Anti-diabetic and anti-hyperlipidemic effects of sea cucumber(Cucumaria frondosa) gonad hydrolysates in type II diabetic rats
Tingting Wang, Lin Zheng, Shuguang Wang, Mouming Zhao,c,*, Xiaoling Liu*
a College of Light Industry and Food Engineering, Guangxi University, Nanning 530004, China
b Guangdong Food Green Processing and Nutrition Regulation Technologies Research Center, Guangzhou 510650, China
c School of Food Science and Engineering, South China University of Technology, Guangzhou 510640, China
ABSTRACT
The present study investigated the anti-diabetic activity and potential mechanisms of sea cucumber gonad hydrolysates (SCGH) in a rat model of type II diabetes induced by streptozotocin (STZ) combined with high-fat diet (HFD). Results showed that SCGH significantly reduced water intake, fasting blood glucose level and glycated hemoglobin level. Moreover, the oral glucose tolerance, insulin resistance and plasma lipid level in diabetic rats were also alleviated. Furthermore, histological analysis showed that SCGH effectively protected the tissue structure of liver. In addition, mechanism studies showed that SCGH improved glucose metabolism via activating the IRS/Akt signaling pathway, and promoted lipid metabolism via activating the AMPK signaling pathway. In summary, these findings suggested that SCGH have potential anti-diabetic effects by improving insulin resistance and lipid metabolism disorders.
Keywords:
Sea cucumber gonad
Bioactive peptide
Anti-diabetic activity
Insulin resistance
1. Introduction
Diabetes mellitus (DM) is a serious chronic metabolic disorder. The International Diabetes Federation (IDF) predicts that 10% of the population of the world will be diagnosed with diabetes by 2035. Among that, 90% of diabetic individuals are type II diabetes mellitus (T2DM) [1]. Insulin resistance, glucose metabolism disorder and lipid metabolism disorder are important characteristics of T2DM. Although the pathogenesis of type 2 diabetes has not been fully elucidated, increasing studies have shown that the mechanisms are associated with the insulin resistance, impaired pancreatic insulin secretion, oxidative stress and disordered lipid metabolism [2].
Insulin exerts physiological function through the post receptor cascades after binding to the cell-surface insulin receptors.Phosphatidylinositol 3-kinase (PI3K) is a key protein involved in insulin physiologic effect, and the activated PI3K phosphorylates serine/threonine-protein kinase (Akt), which could further activate the downstream signaling molecules including glycogen synthase kinase 3β (GSK-3β) and glucose transporter 4 (GLUT4) [3]. Thus, the IRS/Akt signaling pathway regulates glucose uptake and intracellular glycogen synthesis in liver tissues. In insulin resistance state, the IRS/Akt signaling pathway is inhibited [4], and the transportation of glucose to the plasma membrane is hindered. Furthermore, various studies have shown that the insulin resistance could be obviously improved via triggering IRS/Akt signaling [5,6]. Therefore, IRS/Akt signaling activation is a promising therapeutic target for the treatment of insulin resistance and T2DM. Additionally, in recent years, considerable research indicated that the activation of AMP-activated protein kinase(AMPK) in the liver promotes glucose uptake, insulin sensitivity and fatty acid oxidation [7]. AMPK-mediated phosphorylation of acetyl-Co-A carboxylase (ACC) can inhibit the activity of ACC,resulting in the decrease of malonyl-CoA content, FFA levels and triglyceride synthesis, and the increase of β-oxidation. Consequently,AMPK/ACC signaling activation is also a promising therapeutic target for the treatment of insulin resistance and T2DM.
Sea cucumber, is a traditional seafood and important medical material in China. In 2016, the total output of sea cucumber in China reached 174 340 t [8]. During sea cucumber transportation and processing, autolysis frequently occurs due to the existence of endogenous protease [9]. Therefore, sea cucumber was processed to edible parts, and remaining tissues such as intestinal and gonad constituents as low-value by-products are usually processed into fishmeal for animal feed or discarded [10]. The protein content of sea cucumber gonad is higher than 50%, which is a kind of protein-rich resource. However, little is known about utilisation of the sea cucumber gonad. In addition, enzymatic hydrolysis is a safe and effective method for processing sea cucumber, which could obtain a lot of active peptides. Peptides are natural materials with the advantage of wide sources, easy absorption and various biological effects. In view of the high protein content of sea cucumber gonad, the preparation of sea cucumber gonad hydrolysates (SCGH) as potential functional food supplements is promising. Previous studies have demonstrated that sea cucumber body wall hydrolysates exhibited many biological activities, such as antioxidant, anti-hypertension,anti-inflammation and anti-diabetic effects [11-13]. Besides body wall, other organs and gonads with chemical components similar to sea cucumber exhibited antioxidant and angiotensin converting enzyme inhibitory effect [14,15]. However, as far as we know, the preparation of bioactive peptides with hypoglycemic effect from the gonads of sea cucumber has not been reported yet.
Thus, the aim of this study was to investigate the anti-diabetic effect of SCGH in STZ-induced type II diabetic rats, and elucidate the mechanism of SCGH for improving anti-diabetic capacity by alleviating lipid metabolism and insulin resistance.
