Diverse habitat preferences of two sea cucumber species and the seasonal change in a coral reef area*
Chunyang SUN , Duanjie HUANG , , Qiang XU ,**, Fei GAO , Xiubao LI ,Aimin WANG
1 College of Ecology and Environment, Hainan University, Haikou 570228, China
2 College of Marine Science, Hainan University, Haikou 570228, China
3 State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan University, Haikou 570228, China
4 Hainan Provincial Ecological and Environmental Monitoring Centre, Haikou 571126, China
Abstract Diff erent sea cucumbers have diff erent preferred habitats and seasonal changes, which is still lack of detailed research. This study selects two common tropical sea cucumbers ( Holothuria edulis and Stichopus chloronotus) to explore the reasons that aff ect their habitat selection and seasonal changes, so as to provide support for the spatial planning of sea cucumbers reserve in coral reef area. The study area is a compound distribution area ofliving coral reefs and sandy bottom in the north of Wuzhizhou Island.The survey time is January, April-September, 2019. Three fixed transects (G, H, and Q) were selected to reveal the population ecological characteristics of them in the typical dry season (January, April, May, and June) and rainy season (July, August, and September). The variation range of density for H. edulis and S. chloronotus were 2.0-8.9 inds./10 m 2 and 0.2-0.7 inds./10 m 2, respectively. The population density in rainy season was significantly higher than that in dry season ( P< 0.05). H. edulis tends to be distributed in deeper water. The distribution of H. edulis was significant positively correlated with the sand coverage and sand continuity in G transect ( P< 0.05), and significant negatively correlated with live coral coverage in three transects ( P< 0.01). The shift from dry season to rainy season is a key factor aff ecting their habitat preference. In dry season, the preferred habitats of S. chloronotus are small rock (SR), sand (S), and small coral reef (SCR), while the H. edulis is S, SR, and dead coral rubbles (DCR). In rainy season, the preferred habitats of S. chloronotus are shift to SCR and SR, while the H. edulis is SCR, DCR, and sand.
Keyword: habitat preference; coral reef; population density; Holothuria edulis; Stichopus chloronotus
1 INTRODUCTION
Most tropical sea cucumbers have high commercial value, but little is reported about their ecological knowledge (Dissanayake and Stefansson, 2012).Understanding its basic biological and ecological information is conducive to rational utilization and protection of precious tropical sea cucumber resources(Wiedemeyer, 1994; Mercier et al., 1999; Purcell,2004; Conand, 2008). The distribution variation of benthic marine invertebrate populations over time and space are related to complex ecological processes(Mercier et al., 1999). For example, Aspidochirotida sea cucumbers are ubiquitous and conspicuous members in the coral reef ecosystem and play an important ecological role in nutrient cycling and bioturbation processes (Cowen and Sponaugle, 2009;Purcell et al., 2016). Alvarado et al. (2012) found the coral reefs with high species richness and diversity index of echinoderm have a wide variety of coral species, good growth, and large distribution area.Population dynamics are essential for the determination and management of the bio-resource protecting area, but little is known about the temporal dynamics of sea cucumber populations and how these dynamics are related to seasonal ecological change in their habitats (Eriksson et al., 2013), which makes the spatial planning of sea cucumber protecting area on coral reefs diffi cult (Parma et al., 2005; Crowder and Norse, 2008).

Fig.1 Holothuria edulis (a) and Stichopus chloronotus (b)
HolothuriaedulisandStichopuschloronotusare common species in coral reefs in tropical seas, andS.chloronotushave high economic value. The two sea cucumber species inhabit diff erent habitats.H.edulismainly inhabit in the soft bottom habitat dominated by sand (Fig.1a). They have irregular feeding behaviors and prefer to eat large-grained sediments (Liao, 1997).S.chloronotusmainly lives in hard bottom habitat such as coral and rocky reefs (Fig.1b). They feed on the fine sediments on the surface, and their feeding time is relatively regular (Liao, 1997).
Resources are often unevenly distributed in nature,hence animals must constantly adjust in the process of behavioral response (Hutto, 1985; Block and Brennan,1993), and show the characteristics of seasonal habitat selection (Zhang and Li, 2005; Conand, 2008; Slater and Jeff s, 2010; Yamana et al., 2010). The habitat selection of sea cucumbers is aff ected by biological and inorganic environmental factors, mainly involving feeding selection, habitat heterogeneity, hidden behaviors, competition, and water flow (Roberts,1979; Sloan and Von Bodungen, 1980; Entrambasaguas et al., 2008; Morgan, 2011). At present, reports on tropical sea cucumbers mainly focus on population distribution, community structure, and habitat selection. There is still a lack of detailed research on the habitat factors that aff ect the habitat selection of sea cucumbers and the seasonal changes of their preferred habitats.
The coordination between habitat type and habitat selection of benthic animals are an obvious phenomenon in coral reef area (Entrambasaguas et al., 2008; Shiell and Knott, 2010). It is reported that sea cucumber population demography mostly depends on environmental parameters such as food and hydrodynamics (Byrne et al., 1998; Ebert et al.,1999). Besides, substrate type for inhabitation and available detritus food source are also key factors influencing the population metrics of sea cucumbers(Uthicke and Karez, 1999; Hermosillo-Nuñez et al.,2016). Habitat preferences vary among diff erent species, and are also impacted by habitat profiles and seasonal changes. For example,H.edulispreferred fine sand substrate in winter but it moved to coral rubble habitat in spring. There are also researches reporting conflicting results concerning the habitat preference in diff erent areas. For example,Holothuriaatrais reported to prefer seagrass bed (Dissanayake and Stefansson, 2012), while other scholars found they preferred coral sand bottom (Liao, 1997).Therefore, it is particularly necessary to study the habitat preference and seasonal changes of sea cucumbers in the unique coral reef ecosystem in the South China Sea.
Finding out the seasonal changes in the preferred habitat of sea cucumbers can help us analyze the seasonal changes ofits ability to restore a specific coral reef ecosystem and assess the importance of sea cucumbers as an ecological restoration species. Sea cucumbers play a key role in bioturbation and turnover of sediments (Mangion et al., 2004), the deposit fecal can enhance coral calcification and provide a buff er against ocean acidification (Schneider et al., 2011).The seasonal changes of sea cucumber’s preferred habitat are closely related to its ecological adaptability,but the precision of previous studies is low on the time scale, so it is diffi cult to reflect the fine changes of sea cucumber ecological adaptability. It is essential to understand their migration behavior through diff erent coral reef habitats. A fine-scale study is needed to cover the reasons why diff erent species select diff erent habitat types and how it changes throughout the year.
