The key environmental factors driving the succession of phytoplankton functional groups in Hongfeng Reservoir,southwest China*
Libin HAN , Qiuhua LI ,**, Wensheng CHEN , Xing WANG , Shihui ZHOU ,Mengshu HAN , Anton BRANCELJ
1 Key Laboratory for Information System of Mountainous Area and Protection of Ecological Environment of Guizhou Province,Guizhou Normal University, Guiyang 550001, China
2 Guizhou International Science & Technology Cooperation Base-International Joint Research Centre for Aquatic Ecology,Guizhou Normal University, Guiyang 550001, China
3 Key Laboratory for Information and Computing Science of Guizhou Province, Guizhou Normal University, Guiyang 550001,China
4 National Institute of Biology, Vecna pot 111, Ljubljana 1000, Slovenia
Abstract Reservoirs are an important water source in many densely populated areas in southwest China.Phytoplankton play an essential role in maintaining the structure and function of reservoir ecosystems.Understanding the succession in phytoplankton communities and the factors driving it are essential for eff ective water quality management in drinking water reservoirs. In this study, water samples were collected monthly at the surface layers from March 2016 to December 2019 in Hongfeng Reservoir, southwest China.The relationship between functional group succession was analyzed based on nonmetric multidimensional scaling analysis (NMDS), redundancy analysis (RDA), succession rate, and other analysis methods. The results showed distinct shifts in the community structure of phytoplankton functional groups within study period. The Cyclotella sp. was dominant in 2016 and 2017, and Pseudanabaena limnetica was the dominant group in 2018 and 2019. It appears that the phytoplankton composition and biomass are closely related to the water temperature and nutrient status in this reservoir. The results clearly showed that the permanganate index (COD Mn) was the key factor of dramatic phytoplankton functional group succession, and the change in succession rates was closely caused by total nitrogen concentration (TN). Therefore, the succession pattern and key factors of Hongfeng Reservoir revealed in this study were important guidance for the management of drinking water reservoirs in southwest China. A reasonable limit on exogenous nutrient input should be a priority, especially in high water temperature period.
Keyword: phytoplankton functional groups; driving factors; nonmetric multidimensional scaling; succession rates; Redundancy analysis
1 INTRODUCTION
Currently, water ecosystems worldwide are aff ected by eutrophication and water resource degradation (Ho et al., 2019). With the increasing frequency of algal blooms, a growing number of ecologists are focusing on ways to control this phenomenon (Niu et al., 2011;Nwankwegu et al., 2020; Yao et al., 2020). As the primary producer in the water ecosystem, the phytoplankton community composition and biomass variation can directly and quickly reflect changes in the water environment, which usually reveals the degree of eutrophication of the water body (Yang et al., 2016; Kozak et al., 2020). The traditional phytoplankton classification method can reflect the community structure well, but it has some limitations regarding environmental and ecological characteristics.Therefore, functional groups are used to assess water quality and are applicable to lakes and reservoirs, as well as rivers. Phytoplankton functional groups were proposed by Reynolds et al. (2002), based on similarities in the functions of phytoplankton communities in aquatic ecosystems, and supplemented and improved by Padisák et al. (2009). At present, this method is widely used to analyze the relationship between environmental variables and phytoplankton succession in reservoirs, lakes, and rivers (Katsiapi et al., 2011; Demir et al., 2014; Zhu et al., 2020).
Succession is a directional change determined by diff erences in the growth rate of the community,which leads to changes in the biomass of each species.Since the growth cycle of phytoplankton is short(Zhao et al., 2015), succession rate can be used to analyze the process of phytoplankton change. The succession rate can represent the gradual and abrupt changes of phytoplankton (Romanov and Kirillov,2012), and the succession rate based on the biomass of phytoplankton functional groups can better reflect the composition of community structure and environmental ecological characteristics. In addition,the succession of phytoplankton functional groups is closely related to the growth strategy of phytoplankton(Crossetti and de M Bicudo, 2008), which can further explore the internal relationship between phytoplankton and environmental factors during the succession process (Biggs and Stevenson, 1998).
Hongfeng Reservoir is an important water supply reservoir in southwest China, used mainly for drinking water (Lv et al., 2014). Therefore, the development of the reservoir depends not only on water availability but also on water quality. Therefore,it is essential to study the water quality changes and provide theoretical support for water quality protection. Hence, the objectives of this study were as follows: 1) to study the community structure and adaptive strategies of phytoplankton; 2) to identify the key environmental factors of phytoplankton succession; and 3) to explore the main environmental factors aff ecting the succession rates.
2 MATERIAL AND METHOD
2.1 Study area
Hongfeng Reservoir (106°19′E-106°28′E,26°26′N-26°35′N), located in the southwest of China,is the largest karst artificial lake in Guizhou, located upstream of the Maotiao River, the main tributary of the Wujiang River. The water storage area and capacity are 57.2 km2and 6.01×108m3. The maximum depth of the reservoir is 45 m, the average depth is 10.52 m, and its drainage area covers 1 551 km2. For other characteristics of the reservoir refer to Li et al.(2018). It is an important drinking water source in Guiyang and plays an important role in power generation, flood control, irrigation, and tourism(Fig.1).
2.2 Sampling and samples analysis
The water and phytoplankton samples were collected monthly at five sites (S1, S2, S3, S4, and S5) in the Hongfeng Reservoir from March 2016 to December 2019 (Fig.1). Portable multi-parameter water quality analysis tester (HANNA, HI98194,Shenzhen) was used to measure water temperature(WT), conductivity (EC), dissolved oxygen (DO),and pH in the field. Transparency (SD) was measured with Secchi disk in the field. Water samples were taken at the sites at a depth of 0.5 m beneath the water surface using a Van Dorn sampler and were collected in 1-L polyethylene bottles. The concentrations of total nitrogen (TN), total phosphorus (TP), and permanganate index (CODMn) were analyzed using unfiltered water, while filtered water was used to determine the concentration of nitrate nitrogen(NO3-N). The concentrations of chlorophylla(Chla)were determined by the acetone extraction method after a 500-mL water sample was filtered through a 0.45-μm cellulose acetate membrane. All water quality parameters were measured according to the Chinese National Standard Method (The State Environmental Protection Administration, 2002).
For the quantitative samples of phytoplankton,1.5-L surface water samples were collected in polyethylene bottles and fixed with 1% Lugol’s solution. After 24-48 h of sedimentation in the laboratory, the samples were concentrated to 30 mL by the siphon method and then identified under the biological microscope (Olympus CX43 Biological Microscope) according to the literature (Hu and Wei,2006) to quantify cell densities. For the qualitative samples of phytoplankton, samples were collected from the surface water using a #25 plankton net,trawling horizontally and vertically for 1 min.Subsequently, 3%-5% formaldehyde was added to the samples for fixation. Then laboratory identification was based on literature (Hu and Wei, 2006).