2. Materials and methods
2.1 Materials and chemicals
Dried sea cucumber (Cucumaria frondosa) gonad, was obtained from a seafood market in Guangzhou, China. Papain was purchased from Pangbo Biological Engineering Co., Ltd. (Guangxi,China). Protamex was obtained from Novozymes (Bagsvaerd,Denmark). Antibodies against β-actin, IRS1, p-AktSer473, Akt,GSK-3β and p-AMPKα1Ser496were purchased from Proteintech(Wuhan, China). Antibodies against p-GSK-3βSer9, and p-IRS1Ser307,radio immunoprecipitation assay (RIPA) lysis buffer, PMSF and phosphatase inhibitor cocktails were purchased from Beyotime Biotechnology (Shanghai, China). Metformin was obtained from Sino-American Shanghai Squibb Pharmaceuticals Ltd. (Shanghai,China). All the other chemicals and solvents were of analytical grade.
2.2 Preparation of SCGH
First, the dried sea cucumber gonad was kept at 55 °C for 12 h, and washed with tap water for three times to remove other sediments followed by being heated up to 90 °C for 4 h, and then cut into small pieces and homogenized. The homogenate was then hydrolyzed at 55 °C for 4 h with an enzyme mixture of papain and protamex at a protease/substrate ratio of 1% (m/m). After incubation,the hydrolysates were inactivated using a boiling water bath for 20 min followed by being centrifugated at 8 000 × g for 20 min at 4 °C using CR22N refrigerated centrifuge (Hitachi Koki Co., Ltd., Tokyo,Japan). The supernatant was collected as SCGH, and then the SCGH was lyophilized (Alpha 2-4 LDplus, CHRIST, Germany) and stored at-20 °C until use.
2.3 Chemical composition
The polysaccharide content of hydrolysate was determined following the method of Zhou et al. [16]. According to the method of Dong et al. [17], the saponin content of hydrolysate was determined using vanillin-perchloric acid method. The protein content of hydrolysate was determined using Kjeldahl method. The ash content of hydrolysate was determined by Muffle furnace high temperature cineration method. The moisture content of hydrolysate was determined using vacuum drying method.
2.4 Molecular weight (Mw) distribution and amino acids composition
The Mwdistribution was measured according to the method described by Zhao et al. [18]. The standard curve is the logarithm of relative Mwand retention time (RT), y = - 3.095x + 18.25 (R2= 0.938),where y is the logarithm of standard peptide Mw, x is the retention time. Amino acid analysis was determined according to the method described by Su et al. [19] using an A300 auto amino acid analyzer(Membra Pure, Bodenheim, Germany).
2.5 Animals and treatments
Fifty SD rats ((120 ± 30) g, male, 4-week-old) were acquired from Guangdong Medical Laboratory Animal Center (Guangdong, China).Animals were acclimatized at (22 ± 2) °C and (55 ± 5)% relative humidity with a 12/12 h light-dark cycle and had free access to food and water under specific pathogen-free (SPF) conditions. After 7 days of acclimatization, all the rats, except the rats in normal control group (NC, commercial standard chow), were provided with high-fat diet (HFD) (20% protein, 35% carbohydrate and 45% fat, as a percentage of total calories, D12451). After 4 weeks HFD supplementation,experimental diabetes were induced by intraperitoneal injection of freshly prepared streptozotocin (STZ, 25 mg/(kg·day)) dissolved in 0.1 mol/L citrate buffer (pH 4.5) for three consecutive days after fasting for 12 h. In addition, the NC rats were injected with an equivalent volume of buffer solution, all animals were provided with 20% glucose solution for the next 4 h to prevent hypoglycemia. Finally,rats with fasting blood glucose (FBG) level ≥ 10 mmol/L on the third day of SZT injection were considered diabetic. Thirty-two diabetic rats were randomly divided into 4 groups: model control group (MC, n = 8), metformin treatment group (Met, 250 mg/(kg·day),n = 8), the low-dose SCGH group (SCGH-L, 200 mg/(kg·day),n = 8), the high-dose SCGH group (SCGH-H, 400 mg/(kg·day),n = 8). The SCGH and metformin were dissolved in normal saline and administered daily by oral gavage starting at 9:00 am (1.5 mL/rat). In parallel, the NC and MC groups were received an equivalent volume of normal saline. Over the experimental period, food and water intake were measured daily, FBG levels were conducted weekly by using a portable glucometer (HGM-114, OMRON Inc., Japan) in the blood collected from the tail vein.
2.6 Ethics statement
Animal experiments were performed in strict accordance with the guidelines of Sun Yat-sen University for the care and use of laboratory animals, and protocols were approved by the Laboratory Animal Ethics Committee of Sun Yat-sen University (Ethical approval number: SYXK2016-0112).
2.7 Oral glucose tolerance test (OGTT)
To study the glucose tolerance ability of each group, OGTT was performed after overnight fasting on the 56th day of treatment.Blood samples were collected from the tail vein at 0 (just before glucose ingestion), 30, 60, 90 and 120 min after 1.5 g/kg glucose oral administration to measure the blood glucose level using a portable glucose meter. The area under the curve (AUC, mmol/(L·h)) were calculated as follows:
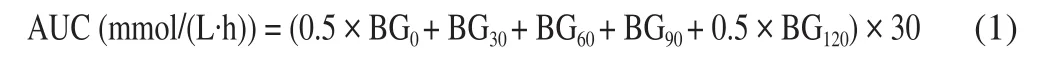
where BG0, BG30, BG60and BG120represent the blood glucose concentrations at 0, 30, 60 and 120 min, respectively.