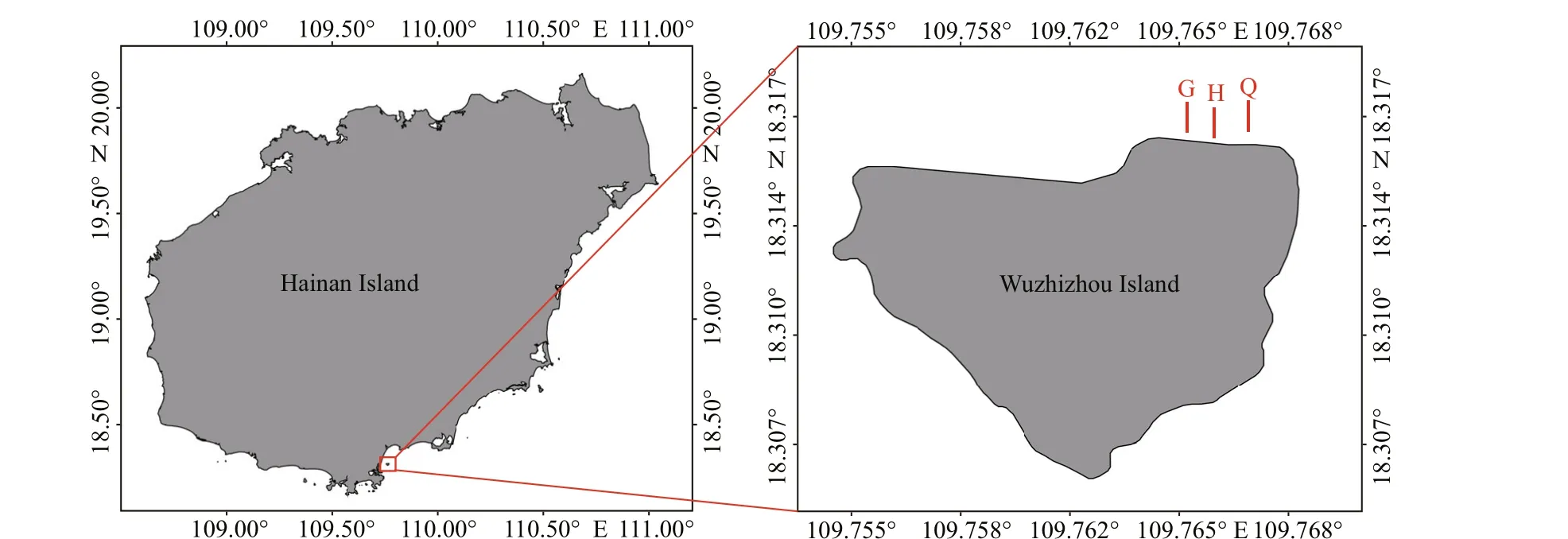
Fig.2 Study area and three fixed transects at Wuzhizhou Island
Most previous studies on sea cucumber population or community were conducted by the method oflarge-scale random sample survey, which is diffi cult to fix the specific habitat profiles and other external biotic and abiotic factors, such as water depth and benthic substrate, so it is not suitable for the study of sea cucumber habitat preference on a fine time scale.In this study, the fixed transect method was used to select three fixed transects with diff erent habitat combinations, and regular underwater video recordings were conducted within one year to study the population migration of sea cucumbers among diff erent habitat types in details. Therefore, the goals were to find the distribution patterns and preferred habitat changes of two predominant sea cucumber species in the coral reef ecosystem; to explore whether the seasonal change of sea cucumber’s preferred habitat is gradually migrated or realized in the short term of seasonal alternation; to explore the eff ect of depth gradient on the distribution of two sea cucumber species; and to find the habitat factors that aff ect the distribution of two sea cucumber species.
2 MATERIAL AND METHOD
2.1 Study area and fixed transect
Wuzhizhou Island (18°31′N, 109°77′E; area 1.48 km2) is a famous tourist region in China with the best coral reef around Hainan Island and excellent water and sediment quality. There are over 90 reef corals with an average coverage of 28.18% (41.85%highest on the south side) (Li et al., 2019). At least 12 sea cucumber species live here with a mean density of 3 individuals per 10 m2(inds./10 m2) (Huang et al.,2020). The good protection is mainly due to the management of the tourist company and the technical support of our research team. Coral reef restoration has been carried out over six years. Commercial trawling fishing is completely prohibited in the study area, and only recreational fishing on one boat belonging to the tourism company is permitted in Wuzhizhou island sea ranching area. It is a perfect place for the biodiversity preservation of sea cucumber species.
During the preliminary benthic habitat survey, it was found that the coastal area on the north side of the Wuzhizhou Island is a compound distribution area ofliving coral reefs and sandy bottom, with gentle seabed topography and abundant sea cucumber population resources (predominant species areHolothuriaedulis,Holothuriaatra, andStichopuschloronotus). It is also the main area for reef diving tourism in summer. Therefore, the study was carried out in this area (18°19.000′-18°19.067′N,109°45.890′-109°46.009′E). Three parallel transects which are perpendicular to the coastline were set from the west to the east and abbreviated as G, H, and Q(Fig.2). The length of each transect is 100 m, using a 100-m long rope (1-cm precision). The interval between transects was 30-50 m. Transects extends from shallow water to deep water. The transects were set within the depth of 15 m, crossing the sand substrate and coral reef substrate, and cover all types of habitat types of the area (the transects snapshots are in the attachment).
2.2 Survey methodology
The survey time is January, April-September,2019, and a 2-person diving team undertook this work. When monitoring, the divers took the videos of each transect with a high-resolution underwater camera (Gopro 5, USA, the resolution is 2.7 kp and the frame rate is 60 frames/s). The average swimming speed of the divers was 0.17 m/s to get high-quality video. During the investigation of each transect,divers started from the left side of the rope to record the habitat and target sea cucumbers within 1 m of the left side, then turned back at the end of 100 m and continued to take the video along the right side of the transect. The target sea cucumbers within 1 m on both sides of the rope were monitored, making a 200-m2survey area (Fig.3). The depth gradient of the transects (10-m interval) was measured by the diver with a diving computer watch. When getting back to the lab, the population density of the two species with the habitat profiles were analyzed based on the video.
In bioresource evaluations, the mean density is commonly used to represent the distribution of sea cucumbers in a certain area, which will potentially simplify a markedly heterogeneous spatial distribution and mask local variation in population structure, and the mean density has limited reference to spatial scales of populations (Purcell et al., 2009; Eriksson et al., 2010). Thus, a population density profile may be better suited to improving resolution in the analysis of evaluation data (Eriksson et al., 2013). In our study,instead of calculating the population density of each transect totally, we divided each transect into 10 subdistricts of 20 m2each (10 m×2 m) to count the population density of each sub-district and then combined them into a complete population density profile. It can better reflect the relationship between sea cucumbers’ population density variations and habitat profiles.