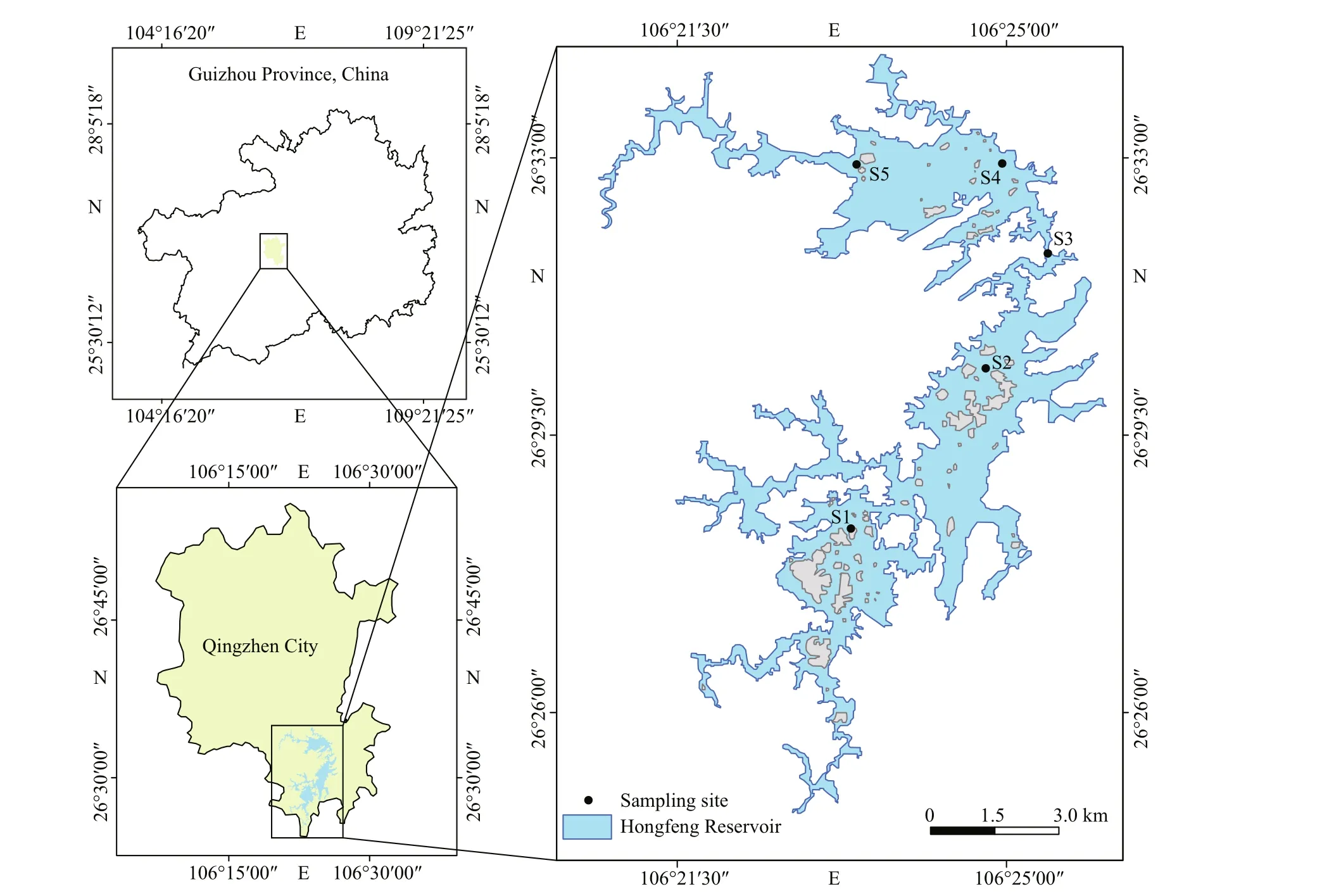
Fig.1 Geographical location of Hongfeng Reservoir and sampling sites
2.3 Data analysis
Succession rates in plankton communities may be both tractable and informative. In particular, the importance of causal factors may be analyzed by comparing the succession rates of phytoplankton in lakes diff ering in environmental equability and primary productivity (Williams and Goldman, 1975).Succession rate is measured by calculating the change in the relative contribution ofindividual species to total biomass diversity. The fraction of total diversity attributable to a single species was calculated as:
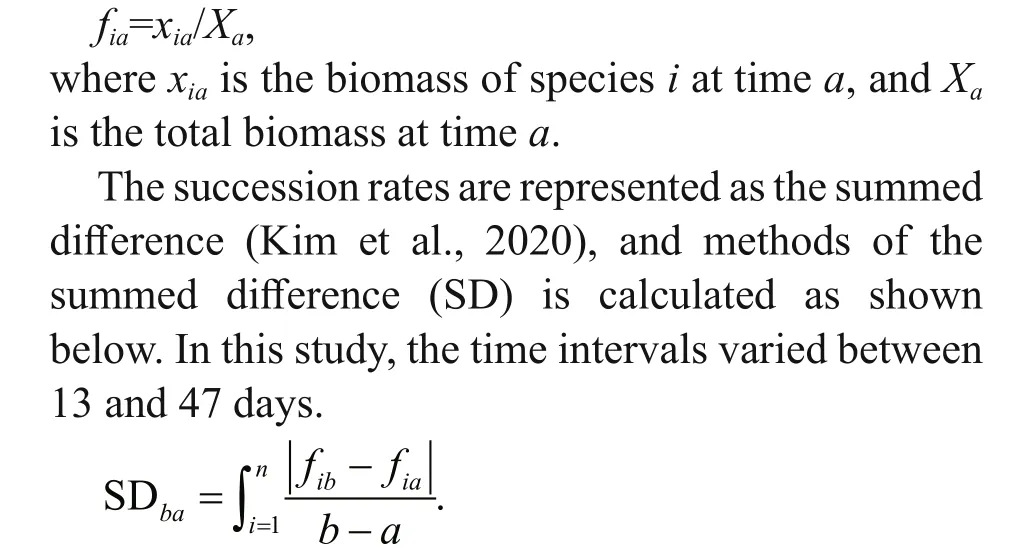
wherearepresents speciesiat timea, andbrepresents speciesiat timeb. The biomass of phytoplankton was calculated using the standard geometric formula(Hillebrand et al., 1999), and the cell was equivalent to the corresponding geometric shape to calculate the biological volume of cells. Assuming that the cell density was equal to 1, the biological volume was converted to biomass. Biomass was expressed as micrograms per liter. One-way analysis of variance(ANOVA) was used to compare the diff erences in environmental factors and phytoplankton biomass between years and to compare the diff erences in succession rates among the five sites. Pearson correlation analysis was used to analyze the correlation between succession rates and environmental factors.Redundancy analysis (RDA) was used to reveal the contribution of environmental factors to variations in the biomass of representative phytoplankton functional groups each year, and Monte Carlo permutation test the values of P and F are calculated(using a test with 999 permutations) to significance test. To extract the gradients in the phytoplankton composition over time based on the Bray-Curtis distance matrix, nonmetric multidimensional scaling(NMDS) was performed based on the biomass of functional groups. The dataset of biomass and environmental factors value (except pH) were log(x+1) transformed before analysis; RDA analysis was analyzed using the CANOCO 5.0 software package,NMDS was analyzed using the R package Vegan (R version 4.0.3), and other analyses were performed using the SPSS 20.0 statistical package software. The other charts were drawn using Origin 2020.
