Identification of egg protein-derived peptides as xanthine oxidase inhibitors:virtual hydrolysis, molecular docking, and in vitro activity evaluation
Zhipeng Yu, Yxin Co, Ruotong Kn, Huizhuo Ji, Wenzhu Zho,*,Siji Wu, Jingo Liu, Dvi Shiun
a School of Food Science and Engineering, Hainan University, Haikou 570228, China
b College of Food Science and Engineering, Bohai University, Jinzhou 121013, China
c Lab of Nutrition and Functional Food, Jilin University, Changchun 130062, China
d Institute of Drug Discovery Technology, Ningbo University, Ningbo 315211, China
ABSTRACT
The purpose of this study was to screen the xanthine oxidase (XO) inhibitory peptides from egg white proteins through virtual hydrolysis, in vitro activity validation, and molecular docking. The results demonstrated that tripeptide EEK from ovalbumin exhibited potent XO inhibitory activity with an IC50 value of 141 μmol/L.The molecular docking results showed that tripeptide EEK bound with the active center of XO via 3 carbon hydrogen bond interactions, 2 salt bridges, 5 conventional hydrogen bond interactions, and 4 attractive charge interactions. The residues Glu802, Phe1009, and Arg880 may play key roles in the XO catalytic reaction.Especially, the key intermolecular forces of inhibiting XO activity may be special type of hydrogen bonds including carbon hydrogen bond interactions and attraction charge interactions. The novel tripeptide EEK is potential candidates for controlling hyperuricemia.
Keywords:
Egg protein-derived peptides
Hyperuricemia
Inhibitor mechanism
Molecular docking
Xanthine oxidase
1. Introduction
Uric acid is thefinal production of purine metabolism in human [1,2].The excessive production and blocked excretion of uric acid will lead to hyperuricemia [3]. In recent years, the prevalence of hyperuricemia has been on the rise due to the increase of high-purine foods intake [4,5]. It has been reported that urate nephropathy also related to hyperuricemia [6]. Reducing the intake of high-purine foods can effectively control the level of blood uric acid in the body by inhibiting the synthesis of uric acid or promoting the excretion of uric acid [7]. Xanthine oxidase (XO, EC 1.17.3.2) is a liver enzyme that catalyzes the two-step oxidation of hypoxanthine to xanthine,followed by xanthine to uric acid [8,9]. Thus, XO is a crucial molecular target for the research and development of drugs to treat hyperuricemia [10]. XO inhibitors, e.g., allopurinol and febuxostat,are widely used in the treatment of hyperuricemia [11]. However,allopurinol and febuxostat have certain side effects [12,13]. Thus, XO inhibitors identified from foods have become the potential candidate for the treatment of hyperuricemia.
Some XO inhibitory peptides have been isolated from seafood, including bonito, tuna, and shark cartilage [14-16]. Egg white proteins are high quality source of bioactive peptides [17].Previous studies had successfully identified bioactive peptides from ovalbumin, ovomucoid, and lysozyme, such as antioxidant peptides,immunologically active peptides, and antimicrobial peptides [18-23].Therefore, ovalbumin, ovomucoid, and lysozyme were used for screening potential XO inhibitory peptides. However, the classical methods of separation and purification of bioactive peptides are time-consuming and costly [22]. Many studies have demonstrated the efficiency of virtual screening which is considered as an effective alternative to traditional enzymatic hydrolysis [24-26]. Therefore,the in silico enzyme digestion methods were applied for producing food-derived bioactive peptides [27].
The purpose of this work was to identify novel XO inhibitory peptides from egg white proteins. Egg white proteins were hydrolyzed using virtual enzymatic hydrolysis. The water solubility and toxicological properties of the obtained peptides were predicted.Subsequently, the in vitro inhibitory activity of XO was validated using high performance liquid chromatography (HPLC). Furthermore,the potential interaction mechanism of XO inhibitory peptides with XO was investigated via molecular docking simulation. This study provides new insights into the application of egg white protein-derived peptides in the treatment of hyperuricemia.
2. Materials and methods
2.1 Chemical reagents
Uric acid (≥ 99%), xanthine (≥ 99.5%), and xanthine oxidase (EC 1.17.3.2, 5 units, milk extract) were obtained from Sigma-Aldrich (St Louis, Missouri, USA). Tri fluoroacetic acid (TFA)and chromatographic grades methanol were obtained from Thermo Fisher Scientific (Waltham, Massachusetts, USA). All other chemicals and reagents were analytical grade.