2.3 Division of dry season and rainy season
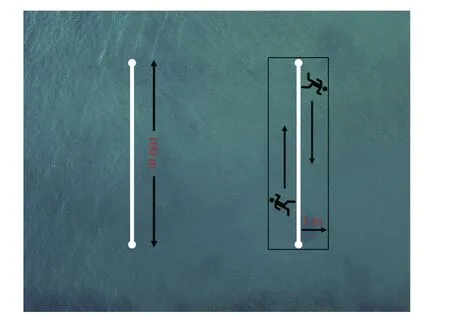
Fig.3 The layout of fixed transects and shooting mode
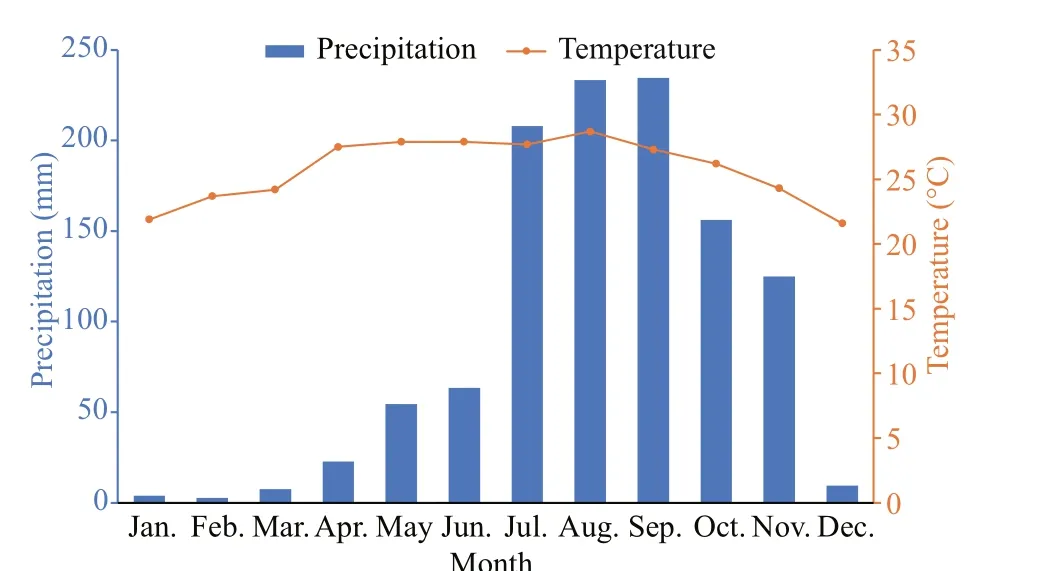
Fig.4 Monthly average precipitation and temperature in Sanya in 2019
Sanya sea area is tropical monsoon climate, the annual variation of temperature in this area is small(22-29 °C), but the seasonal precipitation has a great impact on the marine environment, showing a distinct division of dry season (January to June) and rainy season (July to November.) (Fig.4). The monthly precipitation and temperature data of Sanya in 2019 are taken from the reports of the Agricultural Meteorology of Hainan Province, and delivered by the Institute of Meteorology Sciences of Hainan(http://www.hainanqx.cn/UI/Index/Index.aspx). The division method of dry season and rainy season is as follows: Wuzhizhou Island is located in the tropics,aff ected by the tropical monsoon climate. The precipitation from January to June and December is small, the monthly precipitation is below 65 mm on averaged of 16.8 mm, which is the dry season. The monthly precipitation from July to November is more than 120 mm and averaged as 170 mm, which is the rainy season (Fig.4).
A multi-parameter water quality detector (YSI-650, USA) was used to measure the water temperature,salinity and dissolved oxygen of the surface water(1-m underwater) in the investigated sea area.
2.4 Determination of habitat types in study area
According to the habitat characteristics of the investigated sea area, the habitat types can be divided into the following six types: sand/silt (S), small rock(SR) (diameter below 15 cm), big rock (BR) (diameter above 15 cm), small coral reef (SCR), big coral reef(BCR), and dead coral rubbles (DCR) (Bellchambers et al., 2011; Dissanayake and Stefansson, 2012). The number ofH.edulisandS.chloronotusin each subdistrict were counted, and the habitat types of each sea cucumber were distinguished according to the division method of the above six habitat types, then the number of two sea cucumber species in each habitat type in each month was summarized for further statistical analysis.
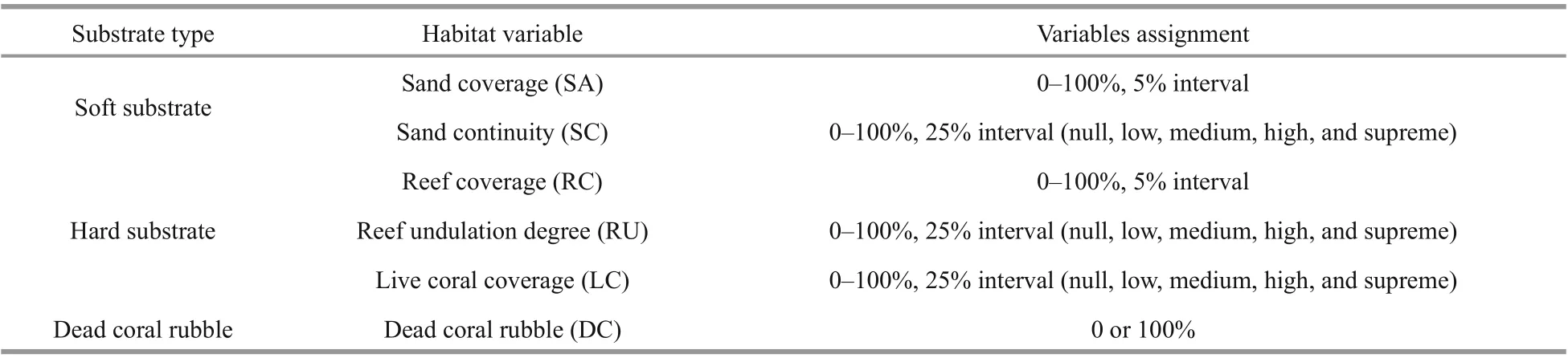
Table 1 Habitat variables and assignment methods
2.5 Determination of habitat variables
After mapping the detailed habitat types of the three transects, we selected six habitat variables to further explore and quantify the impact of environmental factors on the habitat preference of two sea cucumber species (Eriksson et al., 2012; Li et al., 2012). The chosen principle of habitat variables is to extract and integrate the characteristics of habitat types to make them representative, and their changes will aff ect the distribution of sea cucumbers.
We divided habitat variables into three categories:soft substrate (including the plain top surface of big coral rock covered with fine deposit), hard substrate,and dead coral rubble. Soft substrate contained two habitat variables: sand coverage (SA) and sand continuity (SC). Hard substrate contained three habitat variables: reef coverage (RC), reef undulation degree (RU), and live coral coverage (LC). Dead coral rubbles (DC) were scattered in soft and hard substrate, which is diffi cult to be defined as an independent variable. Therefore, DC was taken as a supplemental factor to analyze whether its presence or absence would aff ect the preferred habitat selection of sea cucumber. Table 1 lists the detailed assignment methods of these habitat variables.