3 RESULT
3.1 Variations in environmental factor
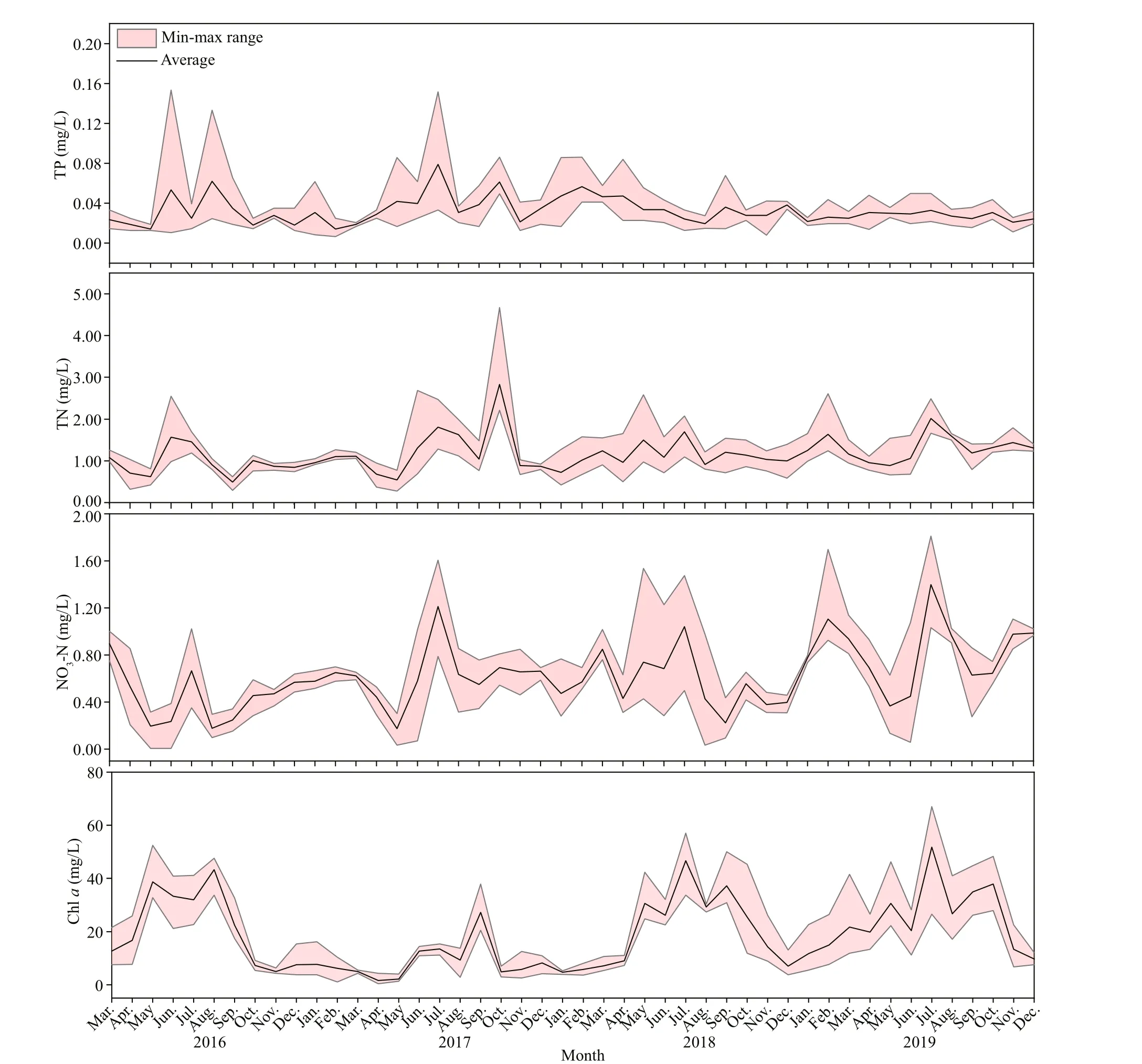
Fig.2 Mean values and min-max ranges of total nitrogen (TN), total phosphorus (TP), nitrate nitrogen (NO 3-N), electronic conductivity (EC), permanganate index (CODMn), transparency (SD), chl- a concentration (Chl a), dissolved oxygen(DO), and pH in Hongfeng Reservoir for each month from March 2016 to December 2019
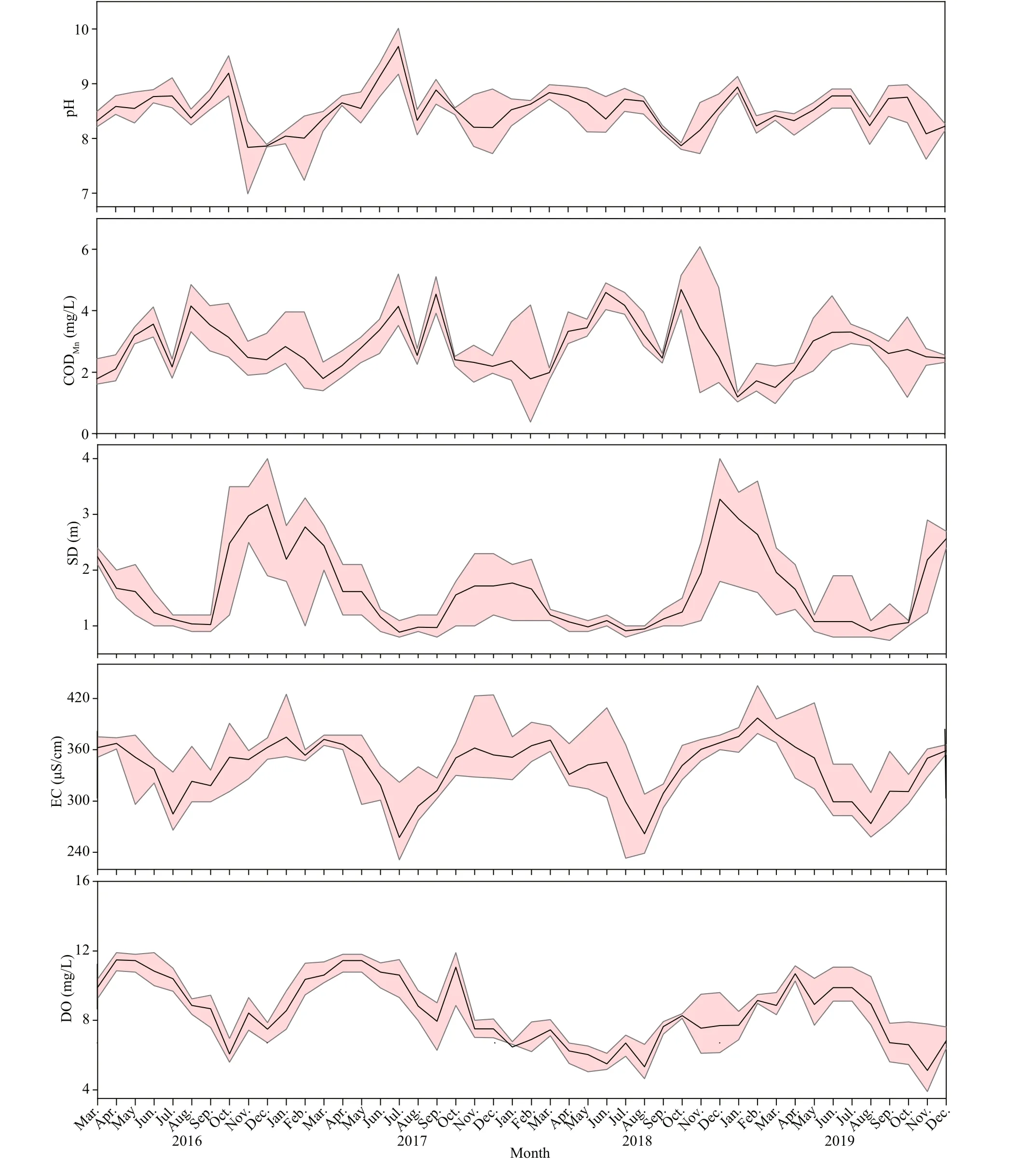
Fig.2 Continued
During the survey period (Fig.2), the monthly concentration of TN ranged from 0.28 to 4.68 mg/L,with a maximum value in October 2017, and TN concentration diff ered significantly between 2016 and 2019 (n=46;P<0.05). Monthly values of TP and CODMnranged 0.01-0.15 mg/L and 0.38-6.09 mg/L respectively, and TP and CODMnshowed significant diff erences between 2018 and 2019 (n=46;P<0.05).Water temperature and pH were higher in summer,and there were both no significant diff erences among the years. Transparency and EC were higher in spring than in other seasons, while no significant diff erences were found between the years (n=46;P<0.05). The values of NO3-N concentrations fluctuated significantly, and values in 2019 diff ered significantly from those of the other three years (n=46;P<0.05).Dissolved oxygen concentrations diff ered significantly between 2018 and 2016 and 2017, whereas Chl-aconcentrations diff ered between 2017 and 2019(n=46;P<0.05).
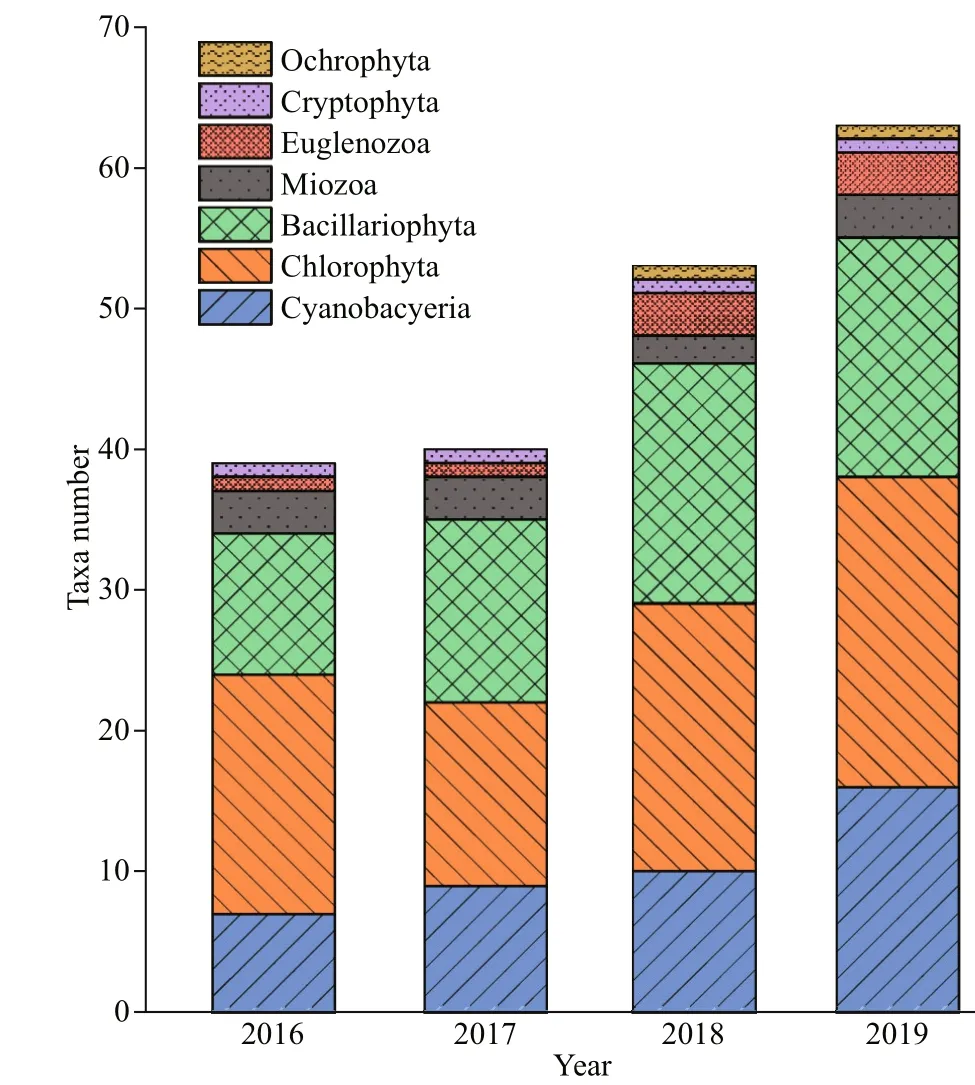
Fig.3 Numbers of phytoplankton taxa in Hongfeng Reservoir from March 2016 to December 2019
3.2 Phytoplankton dynamics
In total, about 70 phytoplankton species were identified in the study area, it varies from 39 to 40 taxa in 2016-2017 and 53-63 taxa were collected in 2018-2019, respectively. The species richness in 2019 and 2018 was greater than that in 2016 and 2017(Fig.3). The phytoplankton biomass through the study varied between 8.09 and 7 797.76 μg/L, with a minimum value in January 2017 and a maximum value in July 2019 (Fig.4). The total biomass in 2019 diff ered significantly from those of the other three years (n=46;P<0.05), and no significant diff erences were found between the other three years.
