Microstructural,magnetic and dielectric performance of rare earth ion(Sm3+)-doped MgCd ferrites
Dandan Wen(文丹丹), Xia Chen(陈霞), Dasen Luo(骆大森), Yi Lu(卢毅),Yixin Chen(陈一鑫), Renpu Li(黎人溥), and Wei Cui(崔巍),‡
1Doctoral Research Station of Chongqing Key Laboratory of Optoelectronic Information Sensing and Transmission Technology,Chongqing University of Post and Telecommunications,Chongqing 400065,China
2State Key Laboratory of Electronic Thin Films and Integrated Devices,University of Electronic Science and Technology of China,Chengdu 610054,China
3Chongqing Key Laboratory of Autonomous Navigation and Microsystem,Chongqing University of Post and Telecommunications,Chongqing 400065,China
Keywords: ferrites,Sm3+ions,substitution,magnetic permeability,dielectric permittivity
1. Introduction
With the advent of the 5G era, functional ceramic materials will usher in a new round of rapid development. Ferrites, as functional ceramics, have been extensively used in modern electronic technologies,making them one of the most significant magnetic materials.[1–3]Around the world, ferrite development and research has been very active, and its progress promotes the rapid development of high-tech industries such as electronics, information, machinery, aerospace,communication and the chemical industry.[4–6]Soft ferrite materials not only possess spontaneous magnetization and electrical properties with chemical and thermal stability but are also pertinent magnetic materials having high permeability with low loss, high resistivity, low cost and high mechanical hardness.[7–9]Recently, Mg-based ferrites (MgFe2O4) have been widely studied for their spinel structure,and show interesting structural, electrical and sensing properties.[10,11]The Cd-substituted MgFe2O4system shows a canted spin arrangement on octahedral B-sites such as Cd-substituted mixed ferrites,for example Cu–Cd,[12]Li–Cd,[13]and Ni–Cd.[14]Gadkariet al.reported Mg1-xCdxFe2O4(x=0, 0.2, 0.4, 0.6, 0.8 and 1)with the addition of 5%Y3+or Sm3+by the oxalate coprecipitation technique.[15–17]They investigated the structural,magnetic and DC electrical performance of the MgCdFe2O4system by means of x-ray diffraction (XRD) and magnetic and electrical measurements. They also used Mg–Cd ferrites as gas sensors to detect liquid petroleum gas, Cl2and C2H5OH. Although Mg–Cd ferrites with different cadmium contents have been researched, compared with other widely studied ferrites there is more to be understood.
It is known that the addition of rare earth elements to ferrites can result in the modification of structural, electrical and magnetic properties.[18–22]Many research groups have researched the effect of rare earth cations in different ferrites to tailor their microstructural, electric and magnetic properties.Bhosaleet al.extensively studied the effect of the addition of Gd3+on the electric and magnetic properties of Mg–Cd ferrite, and the phenomenon of an increase in the lattice constant with increasing Cd2+content was discovered.[20]Wuet al.investigated the effects of RE3+-substituted cobalt ferrite CoFe1.9RE0.1O4(RE=Pr3+, Sm3+, Tb3+, Ho3+) on structural,magnetic and adsorption properties through XRD,transmission electron microscopy, energy dispersive spectroscopy,Fourier transform infrared(FTIR)spectroscopy,Raman spectroscopy and vibrating sample magnetometry.[21]The results indicate that the RE3+substitution leads to a decrease in the particle size,magnetization and coercivity of the CoFe2O4ferrite. The effect of doping with different rare earth elements on spinel Mn–Cr ferrite has been studied by Abdellatifet al.They found that substituted rare earth ions distort the octahedral and tetrahedral sites of the ferrite lattice.[22]