Therefore, each transect was further divided into 20 sub-divisions of 10 m2each (5 m×2 m) for more precise quantification of habitat variables. Two bottom snapshots at both sides of one sub-division were got, and ImageJ was used to quantify habitat variables based on the snapshots (Bottom snapshots of every 10 m of G, H, and Q transects are attached in the attachment). Then correlation analysis was carried out on the distribution of sea cucumber and habitat variables.
2.6 Data processing
2.6.1 Habitat density of two sea cucumber species
The population density of the sea cucumber was calculated as inds./10 m2in diff erent months and water depths. The formula (Eq.1) is as follows:

whereρis the habitat density of sea cucumber,Nis the number of sea cucumbers in the target sub-division,andSis the area of the target sub-division. The unit of habitat density of sea cucumber is inds./10 m2.
2.6.2 Habitat proportion of two sea cucumber species
The total number ofH.edulisandS.chloronotusin three transects was counted every month. The number of them in the six habitat types was also counted separately, and then the result was transformed to the proportion of the target sea cucumbers in a certain habitat type in all of the population. The formula(Eq.2) is as follows:

wherePis the proportion of the target sea cucumbers in a certain habitat type in a certain month,nis the number of the sea cucumbers in a certain habitat type in a certain month, andNis the total number of sea cucumbers in the corresponding month.
2.6.3 One-way ANOVA
One-way ANOVA was used to analyze the diff erences of total number ofH.edulisvs.S.chloronotus, the number ofH.edulisandS.chloronotusin dry season vs. rainy season, and the habitat density ofH.edulisandS.chloronotusin diff erent transects and habitats. Then, one-way ANOVA was also used to analyze the habitatdiff erences between the sandy habitat and coral reef habitat of the three transects under the premise that the data conforms to normal distribution, homogeneity of variance, and independence. R version 4.0.3 was used for the above statistical analyses.
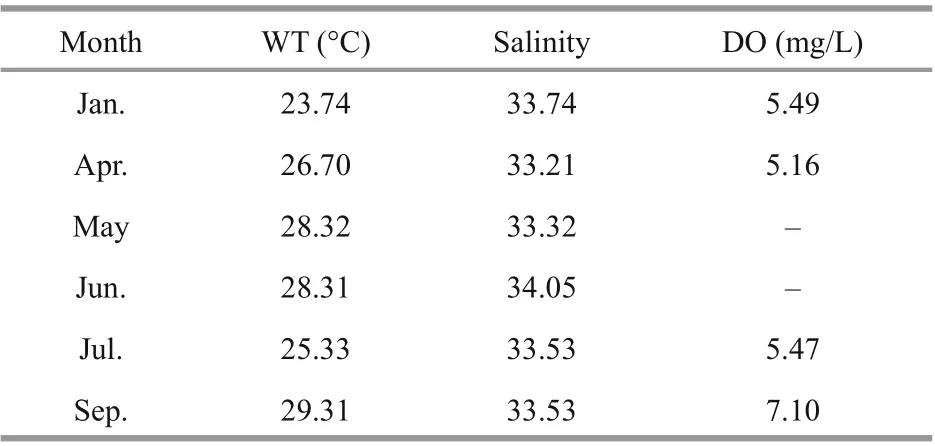
Table 2 Water quality characteristics of Wuzhizhou Island in 2019
2.6.4 Correlation analysis
R version 4.0.3 was used to analyze the correlation between the number ofH.edulisand six habitat factors in each transect, and the method used was Spearman’s correlation coeffi cient.
3 RESULT
3.1 Water quality characteristics of the studied sea area
During the seven monitoring months in 2019, the water temperature (WT) varied from 23.74 °C to 29.31 °C, with the lowest water temperature existed in January and the highest in September. In July, the water temperature showed a relatively low period due to the dominance of an upwelling cold water mass invasion at the east coast of Hainan Island. The salinity ranged from 33.21 to 34.05, with no remarkable diff erence in the monthly survey. The range of dissolved oxygen (DO) was 5.16-7.10 mg/L, with the highest in September and the lowest in April (Table 2).
3.2 Habitat profiles of the three transects
G transect is located at the west. The proportion of sand coverage (SA) and sand continuity (SC)increased with the distance from the shore and water depth, whereas the proportion oflive coral coverage(LC) and reef coverage (RC) decreased. The reef undulation degree (RU) is large in G transect. No dead coral rubble (DC) was observed in the first 10 m of the transect.
H transect is located in the middle, with the sandcoverage (SA) accounted for the lowest proportion among three transects, while the reef coverage (RC)accounted for the highest proportion among three transects. The sand continuity (SC) of this transect is the lowest (sand continuity of six sub-divisions is 0),while the reef undulation degree (RU) is the largest.No dead coral rubble (DC) was observed in the habitat within 60-80 m, and the proportion oflive coral coverage (LC) decreased with the increase of water depth.
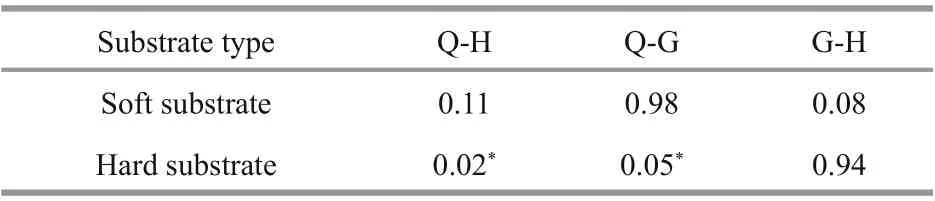
Table 3 P values of the diff erence of the substrate habitats among three transects
Q transect is located in the northeast corner of Wuzhizhou Island. The proportion of sand coverage(SA) gradually increased with the distance from the shore, while the proportion of reef coverage (RC)decreased. The sand continuity (SC) fluctuates greatly in the second half of the transect, and the reef undulation degree (RU) is lower than other two transects. No dead coral rubble (DC) was observed in the 20-30-m habitat. The proportion oflive coral coverage (LC) among diff erent sub-divisions in the transect is relatively stable (S2=0.025).
The results of one-way ANOVA showed that there was diff erence between the soft substrate of H and Q,H and G transects, but not statistically significant(P= 0.11 for H-Q and 0.08 for H-G), while a high similarity was found between transect Q and G(P= 0.98). For hard substrate, there was significant diff erence between Q and H, Q and G transects(P= 0.02 for Q-H and 0.05 for Q-G), while the similarity of hard substrate of G and H transects was high (P= 0.94) (Table 3).