2.8 Collection of plasma and tissue
All rats were fasted for 8 h at the end of the experimental period,blood samples were obtained from abdominal aorta after anaesthesia with chloral hydrate, plasma and blood cells were separated by centrifugation with 1 610 × g at 4 °C for 15 min, and then stored at-80 °C until further use. Afterwards, liver was collected from each animal, washed with cold 0.9% saline, wiped with filter paper and weighed. Sections of liver was snap frozen with liquid nitrogen and stored in a liquid nitrogen tank until subsequent analysis, others were preserved at 4% paraformaldehyde to be fixed, and stored at room temperature for the histopathological study.
2.9 Biochemical analysis in plasma and tissues
The plasma biochemical indexes including triglyceride (TG),total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C),high-density lipoprotein cholesterol (HDL-C) were measured by using an automatic biochemical analyzer (AU680, Beckman Coulter,USA). Glycated hemoglobin (GHb) was tested using a GHb kit(A056, Jiancheng Bioengineering Institute, Nanjing, China). Liver glycogen was measured using a commercial assay kit (A043-1-1,Jiancheng Bioengineering Institute, Nanjing, China). Total protein of liver and kidney were measured using the Bicinchoninic acid (BCA)method. All of the experimental procedures were performed strictly in accordance with the kit recommendations of the manufacturer.Plasma insulin levels were estimated by the ultrasensitive rat insulin ELISA kit (Jiancheng Bioengineering Institute, Nanjing, China) using a arioskan Flash spectral scan Multimode plate reader (Thermo Fisher Scientific, Thermo Electron Co., Waltham, MA, USA).
Homeostatic model assessment of insulin resistance (HOMA-IR) [20]was used to evaluate insulin resistance, insulin sensitivity index (ISI) [21]was calculated to evaluate individual insulin sensitivity.
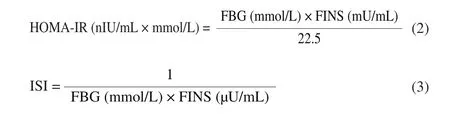
2.10 Histopathological observations
The paraffin section of tissues (5 μm thick) were stained with hematoxylin-eosin (HE), the stained tissues were performed using an optical microscope (B203TR, Optec, China) at 100× magnification.
2.11 Analysis of Western blot
The liver tissues were homogenized in ice RIPA lysis buffer containing 1% PMSF and 2% phosphatase inhibitor cocktails on ice for 30 min, followed by centrifugation at 9 600 × g for 20 min at 4 °C.The extracted proteins were separated in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (10% SDS-PAGE) and transferred to a PVDF membrane. Transferred membranes were blocked with 5% BSA in TBST buffer containing 0.1% Tween-20 for 2 h, and incubated with primary antibody for 2 h at room temperature. The membranes were washed 3 times in TBST and incubated with appropriate HRP-conjugated secondary antibodies for 1 h at room temperature.Following three washes with TBST, the bands were detected by enhanced chemiluminescence (ECL) method and exposed by Chemiluminescefnce Image system (c300, Azure biosystems, USA), and the expression level was performed using the Image J software.
2.12 Statistical analysis
Data were expressed as mean ± standard deviation (mean ± SD).Statistical analysis was performed using SPSS 20.0 statistical software(SPSS Inc., Chicago, IL, USA). One-way analysis of variance(ANOVA) followed by Duncan’s test was used for statistical significance. P-values less than 0.05 were considered statistically significant.
3. Results
3.1 Molecular weight distribution, chemical composition and amino acid composition of SCGH
To investigate the basic composition analysis of SCGH, the chemical composition was determined. As shown in Table 1, SCGH is rich in protein, followed by polysaccharide, water, saponin and ash.Furthermore, Mwdistribution and amino acid composition play an important role in the biological activity of protein hydrolysates [22].Therefore, we determined the Mwdistribution and amino acid composition of SCGH. As shown in Table 2, SCGH is mainly composed of peptides with Mwbelow 3 kDa (98.84%), and peptides below 500 Da accounted for 32.53%. In addition, the contents of Glu,Gly, Asp, Arg, Pro and Ala were relatively high in SCGH (Table 3).

Table 1The chemical composition of SCGH.

Table 2The molecular weight distribution of SCGH.