2.2 Proteolysis simulation of egg white proteins
Egg white proteins were hydrolyzed using the program ExPASy PeptideCutter (http://web.expasy.org/peptide_cutter/) [28]. Ovalbumin(Accession of NCBI: AAB59956), ovomucoid (Accession of NCBI:ACJ04729 and XP_021266564), and lysozyme (Accession of NCBI:AAB31830) of egg white proteins were obtained from the National Center for Biotechnology Information (NCBI), available on https://www.ncbi.nlm.nih.gov/. Then, in silico digestion was performed using three typical enzymes in the gastrointestinal (GI) tract, i.e., pepsin (EC 3.4.23.1), trypsin (EC 3.4.21.4) and chymotrypsin (EC 3.4.21.1) [29].The absorption efficiency of peptides depended on the length of chain,the shorter peptides chain length, the better absorption of peptides [30].Therefore, tripeptides, tetrapeptides, and pentapeptides were chosen to the solubility and toxicity prediction.
2.3 The prediction of solubility and toxicity of peptides
The solubility of the peptides was evaluated by the peptide property calculator (http://www.innovagen.com/) [31]. Subsequently,the mutagenicity, developmental toxicity potential (DTP), and skin sensitization (GPMT) of those peptides were analyzed by Discovery Studio (DS) 2017 R2 software (Dassault Systemes Biovia, San Diego,CA, USA) [32].
2.4 Molecular docking of peptides and XO
Bovine xanthine oxidase preserved all catalytic essential residues of human enzymes, with 90% overall sequence homology [33].And the bovine XO was a common target protein for screening XO inhibitors, thus it was used for molecular docking [34-36]. The crystal structure of quercetin and Bovine XO complex (PDB ID: 3NVY) was downloaded from the Protein Data Bank (PDB) (https://www.rcsb.org/) [16]. The pretreatment of crystal structure was performed using DS 2017 R2 software. The structure of XO was prepared by removing water molecules, adding hydrogen atoms, deleting the original ligand (quercetin) and Mo atoms and defining the active center with coordinates X: 87.70, Y: 7.70, and Z: 15.97, and the docking radius was 9 Å [16,34]. The structure of peptides was draw by DS 2017 R2 software, and the structures of positive control allopurinol and febuxostat were obtained from the PubMed (https://pubmed.ncbi.nlm.nih.gov/about/). Then, the ligand pretreatment process is described as energy minimizing and preparing ligands in the small molecules tab of DS 2017 R2 software (Dassault Systemes Biovia, San Diego,CA, USA). The CDOCKER program of DS 2017 R2 software was used for molecular docking. The best pose was selected based on the CDOCKER-Energy scores.
2.5 Peptides synthesis
Potential XO inhibitory peptides from egg white proteins were synthesized using an AAPPTEC Apex 396 peptide synthesizer as previously described [22].
2.6 In vitro XO inhibitory activity assay
The XO inhibitory activity of potential peptides was determined according to HPLC method [16]. All solutions were dissolved in phosphate buffer (pH 7.4). The test samples (10 μL) and 30 μL XO (0.025 U/mL) were added into the 0.5 mL centrifuge tube and preheated at 37 °C for 10 min. Then, 30 μL (5 mmol/L) xanthine was added to start the reaction and lasted for 20 min at 37 °C. Refrigerate at 5 °C to stop the reaction. Then, xanthine content in thefinal mixture was determined by HPLC with 10 μL of the reaction solution at 254 nm, and the inhibition rate of the sample to XO was calculated.
3. Results and discussions
3.1 Virtual screening of potential XO inhibitory peptides
Virtual screening is quick, reliable, and efficient method for screening the bioactive peptides. A total of 62 tripeptides,tetrapeptides, and pentapeptides were obtained in the hydrolysates of ovalbumin, ovomucoid and lysozyme. Then, the water solubility was predicted, the results showed that 44 peptides had good water solubility. The toxicity prediction results indicated that all peptides were all no-mutagen, 4 peptides (SSSAN, ASR, PEY, and QINSR)had DTP toxic and 4 peptides (GAK, PDAA, GNK, and PDAV)were sensitizer. Therefore, 36 peptides with good water solubility and non-toxicity were used for screening potential XO inhibitory peptides by molecular docking. The docking of 28 peptides with XO was successful, and their CDOCKER-Energy scores were presented in Table 1. The lower CDOCKER-Energy scores indicated that peptides were more likely to be connected with XO, thus obtaining a more favorable conformation [37]. The CDOCKER-Energy scores of peptides EEK (-96.88 kcal/mol), TNDC (-86.44 kcal/mol),EGK (-86.30 kcal/mol), EER (-84.04 kcal/mol), and DNEC (-82.55 kcal/mol)were lower than that of other peptides, and also lower than that of positive control allopurinol (5.91 kcal/mol) and febuxostat (31.62 kcal/mol).Therefore, peptides EEK, TNDC, EGK, EER, and DNEC were synthesized for further study, and mass spectra results of EEK,TNDC, EGK, EER, and DNEC were shown in Fig. 1.