3.3 Seasonal variation of population density of two species
The population density forHolothuriaedulisandStichopuschloronotusvaried from 2.0-8.9 and 0.2-0.7 inds./10 m2, respectively. The overall density ofS.chloronotuswas significantly lower thanH.edulis(t-test,P<0.001). The density of two species showed a diff erent dynamic change throughout the sampling period. The density ofH.eduliswas low in winter,and gradually increased in spring and summer,reaching a peak value in August. The density variation ofS.chloronotuswas not obvious from January to September, only showing a slight increase from June to September. Meanwhile, both of the two species showed a significantly higher population density in rainy season than in dry season (t-test,P= 0.021 forH.edulisand 0.008 forS.chloronotus). In rainy season, the average population density ofH.edulisandS.chloronotuswere 7.99 and 0.66 inds./10 m2,respectively. In dry season, the average population density ofH.edulisandS.chloronotuswere only 4.40 and 0.36 inds./10 m2, respectively (Fig.5).
3.4 Spatial variation of population distribution of two species
There was a spatial variation in the number ofH.edulisandS.chloronotusamong three transects.The results of one-way ANOVA show that the number ofH.edulisin G and H transects was higher than that in Q transects, but it was not significant (P>0.05). The number ofH.edulisin G and H transects within 50-100 m was much higher than that in Q transect, and the number ofH.edulisin G transect was the highest among sampling months (Fig.6a). ForS.chloronotus,the transect of Q that located near the rocky coast at the east had the greatest number ofindividuals than the other two transects’, and the results of one-way ANOVA are significant (P<0.05). The number ofS.chloronotusin Q transect showed a small-clustered distribution in the range of 20-70 m, while it was the least in G transect and absent in April and May (Fig.6b).In addition, the patching distribution phenomenon ofS.chloronotuswas more frequent in rainy season.
3.5 Eff ect of depth gradient on the distribution of two species
The mean depth of the transects ranged 3.3-10.7 m.We divided the transects into 10 sub-districts at the interval of 10 m from shore side as the start point, and each one had a population density that corresponds to depth. Results show that higher density ofH.eduliswas found in deeper water, mainly distributed in the depth of 4.5-10.7 m (Fig.7a), but most ofS.chloronotuslived in the depth of 4.2-8.5 m, and there has no obvious relationship between density and depth (Fig.7b).S.chloronotustended to be distributed in patch, and their number could reach up to 