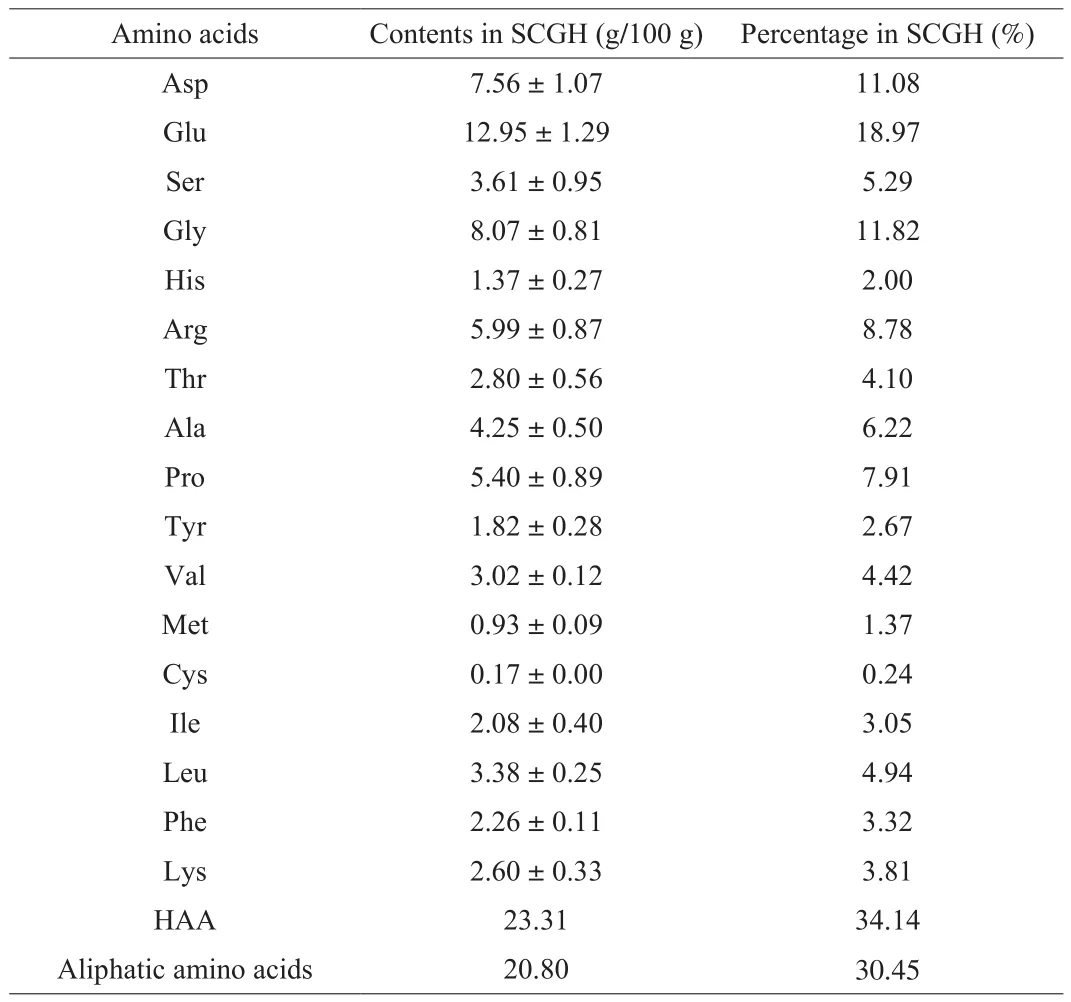
Table 3The amino acid composition of SCGH.
3.2 Effects of SCGH on water intake, body weight, FBG level, GHb and OGTT in STZ-induced diabetic rats
T2DM is a chronic metabolic disorder with hyperglycemia,polydipsia, polyphagia, polyuria and emaciation. The effects of SCGH on the water intake, body weight and FBG were shown in Figs. 1A-C. After STZ injection, the FBG and water intake of the MC group significantly increased compared to the NC group. In parallel, the body weight of the MC group significantly decreased compared with the NC group. In contrast, administration with SCGH and Met reversed the increase in FBG and water intake. In addition,there was no significant difference observed in the SCGH and MC groups in the body weight. However, supplementation with Met markedly reversed the body weight loss compared to that of the MC group. In summary, the results showed that SCGH or Met treatment could improve the symptoms of diabetic rats.
The effects of SCGH on the glucose tolerance after oral administration of glucose to the diabetic rats were showed in Figs. 1D and E. Blood glucose level of all groups rose to a maximum at 30 min after glucose loading. Then the concentrations of blood glucose reduced gradually. The plasma glucose level of NC group decreased to normal levels at 120 min after glucose loading, whereas MC group remained hyperglycaemic. As shown in Fig. 1E, the AUC value of MC group was significantly higher than that of the NC group (by 390.85%). Although the AUC value of the SCGH-L group showed no difference from that of the MC group, SCGH-H and Met groups significantly reduced the AUC by 13.00% and 20.06% in comparison with the MC group, respectively.
GHb is an important clinical parameter in T2DM. which is formed by the reaction of excessive glucose in blood with hemoglobin [23].The effects of SCGH on GHb in STZ-induced diabetic rats were showed in Fig. 1F, revealing that GHb level of the MC group was significantly higher than that of the NC group (P < 0.05). However,the increase of GHb was significantly inhibited in SCGH or Met groups compared with MC group (P < 0.05).