In this paper, we report the effect of Sm3+substitution on the structural,magnetic and electrical properties of Mg–Cd ferrites prepared by the solid-state sintering method. In this work, Sm3+was substituted intensively at low to high concentrations(0.15), and there were no additional phases in the spinel structure.
2. Experiments
Sm-doped Mg–Cd ferrite with the chemical composition Mg0.8Cd0.2Fe2-xSmxO4(0≤x ≤0.15, in steps of 0.03) was synthesized through a solid-state reaction method at high temperature. The pure raw material powders(MgO,CdO,Sm2O3and Fe2O3) were weighed according to their respective stoichiometric ratios and then mixed and ball milled in a planetary mixer with zirconia balls for 18 h. Afterwards,the well-mixed powder was dried and presintered at 1150°C in air for 4 h.The preliminary sintered powder with 3 wt%Bi2O3was ball milled again in deionized water for another 18 h.After the addition of 8 wt%polyvinyl alcohol(PVA)as a binder,the dried powder then ground into particles. High pressure,up to 10 MPa,was applied to press the particles into 2 mm–3 mm thick circular plates. Finally,the molded samples were sintered at 925°C in air for 6 h.
The crystallography of the samples was measured using XRD (DX-2700, Haoyuan Co., China) with Cu-Kαradiation at aθ–2θgeometric angle from 10°to 70°. The microtopography was captured by scanning electron microscopy(SEM;JEOL JSM-6490,Japan).The magnetization hysteresis loops were obtained using a vibrating sample magnetometer(model BHL-525, Japan) with a direct current magnetic field of±2500 Oe. Saturation magnetization and coercivity were calculated from theM–Hloops. The complex magnetic permeability and dielectric permittivity were measured using an Agilent 4991 impedance analyzer(Agilent Technologies,Palo Alto, CA, USA) at various frequencies ranging from 1 MHz to 1 GHz. The bulk density was measured using Archimedes’principle in an autodensity tester (GF-300D, AND Co.). All measurements were carried out at room temperature.
3. Results and discussion
The XRD patterns of Mg0.8Cd0.2Fe2-xSmxO4(x= 0,0.03, 0.06, 0.09, 0.12, and 0.15) with added Sm3+are displayed in Fig. 1. The diffractogram reveals that all the samples crystallized in a normal spinel structure indexed to standard MgFe2O4peaks, referred to as standard PDF card file No. 17-0464. From Fig. 1(a), all the diffraction peaks are in good agreement with the indexed peaks, while no impurity phases were detected,indicating that the phase formation of Mg0.8Cd0.2Fe2-xSmxO4was not interfered with by Sm2O3and Bi2O3oxide. Whenx=0 andx=0.03,there was a small amount ofα=Fe2O3(JCPDS 33-0664) phase in these two samples. The reason for this was uneven mixing during the preparation process. Moreover, a slight shift of the diffraction peaks to a low angle is observed asxincreases from 0 to 0.15,similar to the enlarged figure of the main peak shown in Fig. 1(b). For the main peak [(311)] toward the left, this is due to the increase in the lattice constant as the ionic radius of Sm3+increases compared with Fe3+, and the Sm3+concentration increases. In the case of spinel ferrite, the octahedral sublattice(B-site)contains 16 sites,twice as many as the tetrahedral sublattice(A-site), and is the dominant sublattice.Because Fe3+(ionic radius 0.645 ˚A) in the B-site is substituted by the larger ion Sm3+(ionic radius 0.958 ˚A),substituting Sm3+causes expansion of the lattice in the B-site, which leads to the diffraction peak shifting overall to the left.Similar research,such as ion doping Mg–Cd[16,20]and Li–Zn,[23]has been reported.