9 in one sub-district. The population density ofH.edulisincreased with the water depth; for example, the population density in the first sub-district (near the shore side) was 0.15 inds./10 m2, and increased to 1.16 inds./10 m2in the last sub-district (Fig.7a). When rainy season arrived in July, the density ofH.edulisin the depth of 6.4-10.7 m increased dramatically,indicating that moreH.edulisaggregated in deeper water (Fig.7a).
3.6 Habitat preference and seasonal changes of the two species
The density of the two sea cucumber species at each habitat type varied significantly among diff erent months throughout the sampling period. There was a distinct diff erent temporal dynamic change between two species.
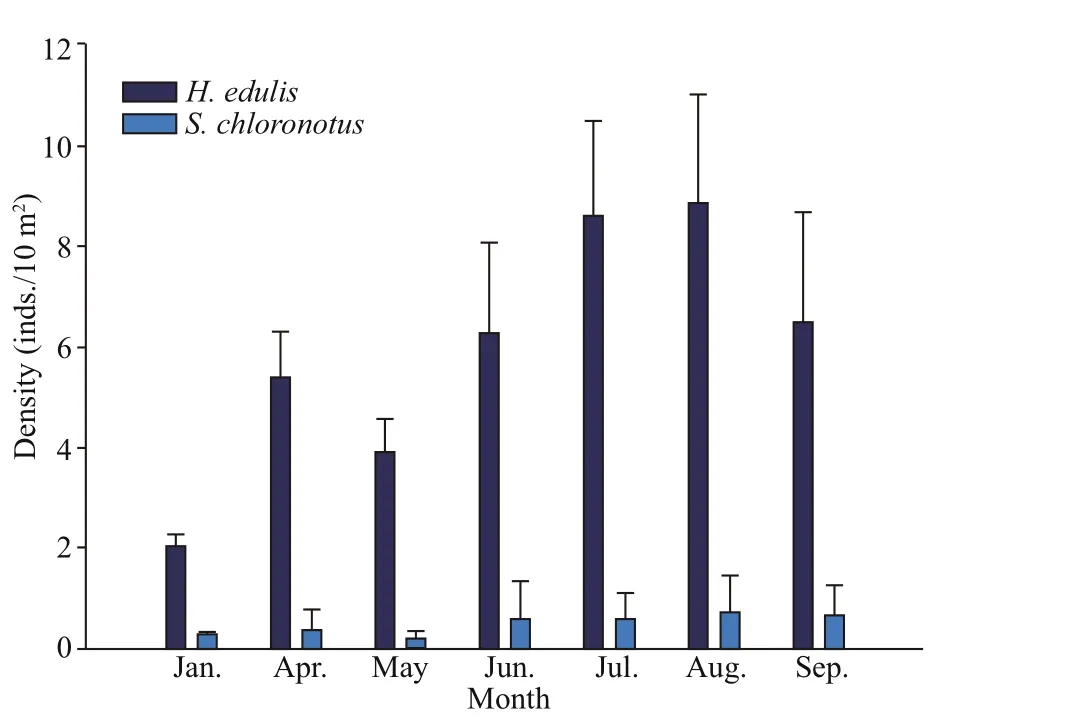
Fig.5 Monthly population density of Holothuria edulis and Stichopus chloronotus
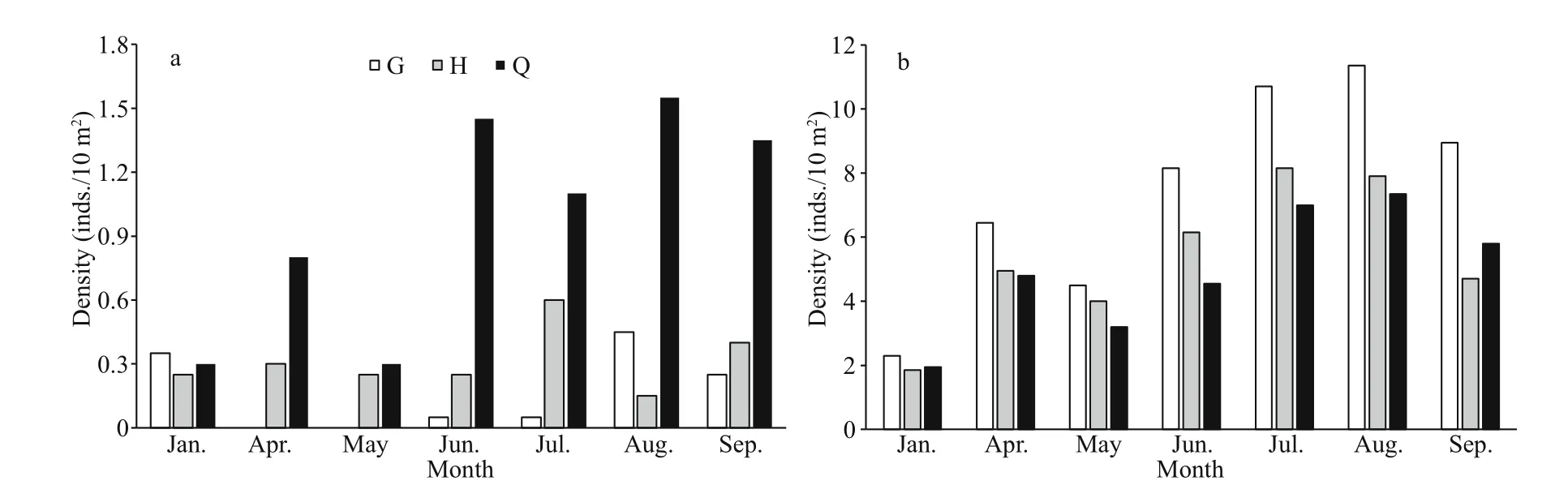
Fig.6 Monthly number of Holothuria edulis (a) and Stichopus chloronotus (b) in three transects
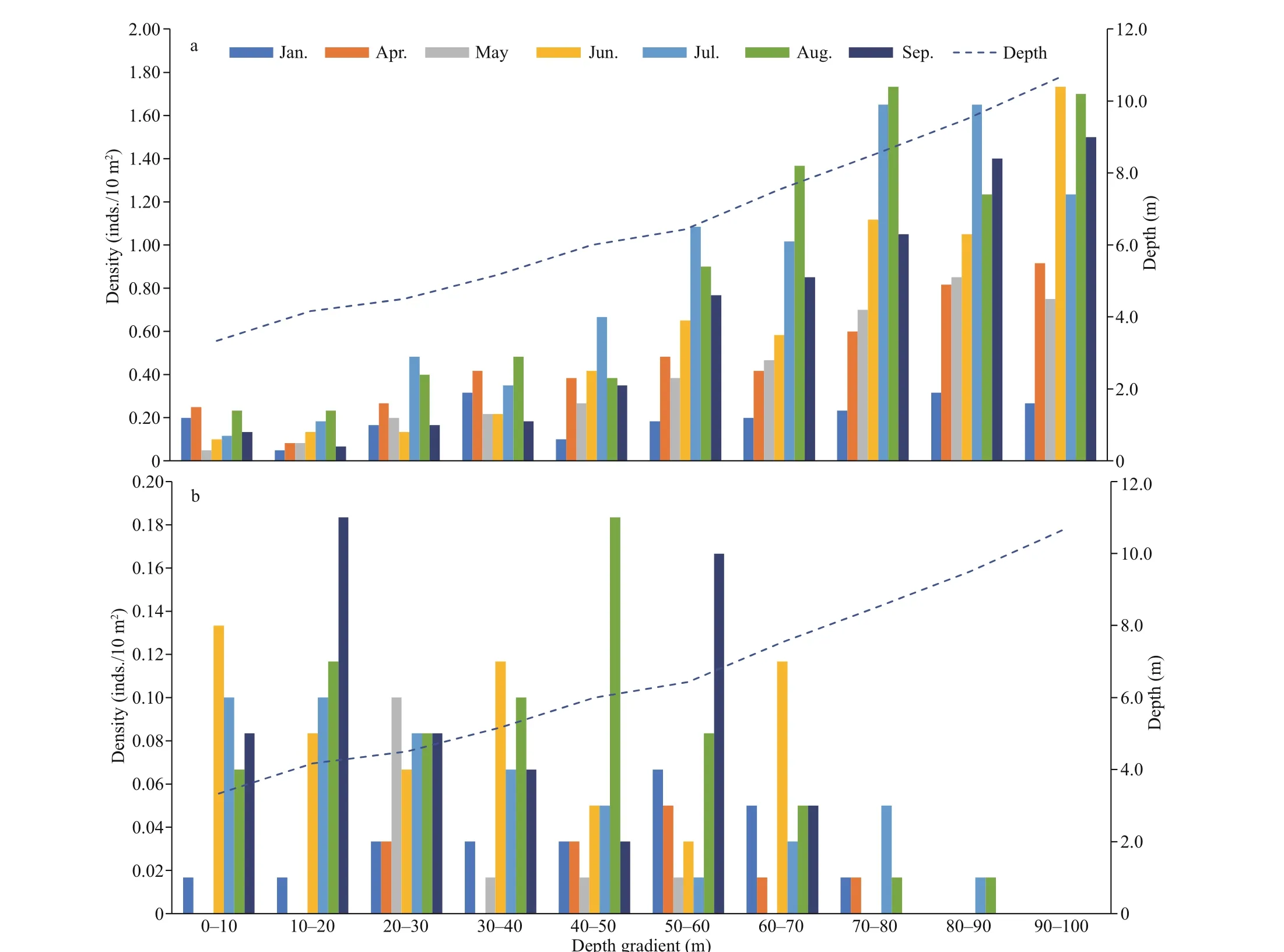
Fig.7 Monthly relationship between the population density of Holothuria edulis (a) and Stichopus chloronotus (b) with depth gradient
3.6.1Holothuriaedulis
In dry season, sand (S), small rock (SR), and dead coral rubble (DCR) were the preferred habitats and the number ofH.edulisinhabited accounted for about 88.5%. In January, over half of theH.edulisindividuals lived in the S (52%) followed by the DCR (21%). From April to July, SR was another most preferred habitat ofH.edulis, accounting for 25.3%-37.2%. As the rainy season came at July,small coral reef (SCR) became one of the preferred habitats ofH.edulis, and its population proportion gradually increased from 9.5% to 37%. The proportion of S and SR habitats decreased in varying degrees. In August and September, 32.3%-35.2% ofH.edulislived in DCR habitat. The habitat preference diff erence ofH.edulisbetween dry season and rainy season was realized through monthly migration(Fig.8a).
In G transect, the results of Spearman’s correlation coeffi cient showed that there was a strong negative correlation between the number ofH.edulisand the live coral coverage (LC) in each month (P<0.05). The number ofH.edulisper month was positively correlated with sand coverage (SA) and sand continuity (SC), and negatively correlated with reef coverage (RC) (P<0.05). In Q and H transects, the monthly number ofH.eduliswas negatively correlated with the live coral coverage (LC) (P<0.05), but no significant correlation with other habitat variables. In all three transects,H.edulishas no significant correlation with reef undulation degree (RU) and dead coral rubble (DC) (P>0.05) (Table 4).