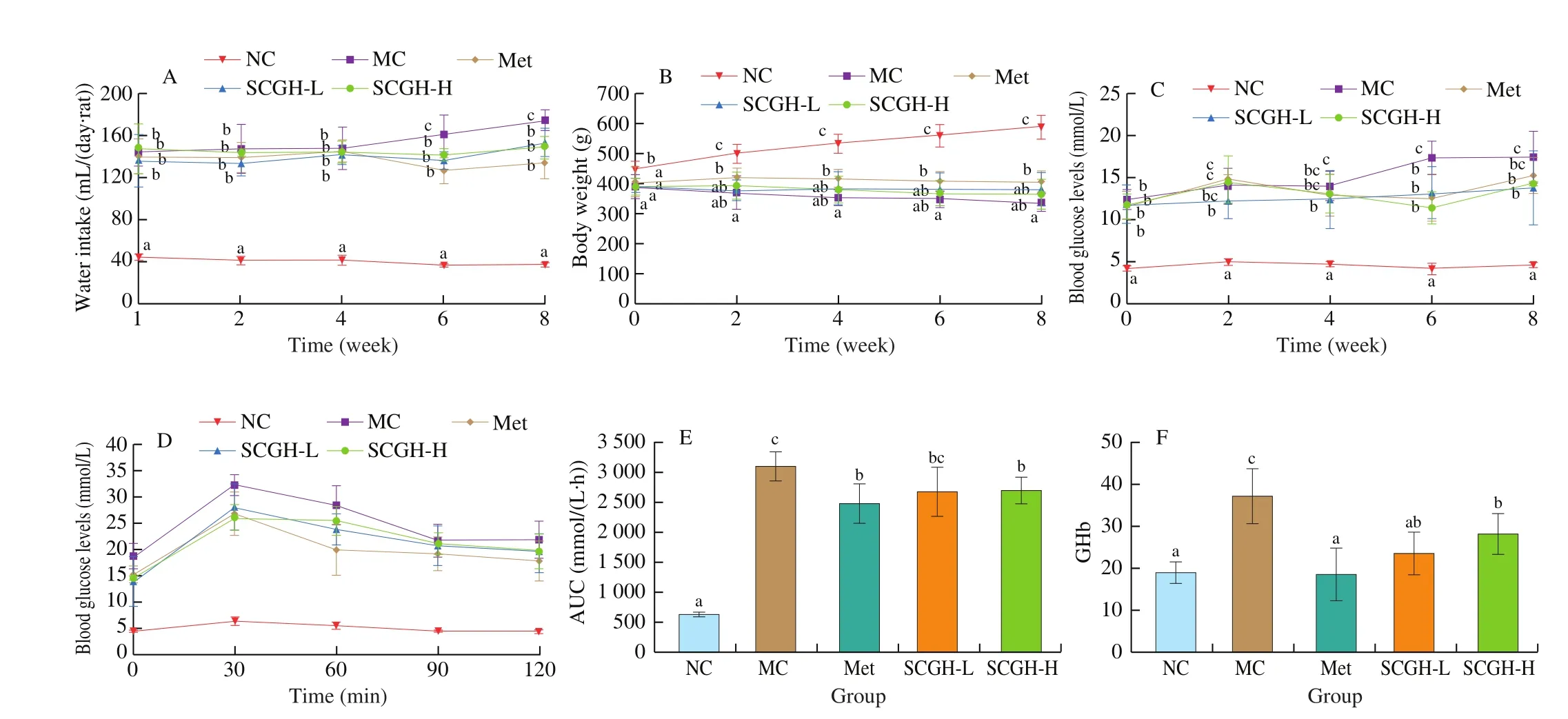
Fig. 1 (A) Water intake, (B) body weight, (C) fasting blood glucose, (D) oral glucose tolerance, (E) AUC and (F) GHb in different animal groups during the experimental period. Values are presented as the means ± SD of 8 animals. Significance was calculated by one-way analysis of variation (ANOVA) followed by Duncan’s test, different letters at the same time represent significant differences (P < 0.05).
3.3 Effects of SCGH on lipid levels in STZ-induced diabetic rats
The impaired utilization of carbohydrate in T2DM led to abnormal lipid metabolism, which is characterized by high LDL-C, TG and TC as well as low HDL-C. As shown in Figs. 2A-D, the MC group showed the elevated serum TG (by 428.99%), TC (by 161.15%),HDL-C (by 55.24%) and LDL-C (by 493.33%) compared with NC group, after 8 weeks administration of SCGH and Met, dyslipidemia conditions were ameliorated, accompanied by lowered TG, TC and LDL-C levels (P < 0.05). Thus, SCGH and Met treatment improved lipid metabolism in STZ-induced diabetic rats.
3.4 Effect of SCGH on insulin level and insulin resistance in STZ-induced diabetic rats
As shown in Fig. 2E, the plasma insulin level of all groups showed no significant difference. To explore the effect of SCGH on insulin resistance, the HOMA-IR and ISI was calculated as described in the methods. As shown in Figs. 2G and H, the value of HOMA-IR and ISI were significantly increased in the MC group compared with the NC group (i.e. approximately 332.83% and 47.42%, respectively),indicating the severe insulin resistance in STZ-induced diabetic rats. In contrast, administration with SCGH reversed the increase in HOMA-IR, especially in SCGH-H group (by 63.43%). However, Met and SCGH intervention showed no significant difference compared with the MC group on the ISI index. These results suggested that SCGH could alleviate the insulin resistance in diabetic rats.