The functional groups in the reservoir were identified according to the functional group classification methods proposed by Reynolds et al. (2002) and Padisák et al. (2009). All the identified species were assigned to 26 functional groups: A, B, C, D, N, P, MP,T, TC, S1, SN, X3, X2, X1, E, Y, F, G, J, K, H1, Lo,LM, M, W1, and W2 (Table 1). Functional groups with relative biomass greater than 5% were defined as the dominant functional groups. Eight groups were defined as dominant groups: B, D, P, MP, S1, Y, J, and LM(marked with “#” in Table 1). During the study period,group B showed absolute dominance in 2016 and 2017,while its dominance was gradually replaced by group S1 in 2019. Group P was abundant in 2018, with a maximum proportion of 39.82% (Fig.5).
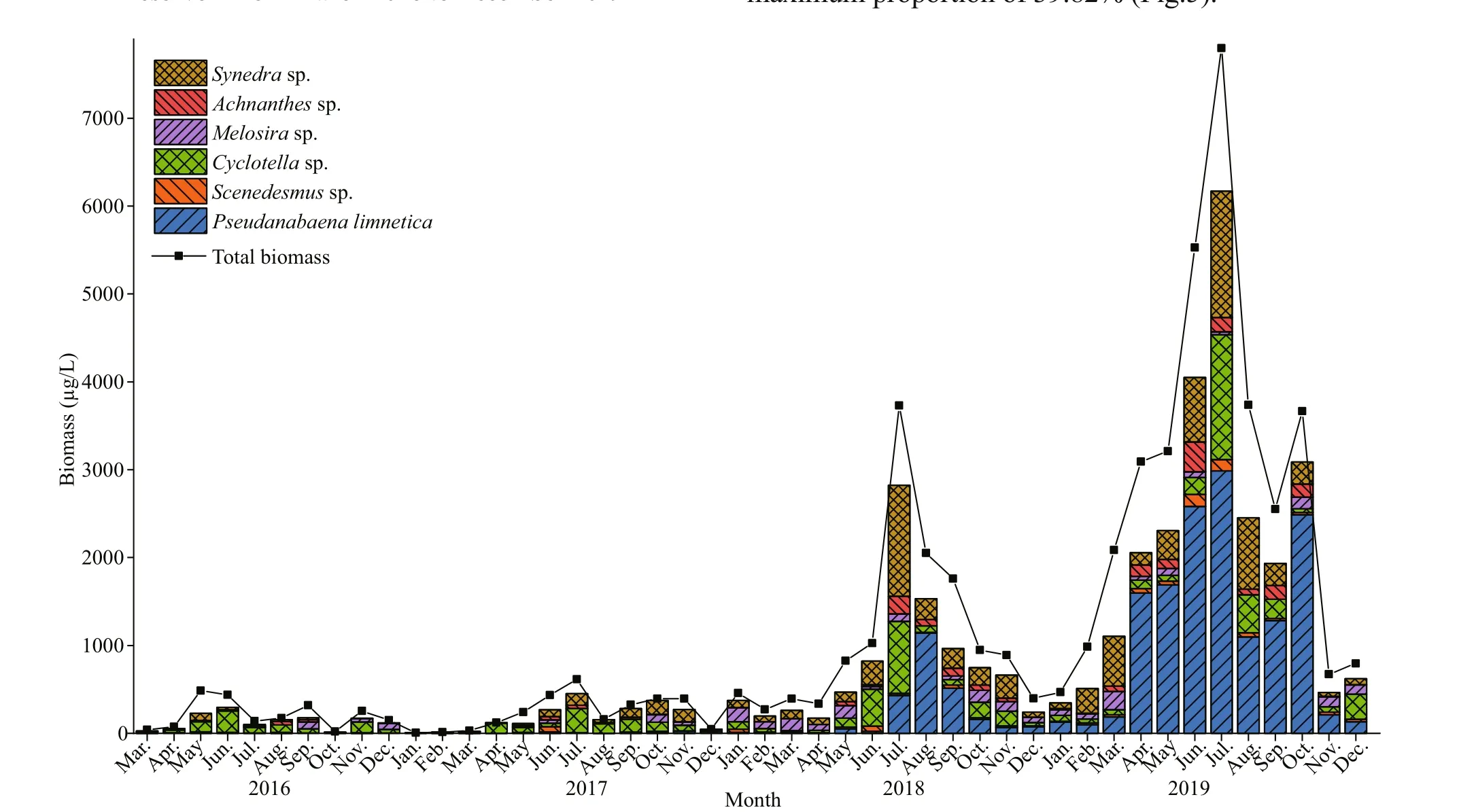
Fig.4 Monthly biomass of main species and total phytoplankton in Hongfeng Reservoir from March 2016 to December 2019
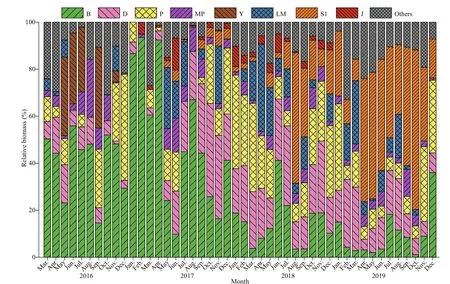
Fig.5 Monthly relative biomass of the dominant functional groups of phytoplankton in Hongfeng Reservoir from March 2016 to December 2019
