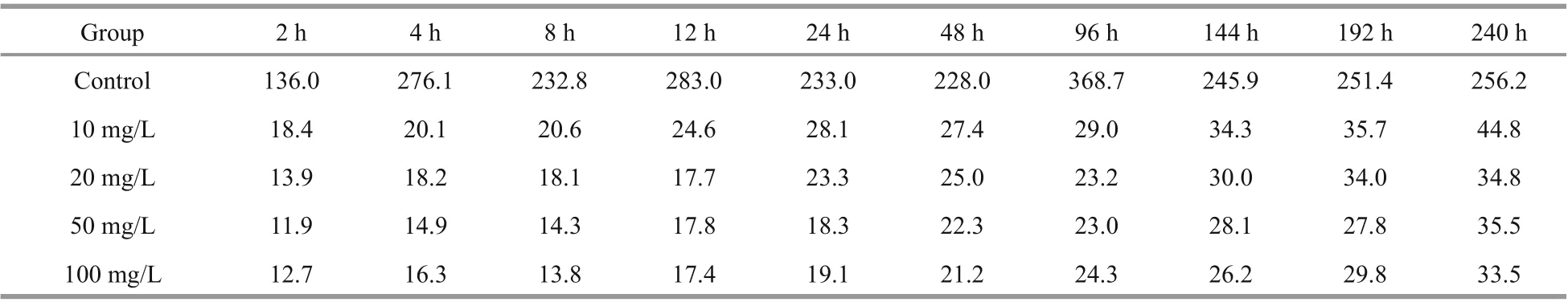
Table 3 Bioconcentration factors (BCFs) of Cs in Ulva prolifera at diff erent times after exposure to four concentrations of Cs-containing culture medium
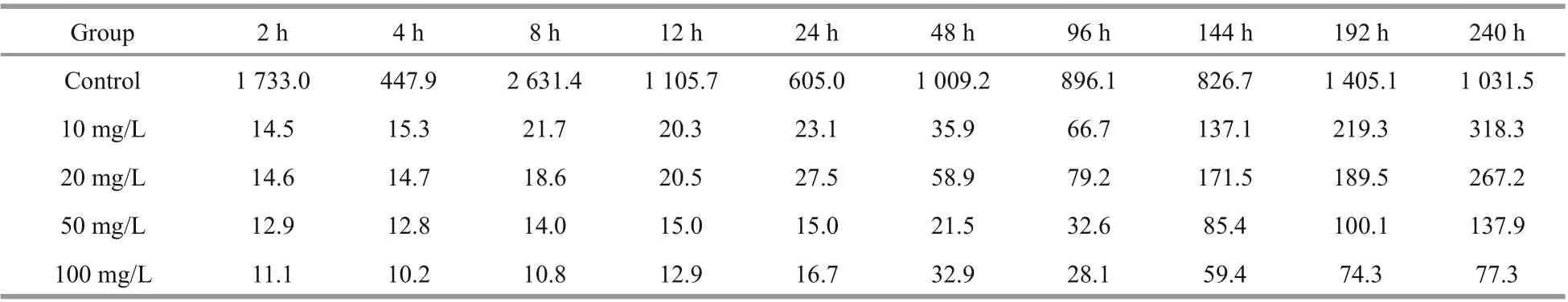
Table 4 Bioconcentration factors (BCFs) of Co in Ulva prolifera at diff erent times after exposure to four concentrations of Co-containing culture medium
3.2 Kinetics model
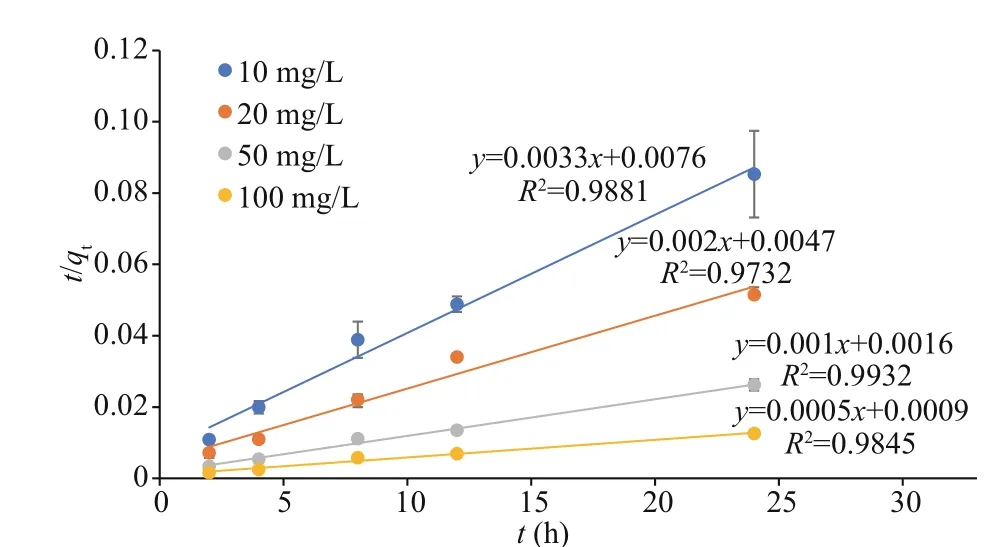
Fig.1 Plots of the pseudo-second-order kinetic model for Cs biosorption using U. prolifera
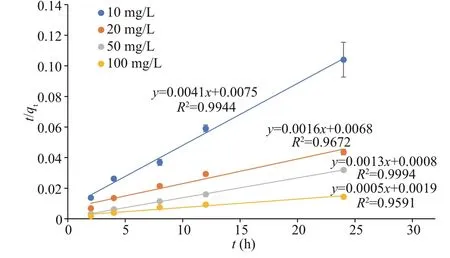
Fig.2 Plots of the pseudo-second-order kinetic model for Co biosorption using U. prolifera
The pseudo-first-order and pseudo-second-order equations were applied to the Cs and Co biosorption processes during the first day before the medium was renewed. The data did not fit the pseudo-first-order model but fit the pseudo-second-order equation well.The physical meaning of pseudo-second-order kinetics is the reaction rate that is proportional to the quadratic power of reactant concentration. It is suitable for reactions with saturation sites, for example, the adsorption of heavy metals. The plots oft/qtversustfor Cs biosorption are shown in Fig.1. The correlation coeffi cients (R2) are higher than 0.95 for all the Cs concentrations. The plots oft/qtversustfor Co