3.5 Histopathological observations
Liver is the main organs of glucose metabolism, and the injury of liver could strengthen insulin resistance, which also affects the glucose metabolism. To determine whether SCGH had the capacity to protect the liver in diabetic rats, histological examinations in liver was performed. As shown in Fig. 2I, the relative liver weight in MC group was significantly higher than that in the NC group (P < 0.05), which indicated that the liver enlargement occurred in diabetic rats. Additionally, liver sections of NC group rats showed normal hepatic cells with a prominent nucleus, distinct cell borders,and visible central veins, which was surrounded by abundant cytoplasm (Fig. 3A). In MC group, the STZ-induced diabetic rats presented with mussy hepatic cords, empty lipid vacuoles, focal necrosis, congestion in central vein, and loss of cellular boundaries.SCGH or Met treatment alleviated the pathological damage in the experimental groups compared with that in MC group, especially in SCGH-H group, histological damage was notably alleviated and the conditions were restored to nearly the normal state. Hepatic cells in SCGH-L group were more or less normal, except a mild degree of mussy hepatic cords.
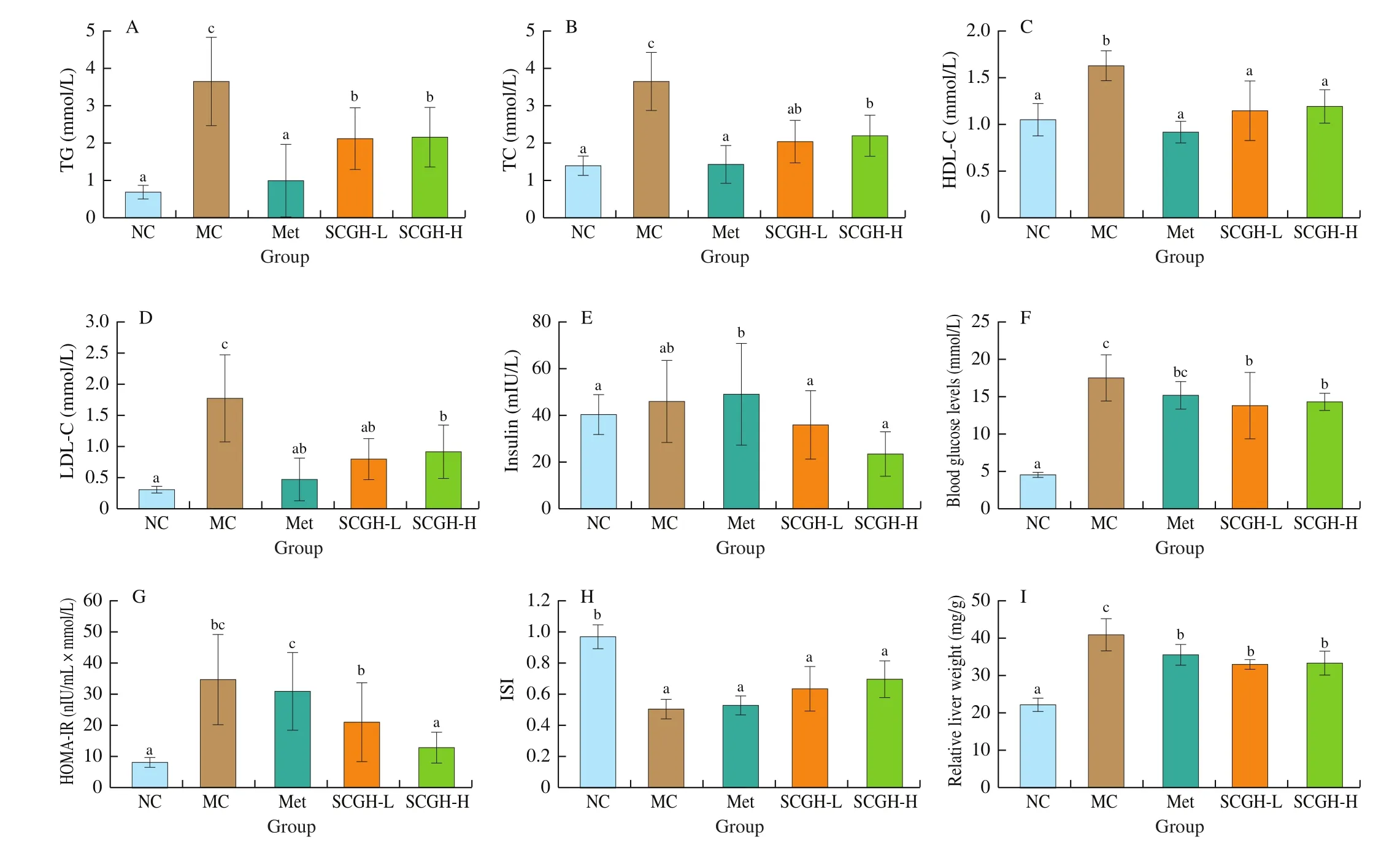
Fig. 2 Effect of SCGH on serum lipid concentrations of rats in different groups. Effects of SCGH on (A) TG, (B) TC, (C) HDL-C and (D) LDL-C in STZ-induced diabetic rats. Effects of SCGH on (E) serum insulin levels, (F) FBG levels, (G) HOMA-IR, (H) ISI and (I) relative liver weight in STZ-induced diabetic rats.Values are presented as the means ± SD of 8 animals. Significance was calculated by one-way analysis of variation (ANOVA) followed by Duncan’s test, different letters represent significant differences (P < 0.05).