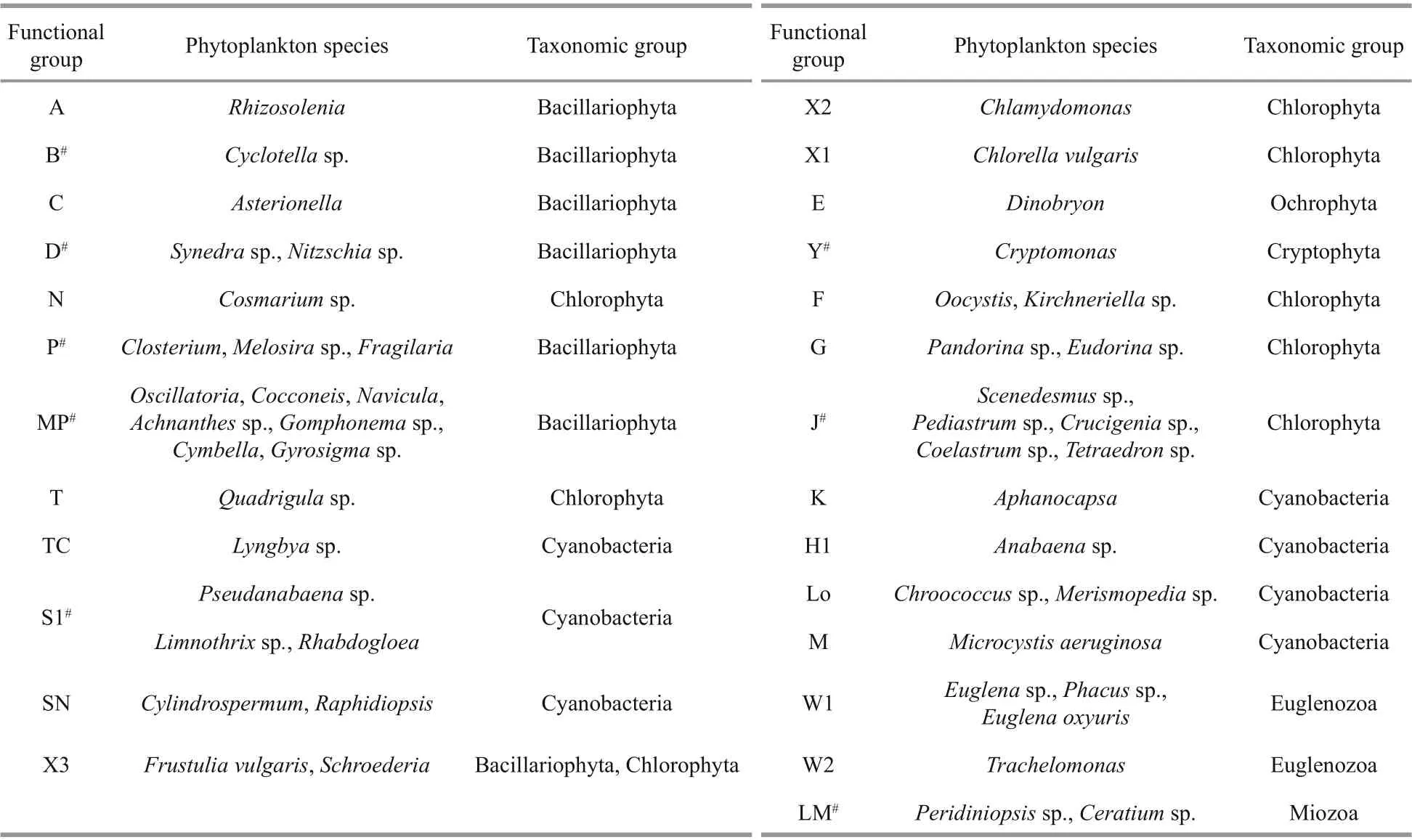
Table 1 Phytoplankton taxa and functional groups in Hongfeng Reservoir from March 2016 to December 2019
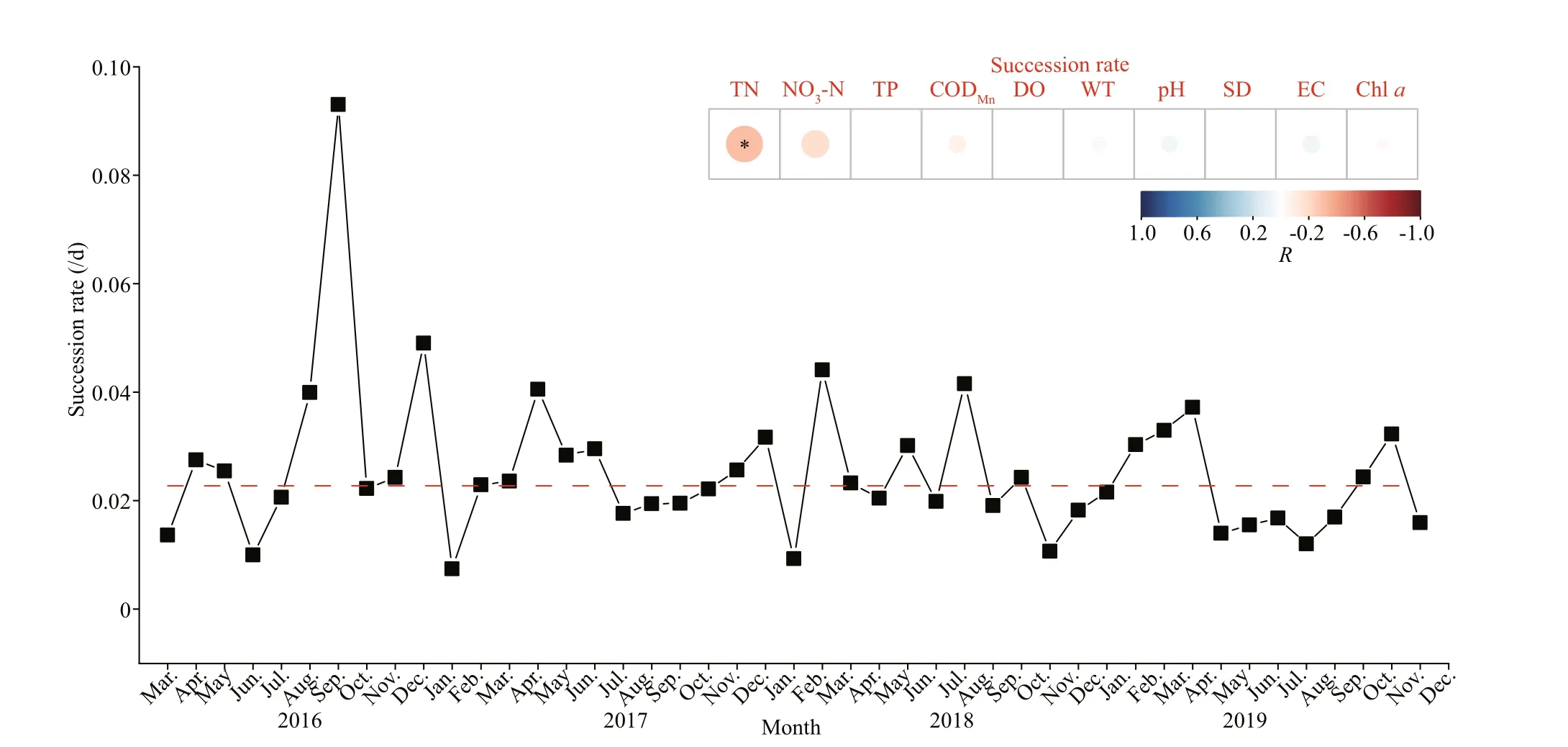
Fig.6 Monthly succession rates of functional groups from 2016 to 2019
3.3 Succession rates of functional groups
According to the results of ANOVA, there were no significant diff erences between the five sites (n=46;P<0.05). Thus, in this study, the average succession rates of the five sites were used to reflect the dynamic changes in the phytoplankton community. Diff erences in succession rates are undoubtedly related to diff erences in phytoplankton growth potential (Lewis,1978). In this study, the change in succession rates was relatively small, and the rates showed obvious seasonal changes. Succession rates increased in the summer (Fig.6). Moreover, as can be seen from the results of Pearson correlation analysis, succession rates were significantly correlated with TN (P<0.05;R=-0.315).