biosorption are shown in Fig.2. The correlation coeffi cients (R2) are higher than 0.95 for all the Co concentrations. Our results revealed that the pseudosecond-order equation provides a high correlation for the biosorption process of Cs and Co byU.prolifera.This indicates that in the total biosorption process,adsorption is the rate-limiting step.
3.3 Tank simulation experiment
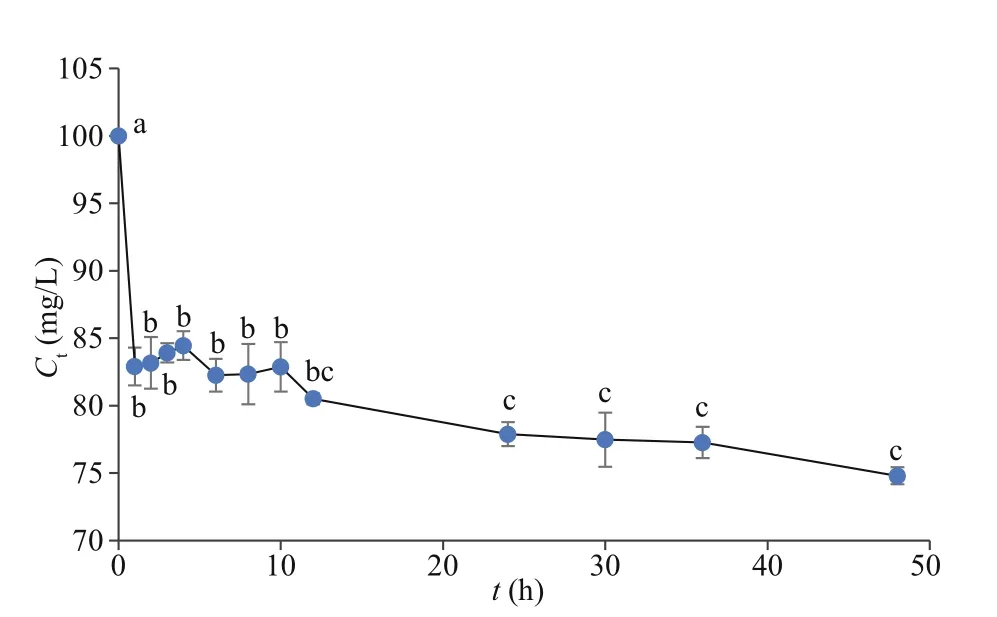
Fig.3 Cs concentrations of culture medium in tank simulation experiment after disposing with U. prolifera for diff erent time in two days
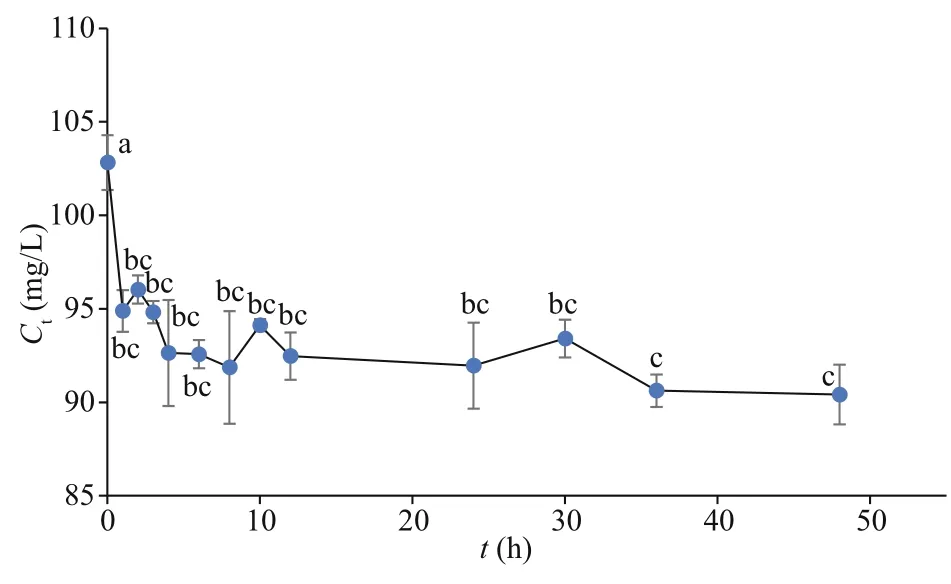
Fig.4 Co concentrations of culture medium in tank simulation experiment after disposing with U. prolifera for diff erent time in two days
The Cs concentrations in the culture medium at diff erent times were calculated using the Cs contents in the algae in the tank simulation experiment (Fig.3).TheCtof Cs decreased sharply in the first 2 h and gradually thereafter. The Cs concentration in the culture medium decreased by 25.2% throughout the experimental period. Additionally, the biosorption process was fast in 12 h, although a slight decline was observed after that. The Co concentrations in the culture medium were determined at diff erent times in 2 days (Fig.4). As with Cs, theCtof Co decreased sharply in the first 2 h and gradually over the remaining time. The Co concentration in the culture medium decreased by 12.1%, less than the Cs reduction percentage, during the experimental period. Similar to Cs, the biosorption process for Co was also rapid in 12 h, followed by a slight decline.
4 DISCUSSION
The macroalgaU.prolifera, the dominant species in the recurrent green tide in the Yellow Sea of China,was found to be eff ective for the remediation of seawater containing the nuclides Cs and Co. Previous research has shown that the biosorption of metal ions by living biomass involves a fast passive adsorptionprocess, followed by a slower active bioaccumulation process, which is dependent on metabolism (Mehta and Gaur, 2005). From the biosorption and tank simulation experiments, we found that the biosorption process of Cs and Co byU.proliferawas rapid on the first day, especially in the first few hours. It may be that adsorption is the dominant process in this remediation process. The fitting results of the pseudosecond-order equation, which identified a strong correlation for the biosorption process of Cs and Co during the first day, substantiated this conclusion.This characteristic of the remediation process can significantly reduce the processing time in the following practical application, achieving the goal of rapid interception once the nuclear leakage accident occurred.