3.6.2Stichopuschloronotus
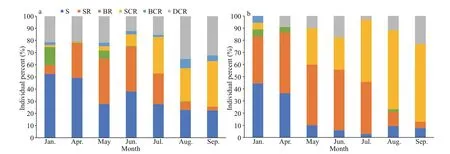
Fig.8 The proportion of Holothuria edulis (a) and Stichopus chloronotus (b) in six habitat types in each month
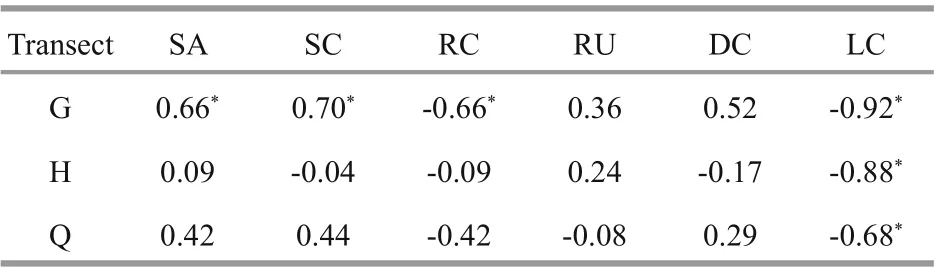
Table 4 Correlation between H. edulis and habitat factors
Comparatively, the total observed number ofS.chloronotuswas much less thanH.edulis(201 vs.2 489), and it showed a diff erent habitat preference and temporal dynamic. In dry season, the preferred habitats ofS.chloronotuswere small rock (SR), sand(S), and small coral reef (SCR), and the number of them account for about 87%. The most preferred habitat ofS.chloronotusin dry season is SR, the number ofit in January, April, May, and June accounted for 38.9%, 50%, 50%, and 50%,respectively. Meanwhile,S.chloronotusliving in sand (S) habitat reduced remarkably (44.4%-5.9%)from January to June, while it gradually increased in dead coral rubble (DCR) habitat (0-17.6%). In rainy season, SCR habitat was the most preferred habitat ofS.chloronotus, accounting for 51.4%-65.1% of the total population. The proportion ofS.chloronotusin SR habitat decreased remarkably (42.9%-5.1%),while the proportion ofit in DCR habitat increased gradually (2.9%-23.1%). The proportion ofS.chloronotusin S habitat was less than 10%.Meanwhile, this species rarely selects big rock (BR)and big coral reef (BCR) habitats (total≤2.3%). The habitat preference diff erence ofS.chloronotusbetween dry season and rainy season was also realized through monthly migration (Fig.8b).
By observing the proportion ofS.chloronotusin each habitat type, we can conclude thatS.chloronotushas obvious preference for hard bottom habitat, and mainly concentrated in SR and SCR habitats.
4 DISCUSSION
4.1 Seasonal population density variation of the two species
The study found that the population density ofHolothuriaeduliswas significantly higher than that ofStichopuschloronotus, and it may because of the strong adaptability or a higher reproductive effi ciency ofH.edulis. Another possible reason is thatS.chloronotushas a high economic and edible value,and “sea cucumber rush” raised in recent years has caused the overfishing ofit. Bellchambers et al.(2011) found that the population densities ofH.edulisandS.chloronotusin the coral reef habitat of the tropical Indian Ocean were only 0.05 and 0.04 inds./10 m2, which is much lower than that in our study sea area. It is reported that there existed overfishing ofS.chloronotusin the Indian Ocean(Dissanayake and Stefansson, 2010), whereas our study area is well protected by the Tourism Management Department of Wuzhizhou Island. The compound habitat ofliving coral reefs and sandy bottom in the northern part of the island showed a highly habitat heterogeneity, which is suitable for the growth of sea cucumbers.
The results show that the population density of two species varied among seasons. Both of the two species showed the lowest population density in winter(January), and the population gradually increased with the rising of water temperature. In winter, the study area is aff ected by the northeast monsoon,leading to a hostile water environment with turbulent flow. Sea cucumbers tend to migrate to deep water to find mild habitats, so the number of sea cucumbers in the fixed transects was low. In addition, the population density of the two species was quite diff erent in dry season and rainy season, which may be related to the food richness. Li et al. (2015) found that there are many kinds of macroalgae in the adjacent coastal area of the Haitang Bay in Sanya. Abundant rainfall in rainy season brought a large amount of fresh water with rich nutrients into the sea, consequently supported the bloom of the macroalgae and pelagic and benthic microalgae, supplying suffi cient food for sea cucumbers.
4.2 Reasons for spatial distribution diff erence of the two species
The results of one-way ANOVA show that the number ofH.edulisin G and H transects was more than that in Q transect (P>0.05), and the number ofS.chloronotusin Q transect was significantly higher than that in G and H transects (P<0.05). It probably relates to the habitat heterogeneity of the three transects. A similar phenomenon was also reported on the species ofStichopusherrmanniin Shark Alley(Australia) with significant population variation among sites, which was related to habitat heterogeneity(Eriksson et al., 2013). We found that moreH.edulisdistributed in deeper water and the number ofH.edulisin G and H transects within 50-100 m was remarkably higher than that in Q transect. Water flow and sediment type can influence the habitat selection ofH.edulis(Dar and Ahmad, 2006; Dissanayake and Stefansson, 2012), and the deeper water area in the north of the island is mainly sandy bottom. The water flow in G and H transects is relatively stable, which are very suitable for the survival ofH.eduliswith sedimentary food habit and hiding behavior. However,Q transect is located in the northeast corner of the island with turbulent flow.
Meanwhile, as a tourist area, the species density diff erence in our study may also be related to human activities. There are frequent diving and sea sport activities in the shallow water area of G and H transects, which may drive sea cucumbers to migrate to deep water or other places not aff ected by human activities. Another possible reason may relate to the weak tube foot adsorption ability ofH.eduliscompared withS.chloronotus, so it cannot stay firm on sand bottom with turbulent water flow. Interestingly,S.chloronotuswith strong adsorption foot mainly distributed in Q transect with more hard substrates and strong waves. In January, April, and May, the population number ofS.chloronotuswas very small among all of the study areas, while in June to September, it increased significantly and appeared small-scale cluster distribution, which may be related to patchy food available. Many sea cucumber species show cluster distribution due to food source, seasonal changes, and habitat types, such asStichopusjaponicusshowed obvious aggregation in summer and relative dispersion in winter (Zhang, 2015).Besides,S.herrmanniwith a homogenous distribution showed dense aggregates in some occasions (Eriksson et al., 2013).
4.3 Relationship between sea cucumber distribution and water depth gradient
The population distribution of the two species varied with water depth. It shows thatH.eduliswhich preferred coral rubbles and sandy bottom likes living in a deeper water area, with the highest density of 2.65 inds./10 m2. However, water depth showed no remarkable impact on the distribution pattern ofS.chloronotus, and they preferred patchy distribution.Dissanayake and Stefansson (2012) found that the distribution ofH.eduliswas greatly aff ected by depth,substrate type, and sediment. In our study, the area of sandy bottom and the sediment increased in deeper water, which is more attractive toH.edulis. It was found that most of theH.edulisindividuals in deep water were large and directly exposed to the sandy bottom by the underwater video, and they need not hide because of the abundant food sources and stable water flow. Conand (1993) found that the size ofS.herrmanniincreased with depth and suggested this species migrated from shallow to deep water with growth, and so might theH.edulis. Lee et al. (2008)noted that the growth ofH.atrahas striking plasticity,and the body size increased by 300% after moving to areas with high food content. This may also be the reason for the larger size ofH.edulisin sandy bottom with rich sediments. However, some scholars point out that few sea cucumbers are directly exposed to the open sand (Moriarty, 1982; Massin and Doumen,1986), and we believe that it may relate to the variation of environmental factors in diff erent study areas.