3.6 Effects of SCGH on IRS/Akt and AMPK signaling pathways in STZ-induced diabetic rats
It has been reported that the IRS/Akt pathway plays a key role in improving insulin resistance signaling pathway and has been considered as a target for the treatment of T2DM [24]. Moreover,considerable research indicated that the activation of AMPK in the liver promotes glucose uptake, insulin sensitivity and fatty acid oxidation [7,25]. Therefore, the expression levels of key proteins related to the regulation of glucose metabolism and lipid metabolism in liver tissues were analyzed by western blotting. According to the data of glycogen content in liver (Fig. 3B), there was no significant difference in liver glycogen contents between the MC and NC groups. However, high supplemental level of SCGH significantly increased the liver glycogen contents compared to the MC group.These results suggested that SCGH might have the potential to increase glycogen synthesis. Furthermore, as shown in Figs. 3C-F,the protein expression of p-Akt and p-GSK-3β in liver tissue of MC group were decreased, while the protein expression of p-IRS1 was significantly increased compared to the NC group, indicating an impairment of insulin signaling in the liver of diabetic rats.However, SCGH or Met treatment markedly enhanced the protein expressions of p-Akt and p-GSK-3β levels and decreased the protein expressions of p-IRS1 of T2DM diabetic rats in a dose-dependent manner. Additionally, the effect of SCGH on the phosphorylation of AMPK was evaluated in this study. As shown in Figs. 3D and G, the induction of STZ markedly reduced the expression level of p-AMPKα1 in diabetic rats compared to the NC group, which was dramatically up-regulated by SCGH administration in the SCGH-L and SCGH-H groups by 154.70% and 141.26%, respectively. Overall, these results demonstrated that the supplementation of SCGH alleviated insulin resistance and improved glucose metabolism and lipid metabolism by triggering the IRS/Akt and AMPK signaling pathways.

Fig. 3 (A) Effects of SCGH on the histological changes of liver tissues in STZ-induced diabetic rats (HE staining, magnification: 100). (B) Effects of SCH on the glycogen content in liver. (C) Densitometric quantification of p-IRS1/IRS1 in liver. (D) Western blotting analysis of p-IRS1, IRS1, p-Akt, Akt, p-GSK-3β,GSK-3β and p-AMPK in liver. Densitometric quantification of (E) p-Akt/Akt, (F) p-GSK-3β/GSK-3β and (G) p-AMPK/β-Actin in liver. Protein band intensities were quantified by using Image J software. Significance was calculated by one-way analysis of variation (ANOVA) followed by Duncan’s test, different letters represent significant differences (P < 0.05).
4. Discussion
Diabetes mellitus is an endocrine and metabolic disorder, which is characterized by biochemical changes and tissue destruction caused by chronic hyperglycemia. Although current synthetic anti-diabetic drugs have proved considerable efficacy, the lack of response to current pharmaceutical drugs in a proportion of patients, as well as therapy discontinuation due to drug-side effects, which are major limitations that have not yet been solved. Therefore, new strategies that modulating diabetes by ameliorating insulin resistance and hyperlipidemia along with improving liver functions are still urgent.
Sea cucumber gonad is a low value by-product and rich in polysaccharides, saponins, peptides and other bioactive substances.Several studies have shown that sea cucumber body wall extracts exhibit beneficial effects on blood glucose levels and insulin resistance in experimental diabetic models [26,27]. However,there was no report on the hypoglycemic effect of the gonads with similar chemical composition compared with the sea cucumber body wall. In the present study, we evaluated the anti-diabetic activity,anti-hyperlipidemia activity, and liver protective effect of SCGH in a rat model of type II diabetes. The results showed that the STZ-lesioned rats suffered pronounced marasmus and hyperglycemia compared with the NC group. In addition, the result of FBG and body weight in MC group supported previous studies in which STZ injection led to the impairment of blood glucose homeostasis [28,29]. On the contrary, the administration of SCGH resulted in a reversal of diabetes-induced hyperglycemia and high level of GHb after SZT injection. Those results suggested that SCGH possessed potential anti-diabetic effects in diabetic rats.
T2DM was usually accompanied by hyperlipidemia, and characterized by high LDL-C, TG, TC and low HDL-C. HDL-C is an anti-atherosclerotic plasma lipoprotein. In contrast, LDL-C particle is classified as bad cholesterol, which could transport the lipid molecules into arterial walls and attract macrophages,eventually forming atherosclerosis or inducing diabetes [30]. Thus,the management of diabetic dyslipidemia has been implicated as a key factor in the treatment of T2DM [31]. AMPK is an intracellular energy sensor implicated in the regulation of cellular metabolism,which plays an important role in whole body energy balance, such as food intake, body weight, glucose uptake and lipid metabolism [25,32].The activation of AMPK may lead to the increased phosphorylation of ACC, which is a key biosynthetic precursor to fatty acids as well as a potent inhibitor of mitochondrial fatty acid oxidation [33,34].AMPK-mediated phosphorylation of ACC can inhibit the activity of ACC, resulting in the decrease of malonyl-CoA content and triglyceride synthesis, and the increase of β-oxidation. In this study, SCGH decreased blood lipid profile level in STZ-induced diabetic rats. The improvement is consistent with the change in western blot results. Insulin resistance leads to the inhibition of AMPK phosphorylation [25,35].In our study, the expression levels of p-AMPK were decreased measurably in the livers of MC group compared with those of NC group. However, SCGH significantly stimulated the phosphorylation of AMPK in STZ-induced diabetic rats. Thus, SCGH contributed to restore the diminished p-AMPK levels in the liver of insulin resistance. The data indicated that SCGH possessed potential anti-hyperlipidemic and anti-hyperglycemic effects in diabetic rats. The hypoglycemic and hypolipidemic effects of SCGH might be related to the potential hypolipidemic peptides in the hydrolysates. Moreover, aliphatic amino acids such as Gly, Ala, Val, Ile and Leu can remove bile acids by forming stable complexes with the hydrophobic bonds [36,37]. In this study, SCGH contained abundant aliphatic amino acids, so we speculated that SCGH had the potential ability to eliminate bile acids and further reduce blood lipid level in vivo.