3.4 Redundancy analysis
The phytoplankton functional group detrended correspondence analysis (DCA) eigenvalue in this study was below 3. Therefore, redundancy analysis linear model (RDA) was applied to analyze the variation between the phytoplankton functional groups and environmental factors. Figure 7 shows the results of the RDA analysis based on the dominant functional groups and environmental factors. In 2016,axis 1 and axis 2 explained 48.17% and 27.92% of the total variation, respectively, and in 2017, the first axis explained 52.84% of the total variation, and the second axis explained 16.65%. Axis 1 and axis 2 explained 52.96% and 14.05% of the total variation,respectively, in 2018. In 2019, the percentage explained variance of axis 1 and axis 2 was 58.93%and 16.46%, respectively. The results showed that axes 1 and 2 could explain the distribution of the major phytoplankton groups.
According to the Monte Carlo permutation test,WT was the most important environmental factor aff ecting phytoplankton functional groups during the entire study period (P<0.05). Key environmental impact factors diff ered among years and the relationship between environmental factors and functional groups also altered. In 2016, WT was positively correlated with group MP, while DO showed a significant correlation with groups Y and B.WT was the most important environmental factor aff ecting groups B and MP in 2017. In 2018, WT was positively associated with group LM, while CODMnwas positively associated with groups D, MP, and B.In 2019, CODMnand WT were positively associated with groups P and S1.
3.5 The nonmetric multidimensional scaling ordination
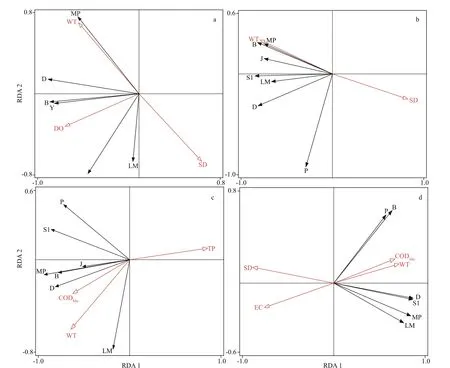
Fig.7 Results of redundancy analysis (RDA) based on the biomass of functional groups and environmental factors
The NMDS results are shown in Fig.8, where the stress values of the NMDS were between 0.02 and 0.11. The points corresponding to chronologically contiguous samples were united by lines to highlight the development over time. In 2018 and 2019, the periodicity of seasonal phytoplankton development was particularly obvious, while in 2016 and 2017, the regularity of time was complex. In 2018 and 2019, the gradient in the composition of the functional groups was strongly separated along the axis of the NMDS configurations, while the samples from 2016 and 2017 showed a disorganized time sequence. Larger distances on functional groups were found in 2016 and 2017, which showed clear diff erences between months. However, the close similarity of functional groups was observed between 2018 and 2019 due to the predominance ofPseudanabaenalimnetica.
4 DISCUSSION
4.1 Eff ects of environmental variables in phytoplankton community structure
From 2016 to 2019, the total biomass showed an increasing trend, and the maximum biomass of phytoplankton was 487.23, 615.04, 3 729.32, and 7 797.76 μg/L in 2016, 2017, 2018, and 2019,respectively. The significant biomass increased ofP.limneticaled to the annual increase of the total biomass in Hongfeng Reservoir. During the study period, the biomass in summer was significantly higher than in the other seasons because, in summer,the temperatures were higher, and solar radiation caused the surface water temperature to increase.Water temperature is an important direct environmental factor aff ecting phytoplankton growth and succession(Jiang et al., 2014). An increase in water temperature can enhance the photosynthetic rates of phytoplankton and cause an increase in biomass.
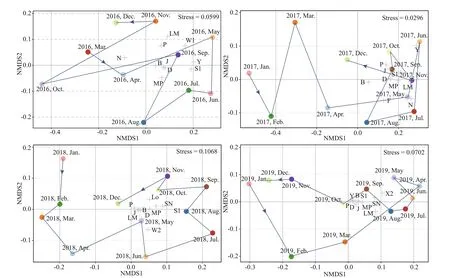
Fig.8 Results of nonmetric multidimensional scaling ordination (NMDS) based on the biomass of functional groups among years
Overall, Bacillariophyta, Cyanobacteria, and Chlorophyta were the main phyla in Hongfeng Reservoir, which is consistent with previous studies(Li et al., 2014). The Cyanobacteria were dominated byP.limnetica(S1), the Chlorophyta byScenedesmussp. (J), and the Bacillariophyta bySynedrasp. (D),Cyclotellasp. (B),Melosirasp. (P), andAchnanthessp. (MP). The growth of these species varied over time in Hongfeng Reservoir, and this was related to the growth strategy of the phytoplankton and the reservoir’s water quality. For example, diatoms are high-growth species adapted to a nutrient-rich environment (Salmaso, 1996). In 2016 and 2017, the total phosphorus concentrations changed significantly;therefore,Cyclotellasp. was at an advantage.P.limneticabelongs to the R adaptive strategy and is an aggressive,opportunistic species, and its competitive ability is superior to that of other species (Reynolds, 1998).Therefore, the biomass ofP.limneticacontinued to rise after 2018, although the fierce nutrient competition between species.