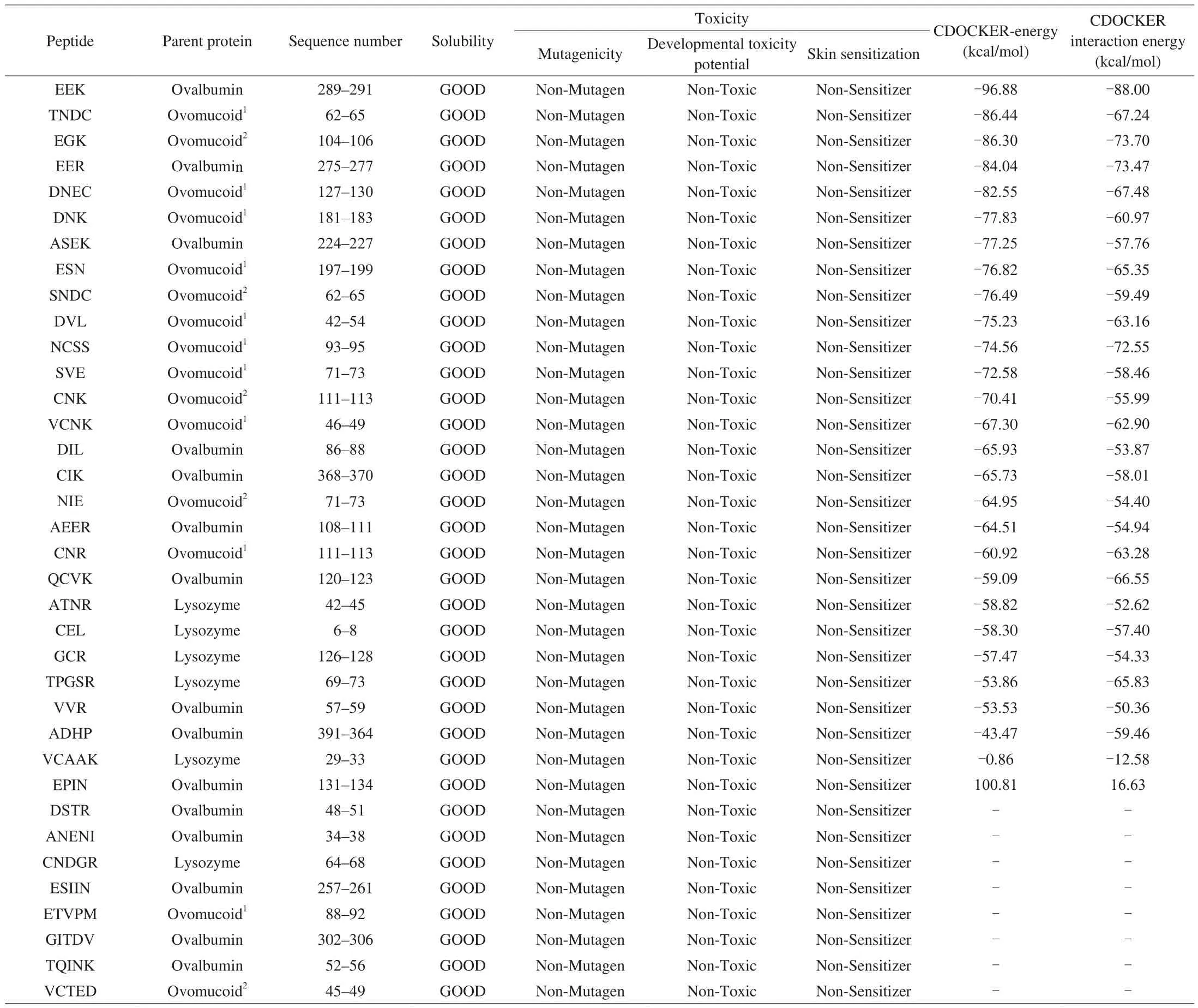
Table 1Water solubility, toxicity prediction of peptides and docking with XO.