The patchy distribution pattern ofS.chloronotusis the result of the interaction of multiple factors.Uthicke and Karez (1999) and Bellchambers et al.(2011) also found that theS.chloronotusin the coral reef of Cocos (Keeling) Islands distributed in patches.Many scholars have found that the wild sea cucumber population will show uneven patchy distribution due to the combined eff ects of biotic and abiotic factors(Woo et al., 2010; Morgan, 2011; Mmbaga, 2013;Navarro et al., 2013). Uthicke and Karez (1999) also pointed out thatS.chloronotuswould actively select sediments. The habitat types are scattered in three transects andS.chloronotusare distributed in patches,which may be related to its feeding selection.
In summary, the distribution of the two sea cucumber species with depth gradient may be the comprehensive influence of depth, sediment types together with food patches.
4.4 Reasons for habitat preference and seasonal changes of two species
Many foreign scholars pointed out that diff erent sea cucumber species have diff erent habitat preferences (Conand and Mangion, 2002; Conand,2008; Bellchambers et al., 2011). In the current study,the preferred habitat also showed a distinct speciesspecific character, and seasonal variation was obvious.Conand (2008) and Dissanayake and Stefansson(2012) found that theH.edulishad a distinct aggregation phenomenon in the coral reef and rocky reef area. In this study, theH.edulisalso showed an evident preference for small rock (SR) and small coral reef (SCR), which may be related to the hidden behavior. According to underwater video and divers’observation,H.edulisin shallow water hides inside coral reefs or under reefs to avoid adverse sea conditions, such as the torrent surge in January. The hidden behavior has been confirmed inHolothuriadiffi cilisandApostichopusjaponicus(Conand and Mangion, 2002; Zhang et al., 2015). In addition,H.edulisin this study has a clear preference for sand(S) habitat, which may be related to the feeding habits.H.edulisprefer to eat coarse-grained sediments(Dissanayake and Stefansson, 2012), while S habitat is rich in coral sand. The result of correlation analysis betweenH.edulisand six habitat variables is consistent with other scholars:H.eduliswill live in hard habitats, while also like to ingest sediments in sandy bottom. It is worth noting that there was a strong negative correlation betweenH.edulisand live coral coverage (LC) in all three transects (P<0.01),and the influence of LC is remarkably greater than that of the other five habitat factors, which indicates that theH.eduliswill deliberately avoid the places with living corals, such as small coral reef (SCR) and big coral reef (BCR).
Stichopuschloronotusprefers small rock (SR) and small coral reef (SCR) habitats in this study, which was consistent with the research conclusion of other scholars (Bellchambers et al., 2011; Zhang et al.,2015). In addition, the habitat preference of sea cucumbers is diff erent in varying life history stages.Eriksson et al. (2012) found the juvenileS.chloronotuspreferred soft-bottom habitat, while adultS.chloronotuspreferred to live in hard bottom habitats such as rocks and coral reefs, which may be related to its feeding habits and avoidance of current. We found part ofS.chloronotuslikes feeding on the rocks and coral reefs (with excrement nearby) according to underwater video and divers’ observation, while others hide in hard bottom habitat. Uthicke (1994)found the water flow is the main factor aff ecting the distribution ofS.chloronotus, and they tend to live on reef flat with steady water flow. Hard habitats such as coral reefs and rocks are good attachment substrate and can eff ectively block part of the water flow,providing a mild living place forS.chloronotus.Moreover, Uthicke (1999) and Uthicke and Karez(1999) found thatS.chloronotuswould actively select food patches and ingest sediments with high microalgae content. There are abundant microalgae in SR and SCR habitats (Zhang et al., 1995), which is more attractive toS.chloronotus.
Both species showed diff erent changing processes during the seasonal changes. Changes in environmental factors (Ocean current, sediment source, precipitation,and temperature) were not instantaneous, therefore,the change of sea cucumber’s preferred habitat was also gradually migrated. The main habitat of the two species was S in January, and it gradually moved to SR and SCR after April, which may be related to avoiding hostile sea conditions and choosing food patches. In January, both species tended to migrate to deeper waters to seek shelter against violent waves.The individual size ofS.chloronotusis large, and the ventral tube foot can make it firmly adsorb on the hard substrate. Therefore,S.chloronotusalso showed a preference for SR habitat in January and April. The preference of sea cucumbers for reefs with microalgae was obvious. Uthicke and Karez (1999) found thatS.chloronotushad a higher requirement on microalgae content in habitat patches. Zhang et al. (2015) further confirmed it in the behavior ofA.japonicus. In our study area, abundant rainfall in the rainy season is conducive to the growth of algae, consequently bringing abundant algae-derived detritus food to the sediment. Therefore, the number of sea cucumbers in small rock (SR) and small coral reef (SCR) habitats gradually increased from June to September,especially theS.chloronotus. Comparatively,H.edulisstill preferred sand (S) habitat for its feeding selection of coarse-grained sediments.
5 CONCLUSION
In this study, the population density ofH.eduliswas significantly higher than that ofS.chloronotus,and the density ofH.edulisincreased with the depth of water. In winter, sea cucumbers tended to migrate to deep water due to poor sea conditions, and then the density of two target sea cucumber species increased with the increase of temperature and abundant food source in rainy season.H.edulispreferred soft substrates with stable water flow, whileS.chloronotuspreferred hard substrates and distributed in small clusters in rainy season. The preferred habitats ofH.edulisandS.chloronotuschanged seasonally and gradually migrated. The shift from dry season to rainy season was the key factor aff ecting their habitat preference. The results of correlation analysis show thatH.eduliscould deliberately avoid the habitats with living corals, such as small coral reef (SCR) and big coral reef (BCR). These results are helpful to analyze the seasonal variation of the two sea cucumber species to restore a specific coral reef ecosystem, and to assess their importance as ecological restoration species.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are not publicly available as the datasets also forms parts of an ongoing study, but are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
The authors are grateful to Fengguo WANG and Zhigang ZHOU from Wuzhizhou Island Tourism Company for their help in fieldwork.
 Journal of Oceanology and Limnology2022年4期
Journal of Oceanology and Limnology2022年4期
- Journal of Oceanology and Limnology的其它文章
- Validation and error analysis of wave-modified ocean surface currents in the northwestern Pacific Ocean*
- The energy conversion rates from eddies and mean flow into internal lee waves in the global ocean*
- Observation of physical oceanography at the Y3 seamount(Yap Arc) in winter 2014*
- Decadal variation and trend of the upper layer salinity in the South China Sea from 1960 to 2010*
- Observations of turbulent mixing and vertical diff usive salt flux in the Changjiang Diluted Water*
- Experimental research on oil film thickness and its microwave scattering during emulsification*