Fig. 1. The XRD patterns of Sm-doped MgCd ferrites with different Sm3+contents.
The increase in bulk density first increases with Sm3+content from 4.552 g/cm3to 4.703 g/cm3and then remains stable, as shown in Fig. 2(a). This is because the bulk density is related to the lattice constant and lattice distortion. Lattice constants are calculated using Bragg’s law as a function of Sm3+content in Mg–Cd ferrites,which is shown in Fig.2(b).Moreover, the lattice constant shows nonlinear behavior and increases from 8.375 ˚A to 8.415 ˚A with increasing Sm3+content, as shown in Fig. 2(b), which may be attributed to the larger ionic radius of Sm3+(0.958 ˚A) compared with Fe3+(0.645 ˚A). In the Sm-substituted Mg–Cd spinel structure, all Cd2+occupies the tetrahedral A-site and Sm3+occupies the octahedral B-site. Similar behavior was observed in Mg–Cd ferrite with added Sm3+,suggesting that rare earth ions occupied the octahedral B-site.[15]Additionally,the grain size and pores among grains can also affect the bulk density,as shown in Fig.3. Noticeably,the lattice constant and bulk density are important factors in the magnetic and dielectric properties of ferrites.

Fig. 2. Dependence of bulk density (a) and lattice constant (b) on Sm3+content.
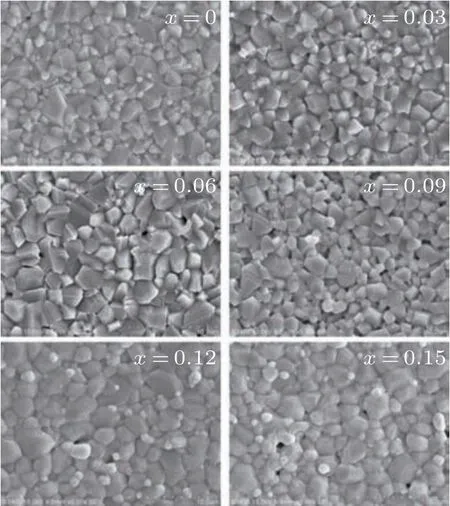
Fig.3. SEM images of samples with different Sm3+ ion contents.
Figure 3 shows SEM images of Mg–Cd ferrites with different amounts of added Sm3+(x=0–0.15). It can be concluded that all the Sm-substituted Mg–Cd ferrites have a relatively homogeneous grain distribution. The particle morphology is cubical,and the grain growth accelerates asxincreases from 0 to 0.06. Sm3+has a larger ionic radius than Fe3+,which can explain this phenomenon. Forx ≥0.12, the degree of Sm3+substitution leads to a further increase in the grain size of the ferrite,and a few grain pores begin to appear.Meanwhile,the grain shape gradually changes from polygonal polyhedron to arc polyhedron, and some grains even become spherical. The increase in grain size and the appearance of pores cause the magnetic properties of ferrite to deteriorate,but the dielectric properties are improved. Remarkably, almost all the particle morphology remains polyhedral and the grain size is roughly uniform whenx=0.06,which gives Smsubstituted Mg–Cd ferrite the best magnetic and dielectric performance.
Figure 4(a)shows the hysteresis loops of the Mg–Cd ferrites with added Sm3+at room temperature. TheM–Hloops of all samples have narrow hysteresis,which displays excellent soft magnetic characteristics. The saturation magnetization and coercivity,derived from theM–Hloops,are also displayed in Fig.4(b). These results are in close agreement with another report for Mg–Cd ferrite.[16]From Fig. 4, it is apparent that the saturation magnetization increases first from 33.8 emu/g(x=0) to the maximum value of 36.8 emu/g (x=0.06) and then decreases with a further increase inxuntil the minimum value of 22.7 emu/g is obtained atx=0.15. Evidently, magnetization is relatively dependent on the Sm3+concentration.This is due to the different magnetic moments between the A and B sublattices,depending on their respective cation distributions. It has been reported that in Sm-supplemented Mg–Cd ferrites, all divalent Cd2+ions occupy the tetrahedral A-site and Sm3+ions occupy the octahedral B-site. Mg2+and Fe3+ions occupy the A-sites and B-sites at random. The total magnetizationMscan be expressed as

whereMBandMAare the magnetic moments of the A-and Bsites,respectively,andαY–K is the Yafet–Kittel angle. Using the triangle spin arrangement model to calculate the Yafet–Kittel angle can help us understand the degree of spin canting.αY–K decreases with Sm3+substitution forx ≤0.06 and there is an increase in the magnetization of the ferrite. Similar results are found in other works based on FTIR results.[16]In addition,the increase in saturation magnetization is also related to the increase in bulk density. The density increased whenxincreased 0 to 0.06 (Fig. 2(a)), contributing to the saturation magnetization. Forx >0.06, the great mass of paramagnetic Sm3+ions would further replace Fe3+in B-sites. However,a small number of Sm3+ions would prefer to occupy A-sites,leading to a decrease in the magnetic momentnBof ferrite,and hence the totalMswould decrease.
However, as shown in Fig.4(b), the coercivity gradually decreases and then increases sharply with increasing Sm3+concentration,which displays an inverse trend compared withMs. The coercivity first decreases from 33.6 Oe (atx=0)to 29.2 Oe (atx= 0.06) and then increases to 36.1 Oe (atx=0.15). Brown’s relation could explain this behavior:

whereK1is the crystalline anisotropy constant.The amount of Fe3+decreases because of the increasing Sm3+content on the B-site. Generally,the anisotropy field of ferrites is caused by the presence of Fe3+.[24]In other words,as the Sm3+content increases, the anisotropy constant decreases, and the magnitude of coercivity also decreases whenx <0.06. With a further increase in Sm3+, the coercivity increases sharply. This is because the Sm–Fe interaction is stronger than the Sm–Sm interaction on the B-site. In addition, according to Eq. (2),the coercivity is positively proportional to the anisotropy constant and inversely proportional toMs. Therefore,the coercive force increases with decreasingMsand increasingK1whenx >0.06. Moreover, the coercivity is also influenced by the internal stress. When the Sm3+ions enter the spinel lattice,due to their electronic configuration the lattice or crystalline field will distort and then generate an internal stress.