3.2 In vitro XO inhibitory activity of peptides
The XO inhibitory activity of the peptides EEK, TNDC, EGK,EER, and DNEC were determined. At the concentration of 0.50 mg/mL,the XO inhibitory rates of peptide EEK was 73.67%, and that of peptides TNDC, EGK, EER, and DNEC were 4.38%, 10.49%, 5.41%,and 13.24%, respectively. Therefore, peptide EEK was selected to continue the following study. Peptide EEK which matched 289–291 residues of ovalbumin in egg, showed the best XO inhibitory activity with IC50value of 141 μmol/L (0.40 mg/mL). The XO inhibitory activity of peptide EEK was lower than that of commercial inhibitor allopurinol (IC50= 10.10 μg/mL) [11], but was significantly better than that of XO inhibitory peptides WPPKN (IC50= 17.75 mg/mL),ADIYTE (IC50= 19.01 mg/mL), and EEAK (IC50= 0.58 mg/mL)reported from previous studies [11,16].
3.3 Molecular interaction mechanism of tripeptide EEK with XO
XO is a dimer; each monomer contains an active site, Glu802,Glu1261, and Arg880 residues in the molybdenum center of the gorge play key roles in catalyzing xanthine oxidation, whereas some residues at the entrance of the cavity, such as Leu648, Phe649,Phe914, Phe1009, Val1011, Phe1013, and Leu1014, modulate the entry of small molecules including substrates or inhibitors into the center [38-42]. The best docking pose of EEK and XO was shownin Fig. 2. EEK and XO were stably bonded by 3 carbon hydrogen bond interactions (carbon hydrogen bond interactions are considered weaker hydrogen bonds where the donor is a polarized carbon atom [43]), 2 salt bridges, 5 conventional hydrogen bond interactions,and 4 attractive charge interactions. Two carbon hydrogen bond interactions were formed between the hydrogen atoms (HB1 and HB2) of the residues Ser876 of XO and the oxygen atom (O17)of EEK, both with a length of 2.82 Å. The carbon hydrogen bond interaction was also formed between the hydrogen atoms (HA)of the amino acid residue Phe1009 and the oxygen atom (O14) of EEK (2.45 Å). The oxygen atom (OE2) of residues Glu879 and the oxygen atom (OE1) of residues Glu802 of XO bond with EEK by salt bridges interactions at distances of 1.82 and 1.87 Å, respectively.Five conventional hydrogen bond interactions were formed between H52, O15, O14, O15, and O30 of EEK and the nitrogen atom (NE2)of His875, the hydrogen atoms (HE, HH21) of Arg880, the hydrogen atoms (HG1) of Thr1010, the hydrogen atoms (HD22) of Asn768 of XO, at distances of 2.58, 2.08, 2.08, 1.90, and 2.17 Å, respectively.Four attractive charge interactions were found in the EEK-XO complex. The first one involved the oxygen atom (OE2) of residue Glu879 with the hydrogen atom (H51) of EEK with a length of 1.82 Å;the second one involved the nitrogen atom (NH2) of the residue Arg880 with the oxygen atom (O15) of EEK with a length of 3.35 Å;the third one involved the oxygen atom (OE1) of the residue Glu802 with the hydrogen atom (H3) of EEK with a length of 1.87 Å;and the fourth one involved the nitrogen atom (NZ) of the residue Lys771 with the oxygen atom (O30) of EEK with a length of 4.70 Å.