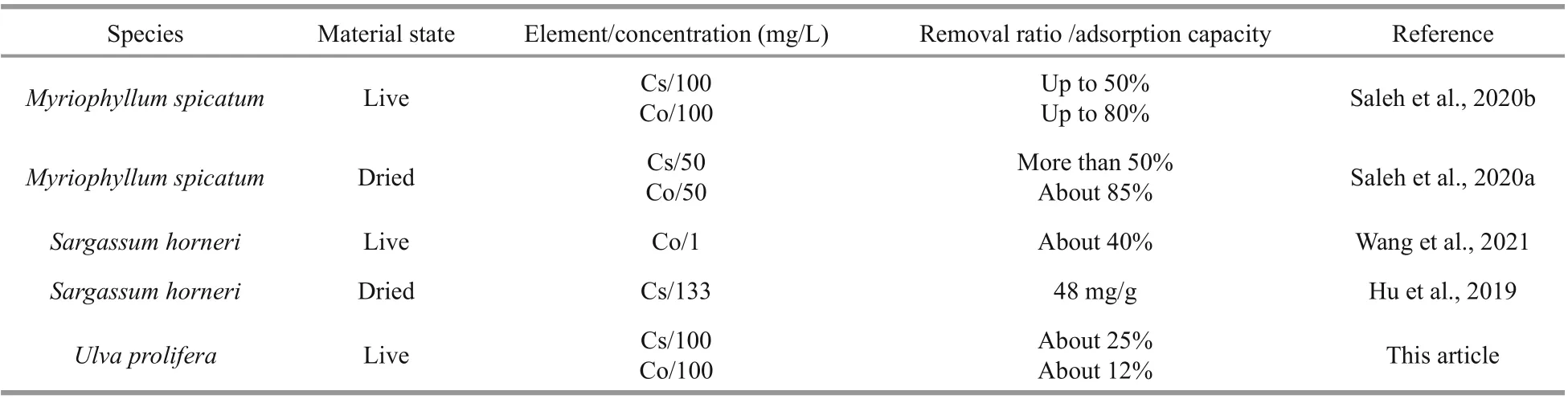
Table 5 The effi ciency of diff erent algal species to remove Cs and Co contamination from water
To compare the nuclide adsorption capacity ofU.proliferawith other algal species, the effi ciency of diff erent algal species to remove nuclides from contaminated water is listed in Table 5. The removal rate (%) for Cs and Co ofU.proliferawas lower thanMyriophyllumspicatum, a kind of common freshwater alga. For another algal bloom species,Sargassumhorneri, the removal rate for Co was about 40% when the concentration of Co was 1 mg/L. However, we cannot draw the conclusion which alga has higher adsorption capacity for Co, as the concentration of Co was diff erent.
The biosorption capabilities of diff erent algal species likely vary due to their specific cell wall structure and composition. The adsorption force is mainly the crosslinking between the positively charged nuclides and negatively charged chemicals in the cell wall, such as the carboxyl in the alginate (Mata et al., 2009).U.proliferahas tubular structure of monolayer cells,favorable to adsorbing pollutants in theory. However,in our previous study, brown algae exhibited higher removal capacity for Sr compared with green algae such asUlva(Wang et al., 2018b). This is likely caused by fewer negatively charged binding groups on the cell wall ofUlvathan brown algae, which contain a large amount of alginates in their cell walls.
The survival ofU.proliferawould decrease without renewal of the culture medium owing to nutrient depletion or exudate build-up. This would complicate evaluations of the remediation potential ofU.proliferain an open sea area. However, renewing the culture medium would have interrupted the kinetics process during the experiment. Therefore, we only used the data from the first day in the biosorption process to investigate the kinetics models. In fact, we attempted to apply the pseudo-second-order equation to the whole Cs and Co biosorption process, but the experimental data did not fit the pseudo-first-order model (results not shown). By the end of the biosorption experiment, the Cs and Co contents in algae were relatively high and still increasing.Equilibrium states have been reached in the remediation of Sr and Cs by the algaeSargassumhorneri(Wang et al., 2018b; Hu et al., 2019). Here,however, equilibrium was not reached until the tenth day in the constant external concentration conditions of Cs and Co. This is likely caused by the diff erent components of cell wall between brown and green macroalgae. Overall, our results suggest that live macroalgaeU.proliferalikely has remediation potential for the nuclides Cs and Co, and future studies should focus on the removal mechanism.
5 CONCLUSION
The live green algaU.proliferacan potentially remove Cs and Co from contaminated seawater.Given thatU.proliferais the dominant species in green tides and that a large quantity of biomass is usually available, it has a potential to be employed in the bioremediation of seawater contaminated by Cs or Co. Its use may resolve, to some extent, the high demand for biomass in the bioremediation of seawater polluted by nuclides. After all, the large harvests ofUlvaduring green tide events have made disposal a challenge in recent years. This study provides some direction for the potential practical exploitation ofUlvabiomass. Practical application would be doubly beneficial by providing an outlet for excessUlvabiomass during large-scale green tides and remediating seawater nuclide pollution.
6 DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2022年4期
Journal of Oceanology and Limnology2022年4期
- Journal of Oceanology and Limnology的其它文章
- Validation and error analysis of wave-modified ocean surface currents in the northwestern Pacific Ocean*
- The energy conversion rates from eddies and mean flow into internal lee waves in the global ocean*
- Observation of physical oceanography at the Y3 seamount(Yap Arc) in winter 2014*
- Decadal variation and trend of the upper layer salinity in the South China Sea from 1960 to 2010*
- Observations of turbulent mixing and vertical diff usive salt flux in the Changjiang Diluted Water*
- Experimental research on oil film thickness and its microwave scattering during emulsification*