Fig.4. The M–H loops(a)and variation in the saturation magnetization Ms and coercive field Hc of MgCd ferrite with Sm ion contents
The frequency-dependent trends of the complex magnetic permeability and dielectric permittivity for all samples,whenxincreases from 0 to 0.15,are shown in Fig.5,for change in frequency ranging from 1 MHz to 1 GHz.As seen in Fig.5(a),the real magnetic permeability (μ′) remains at a stable level,and whenxchanges from 0 to 0.06, the real magnetic permeability of Sm-substituted Mg–Cd ferrites first increases and then decreases whenx >0.06. In ferrite materials, the initial permeability mainly relies on the saturation magnetization and first-order anisotropy constant(Ku1),and the relationship is as follows:[25]

whereλsandδrepresent the saturation magnetization coefficient and internal stress, respectively. In Eq. (3), the value ofλsδis so small (because of the smallδ) that it can be ignored.[26,27]Therefore, a proportional trend holds between the initial permeability andMs. In other words, permeability mainly depends onMs. Therefore, the changing trend ofMsunder different Sm3+contents could account for the phenomenon of real permeability.
Considerable values (approximately 4–9 for all samples exceptx=0) over the wide frequency range are observed in the imaginary part(μ′′)of the samples in Fig.5(a). Hence,the magnetic tangent tanδμis obtained by the equation

Compared with other work,[28]this work renders a relatively large order of magnitude of tanδμ(approximately 3×10-1to 5×10-2). In addition, this work also found that the value of the imaginary part can be reduced by adding Sm3+.
Figure 5(b) shows that the dielectric permittivity (ε′andε′′)of the samples depends on the Sm3+doping content. The real part of the dielectric permittivity(ε′)increases from 7 to 23 with Sm3+content increasing from 0 to 0.15. Note thatε′displays flat responses of nearly 300 MHz. Moreover, the imaginary part of the dielectric permittivity(ε′′)also has a flat response but at low values of nearly 500 MHz. For ferrite,assuming that the mechanism for electrical conduction is similar to that of dielectric polarization, the increase in dielectric permittivity can be explained. Usually, when an electric field is applied on ferrite, hence increasing the polarization,the electronic exchange between Fe2+and Fe3+at the octahedral sites will cause a local displacement.[29]In Mg ferrite, Sm3+occupies octahedral sites (B-sites) because of its larger ionic radius and its electron configuration. The concentration of Fe3+at B-sites decreases gradually with increasing Sm content. Therefore, the probability of hopping between Fe3+and Fe2+ions is improved, which causes the polarization to increase. Consequently, the dielectric permittivity increases with increasing Sm concentration. Moreover,material microstructure, grain size, porosity and impurities also have a great influence on the dielectric properties of materials. The grain size gradually increases then stays stable,and the density had the same trend in Figs. 3 and 2, respectively, which also increases the permittivity.In summary,all ferrite samples with Mg0.8Cd0.2Fe2-xSmxO4show a constant increase in dielectric permittivity,which is consistent with other work.[28]

Fig.5. Complex magnetic permeability(a)and dielectric permittivity(b)of Mg–Cd ferrites for different Sm3+ contents.
4. Conclusion
Sm3+-substituted Mg–Cd ferrites were investigated for Sm3+doping concentrations between 0 and 0.15. Phase formation and surface morphology were changed substantially.Moreover,the magnetic and dielectric properties of the spinel Mg–Cd ferrites were also studied in detail. The saturation magnetization first increases and then decreases;however,the coercivity first decreases and then increases. Whenx=0.06,high saturation magnetization and low coercivity can be obtained. Meanwhile,the permeability and permittivity can also achieve their best values.
Acknowledgments
Project supported by the National Key Research and Development Program of China (Grant No. 2018YFE0115500),the National Natural Science Foundation of China (Grant Nos.51902037 and 62005033),the Open Foundation of State Key Laboratory of Electronic Thin Films and Integrated Devices (Grant No. KFJJ201912), the Science and Technology Project Affiliated to the Education Department of Chongqing Municipality (Grant No. KJQN201900615), and the Nature Science Foundation of Chongqing (Grant No. cstc2019jcyjmsxmX0696).
- Chinese Physics B的其它文章
- Real non-Hermitian energy spectra without any symmetry
- Propagation and modulational instability of Rossby waves in stratified fluids
- Effect of observation time on source identification of diffusion in complex networks
- Topological phase transition in cavity optomechanical system with periodical modulation
- Practical security analysis of continuous-variable quantum key distribution with an unbalanced heterodyne detector
- Photon blockade in a cavity–atom optomechanical system