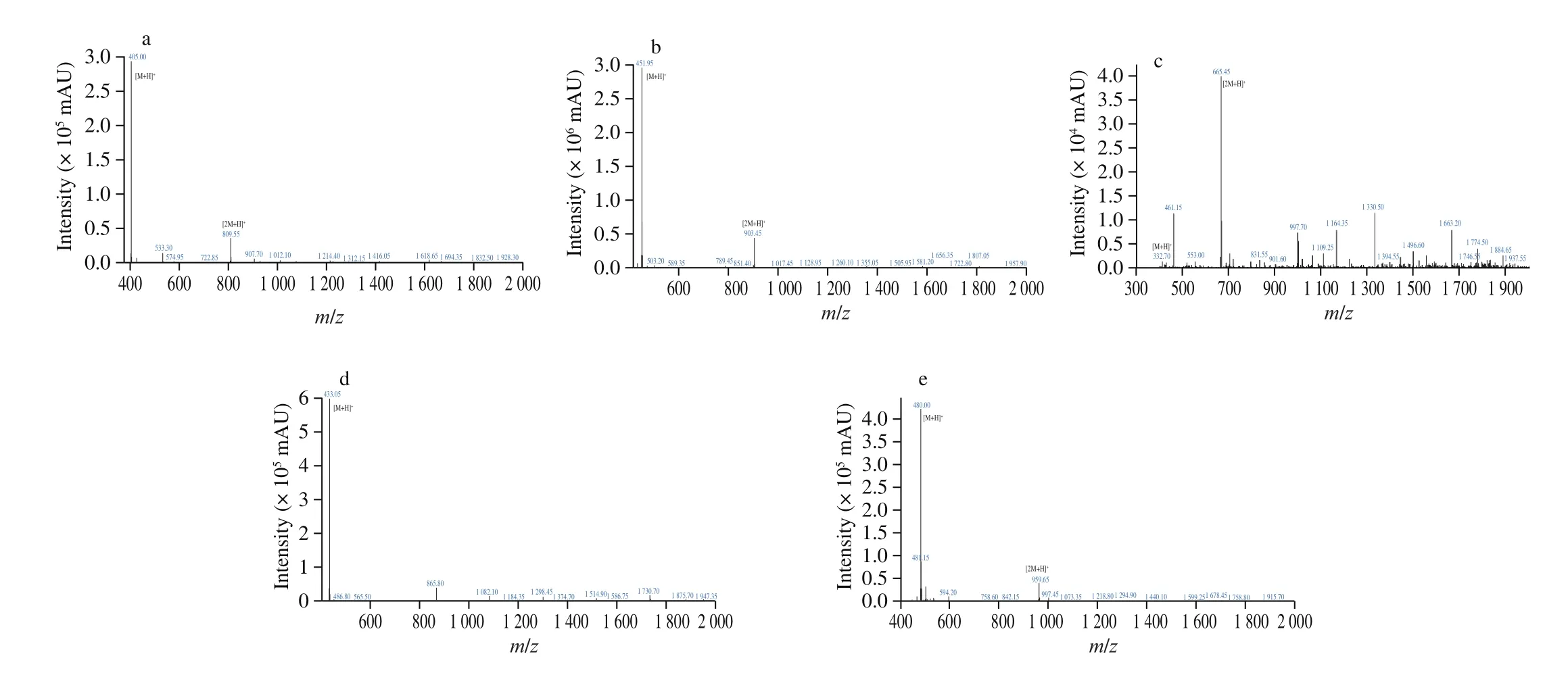
Fig. 1 The mass spectra results of peptides (a) EEK, (b) TNDC, (c) EGK, (d) EER, and (e) DNEC.

Fig. 2 The docking interactions of EEK with XO. (a) 3D structure of EEK-XO complex, red represents residues amino acids of XO, black represents atom numberings in XO; blue represents atom numberings in peptide EEK. (b) 2D diagram of the EEK-XO molecular interactions. Light blue represents carbon hydrogen bond. Green represents conventional hydrogen bond. Orange represents salt bridge and electrostatic interactions, and red color represents unfavorable negative-negative interactions.
Previous studies have demonstrated that quercetin interacted with catalytic residues Phe914, Glu802, Val1011, Leu648, Leu1014,Leu873, Phe1009, Ala1079, Arg880 and Thr1010 in the L chain of XO [33]. As shown Fig. 3a, EEK bond the XO by residues Lys771,Asn768, Ser876, Glu802, Thr1010, Arg880, Phe1009, His875, and Glu879 of the chain L. Therefore, the residues Glu802, Thr1010,Arg880, and Phe1009 of XO might play important roles in EEK combination, and they may also be important screening criterion.Additionally, as shown in Table 2, peptides EEAK, FH, DN, and YLD all combined with the residues Glu802, Phe1009, and Arg880 of XO. Thus, Glu802, Phe1009, and Arg880 may play major roles in XO binding.