Liver is the most important organ in regulating glucose metabolism by transforming glucose into glycogen [38], and it was stimulated by insulin to take more glucose from the blood and synthesize glycogen, thereby reducing the postprandial hyperglycemia.Impaired insulin signaling cascade in insulin-resistance state leads to a significant decrease in glucose uptake [39]. The biological effect of insulin is initiated with the binding of insulin to insulin receptor (IR), and then the activation of IR cause tyrosine phosphorylation of IRS [40,41], the phosphorylated IRS activates PI3K [42], and the activation of PI3K leads to the phosphorylation of Akt, and subsequently, activation of Akt can phosphorylate GSK-3β,and lead to promotion of glucose synthesis [43]. In our study, MC group exhibited symptoms of hyperglycemia and hyperinsulinemia,with significantly higher HOMA-IR scores and lower ISI scores compared to the NC group (Figs. 2G and H), indicating that the diabetic rats developed insulin resistance. Interestingly, SCGH significantly improved oral glucose tolerance and reduced HOMA-IR scores in diabetic rats. The results suggested that SCGH improved abnormal oral glucose tolerance, hyperinsulinaemia and insulin resistance, the improvement is consistent with the change in western blot results. In our study, administration of SCGH decreased the protein expression of p-IRS1 and increased the protein expression of p-Akt and p-GSK-3β in liver. Phosphorylation of IRS1 at Ser307 is considered to be involved in IRS1 degradation [44,45]. Thus the inhibition of p-IRS1 reduced the degradation of IRS1, thereby promoting the phosphorylation level of Akt. GSK-3β is a widely expressed Ser/Thr protein kinase, located downstream of Akt,which can phosphorylate and inactivate glycogen synthase [46].Moreover, the activity of GSK-3β can be inhibited by Akt-mediated phosphorylation at Ser 9 of GSK-3β. Therefore, the inhibition of GSK-3β could reduce blood glucose level by promoting glycogen synthesis, which is consistent with the increase of glycogen content in SCGH group (Fig. 3B). The results showed that SCGH treatment could promote glycogen synthesis and insulin signal transduction. A previous study proved that amino acids including Pro, Phe, Leu/Ile and Ala could alleviate glucose uptake and insulin resistance by activating IRTK/PI3K/GLUT4 signaling pathway [47]. Therefore, the abundant Pro (7.91%), Leu + Ile (7.99%) and Ala (6.22%) or peptides containing these amino acids within SCGH might be involved in the activation of PI3K/GLUT4 signaling pathway.
In summary, SCGH exerted potent and effective hypoglycemic,hypolipidemic and insulin-sensitizing effects in STZ-induced diabetic rats. The potential molecular mechanism investigations indicated that SCGH activated PI3K/Akt and AMPK signaling pathways,further regulating the expression of the GSK-3β and ACC proteins,thus promoting lipid metabolism and improving insulin sensitivity.The study firstly reported the anti-diabetic, anti-lipidemic and hepatoprotective effects of the protein extract from the sea cucumber gonad, and provides experimental basis in support of its use in the treatment of diabetes-related complications.
5. Conclusion
The SCGH administrated orally could ameliorate hyperglycaemia and impaired glucose tolerance, and improve lipid metabolism disorders, hepatoprotective and ameliorated insulin resistance. This investigation reveals the potential of SCGH for use as a natural oral agent with antidiabetic and antihyperlipidemic effects.
Con flicts of interest
The authors declare no con flict of interest.
Acknowledgement
This research was supported by the Natural Science Foundation of Guangxi (2016GXNSFEA380003), Guangxi Science and Technology Major Special Project (AA17204075), Guangxi Science and Technology Major Special Project (AA4102) and Shandong Provincial Key R&D Program (LJNY202018).
- 食品科学与人类健康(英文)的其它文章
- A review on mechanisms of action of bioactive peptides against glucose intolerance and insulin resistance
- Adropin as an indicator of T2DM and its complications
- Isolation and characterization of novel peptides from fermented products of Lactobacillus for ulcerative colitis prevention and treatment
- The immunity-promoting activity of porcine placenta in mice as an immunomodulator for functional foods
- Nutritional properties of Europen eel (Anguilla anguilla) bone peptide-calcium and its apoptosis effect on Caco-2 cells
- A comprehensive method to explore inhibitory kinetics and mechanisms of an anticoagulant peptide derived from Crassostrea gigas