It was clear that the same water body had diff erent dominant species at diff erent times; that is, each period had a specific ecosystem. It is well known that the phytoplankton community structure and composition are greatly aff ected by environmental factors. Indeed, phytoplankton are quite sensitive to minute disturbances in the environment and are thus a useful indicator of water quality changes (Rangel et al., 2016). From the analysis results, it can be concluded that the changes in phytoplankton functional groups were related to the changes in environmental factors, and the key influencing factors changed with the change in dominant functional groups over time. Many studies have shown that small changes in nutrient concentrations, such as TN and TP, can cause significant spatial changes in the phytoplankton community (Cao et al., 2018). This was consistent with the results of the research in Hongfeng Reservoir, a significant diff erence in the TN and TP concentration caused obvious variation in phytoplankton community structure. In addition, the total biomass of phytoplankton also increased significantly, firstly the pH value of water in Hongfeng Reservoir was above 8.0, and the adaptability of Cyanobacteria to high pH values was conducive to the growth ofP.limnetica, leading to an annual increase in total biomass. Secondly, the obvious fluctuation of NO3-N concentration in 2019 was conducive to the growth ofSynedrasp. andMelosirasp., which were the species with strong nutrient absorption capacity. In addition, the concentration of DO in Hongfeng Reservoir was relatively high in summer, probably because Hongfeng Reservoir is located on the karst plateau in southwest China and the air quality is good. The DO in the water is continuously supplemented by oxygen in the air and the vigorous photosynthetic activity of green aquatic plants in summer.
4.2 The relationship between phytoplankton functional groups and the concentration of COD Mn
The RDA results indicating annual diff erences in environmental factors between quadrants explained that the redundancy of environmental factors diff ered among years, and the relationship between environmental factors and functional groups also fluctuated annually. In 2018, CODMnbecame an important factor aff ecting the functional groups. In addition, the dominant functional groups changed significantly. The functional group S1 gradually occupied the dominant position in 2018 and became the dominant group in 2019, while the dominant position of functional group B was gradually replaced.The RDA results showed that CODMnwas highly correlated with functional group B in 2018 and significantly correlated with functional group S1 in 2019. This pattern indicated that 2018 was a transitional period in the succession, and there was a closer relationship between the concentration of CODMnand functional groups succession. The CODMnin reservoir water mainly comes from exogenous inputs, such as rivers, sewage discharge, and biological metabolism and decomposition (Yao et al.,2011). Hongfeng Reservoir is located in a densely populated agricultural production area. Pesticides,fertilizers, and other organic or inorganic pollutants in agricultural production activities enter the water body through artificial or natural ways, which causes organic pollution in the water body of the reservoir.The results of Pearson correlation analysis showed that the concentration of CODMnin Hongfeng Reservoir had a significant positive correlation with Chla(R=0.505;P<0.05), and a significant negative correlation with SD (R=-0.505;P<0.05). Many studies have shown that degradation of cyanobacteria and chlorophyll would cause an increase in organics,which resulted in a change of CODMnin the water(Tang et al., 2007). Moreover, the increased biomass of phytoplankton in Hongfeng Reservoir also led to a decrease of the SD and increase in chla. The synchronization between succession and CODMnindicated that CODMnmust be a key factor in change period, but the interrelation between the succession of functional groups and CODMnneeds to be further explored.
In addition, the monthly distribution of the dominant functional groups over the years was tested by NMDS analysis based on the biomass of phytoplankton (Fig.8). The analysis results indicated that the monthly distance and succession of the functional groups of Hongfeng Reservoir diff ered from year to year. The monthly succession of 2016 and 2017 was disorganized, and the distribution of functional groups was scattered. The monthly succession trajectories of 2018 and 2019 could be clearly distinguished along axis 1, and the distribution distance of the functional groups was close. The NMDS analysis results showed two diff erent periods as well, which were consistent with the above analysis results, indicating that Hongfeng Reservoir had an obvious succession of functional groups in 2018.Moreover, it can be concluded from the NMDS results that the distance between functional group B and other functional groups in the previous period was large, indicating that competition between the functional groups was small; hence, group B maintained a dominant position. However, the distance was reduced in the later period, and the biomass increased, so group S1, which had strong competitive ability, began to occupy the dominant position.
4.3 Succession rates indicating the gradual eff ect of environmental factors in phytoplankton succession
Comparing the succession rates in the same water body in diff erent years can be used as the basis for evaluating the characteristics of phytoplankton succession (Romanov and Kirillov, 2012). Using succession rates to analyze the composition of phytoplankton functional groups can distinguish the time of significant change in a reservoir. In Hongfeng Reservoir, biomass-based succession rates varied from 0.007 to 0.093/d, and high succession rates occurred in the months with high functional group diversity. Additionally, high biomass of phytoplankton does not necessarily lead to high succession rates (de S Cardoso and da Motta Marques, 2003). From the analysis results, the variation of succession rates in Hongfeng Reservoir were significantly aff ected by the concentration of TN, which is an essential nutrient for long-term growth and succession of phytoplankton.Therefore, the long-term succession of functional groups is significantly related to nutrient concentration,indicating that it is necessary to control nutrient concentration in reservoir management. In addition,the sequence of changes in phytoplankton structure and abundance observed at stable sampling sites in the reservoir may not be “true succession”. Instead,these eff ects may be the overlap of several processes,such as the residence time of water flow, the resuscitation of phytoplankton in sediments, and the seasonal succession of phytoplankton. Therefore, the long-term succession of phytoplankton species in reservoirs might depend upon factors not entirely understood and the eff ect of chance cannot be ignored.
5 CONCLUSION
The major succession processes of phytoplankton functional groups in Hongfeng Reservoir changed from B/D/P/Y to S1/D/P, with a significant increase in the total biomass. This study showed that, in a eutrophic reservoir, nutrients and water temperatures are prominent drivers for shaping the phytoplankton community structure. The phytoplankton functional groups combined with the growth strategy of phytoplankton, provide a detailed description of phytoplankton community behavior and ecosystem functioning. The phytoplankton functional groups changed rapidly in 2018 were significantly related with the concentration of CODMn, and the gradual change in succession rates was significantly related to the concentration of TN. We suggested that a sudden and gradual increase of energy resources in reservoirs and the increase in biomass could induce the dominance of enriched competitive species and result in phytoplankton succession as seen in this study.
6 DATA AVAILABILITY STATEMENT
The data for this study are available through the corresponding author.
7 ACKNOWLEDGMENT
We appreciate our colleagues for assisting with fieldwork and laboratory experiments.
 Journal of Oceanology and Limnology2022年4期
Journal of Oceanology and Limnology2022年4期
- Journal of Oceanology and Limnology的其它文章
- Validation and error analysis of wave-modified ocean surface currents in the northwestern Pacific Ocean*
- The energy conversion rates from eddies and mean flow into internal lee waves in the global ocean*
- Observation of physical oceanography at the Y3 seamount(Yap Arc) in winter 2014*
- Decadal variation and trend of the upper layer salinity in the South China Sea from 1960 to 2010*
- Observations of turbulent mixing and vertical diff usive salt flux in the Changjiang Diluted Water*
- Experimental research on oil film thickness and its microwave scattering during emulsification*