Fig. 3 (Continued)

Fig. 3 (a) The docking poses of original ligand quercetin and XO. Green represents conventional hydrogen bond. Light blue represents Pi-Donor hydrogen bond.Hotpink represents Pi-Pi stacked and Pi-Pi T-shaped interaction. Pink represents Pi-Alkyl interaction. Purple represents Pi-Sigma interaction. Red represents unfavorable bump and unfavorable acceptor-acceptor interaction. Molecular interactions of (b) allopurinol, (c) febuxostat, peptides (d) EEAK, (e) FH, (f) DN, and(g) YLD with XO.

Table 2The interaction residues of XO with EEK, quercetin, allopurinol, febuxostat,EEAK, FH, DN, YLD, LD, LND, and NY.
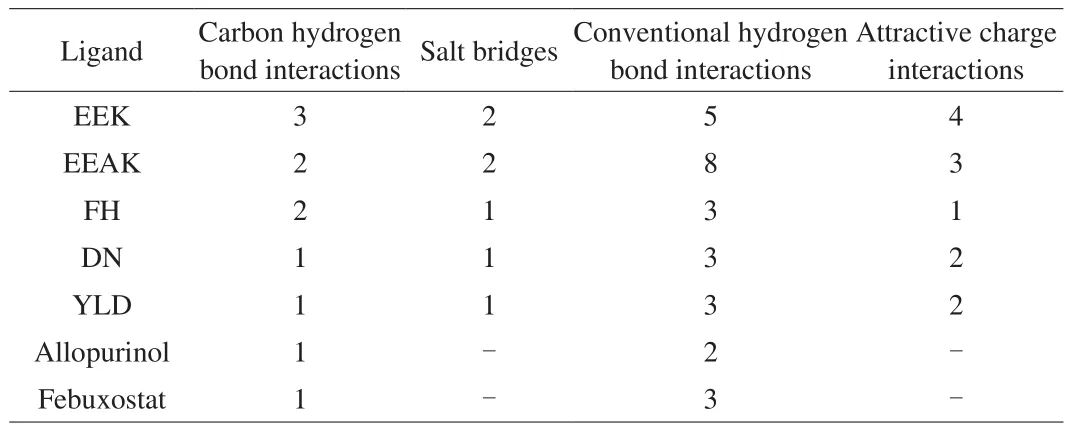
Table 3The number of interactions between ligands and the amino acid residues of XO.
Compared with the original ligand quercetin in XO, tripeptide EEK had more advantages in binding with the predicted amino acid residues (Glu802, Phe1009, and Arg880) of XO. Quercetin formed two unfavorable chemical bonds with Glu802 of XO, while tripeptide EEK combined with Glu802 steadily by a salt bridge and an attractive charge interaction. Quercetin contacted with Phe1009 of XO by two Pi-Pi-T-Shaped interactions. In contrast, tripeptide EEK bond Phe1009 of XO by a carbon hydrogen bond interaction. Quercetin combined with Arg880 of XO by a conventional hydrogen bond interaction, tripeptide EEK formed an attraction charge interaction with Arg880. As shown in Table 3, the number of carbon hydrogen bond interactions and attraction charge interactions formed by the combination of EEK with XO was higher than allopurinol, febuxostat,EEAK, FH, DN, and YLD [15,16,34]. Therefore, carbon hydrogen bond interactions and attraction charge interactions may play key roles in screening for the XO inhibitors.
The structure and IC50value of peptides EEK were similar with known XO inhibitory peptide EEAK. The EEK-XO complex had more carbon hydrogen bond interactions and attractive charge interactions compared with the EEAK-XO complex. Additionally,EEK could bind to Phe1009 of XO which was important for the binding of other XO inhibitory peptides (FH, DN, and YLD).Therefore, the XO inhibitory activity of EEK in vitro was higher than that of EEAK. And it is further proved that Phe1009 residue of XO might play a key role in XO-ligand interaction.
4. Conclusion
Tripeptide EEK with XO inhibitory activity was identified from Ovalbumin with an IC50value of 141 μmol/L. The tripeptide EEK bond to the active center of XO via 3 carbon hydrogen bond interactions, 2 salt bridges, 5 conventional hydrogen bond interactions, and 4 attractive charge interactions. Glu802, Phe1009,and Arg880 are key residues in the interaction of peptides and XO. The carbon hydrogen bond interactions and attraction charge interactions play major roles in the interaction between peptide and the predicted amino acid residues (Glu802, Phe1009, and Arg880) of XO. Tripeptide EEK was promising to use as a natural XO inhibitor to control hyperuricemia. Although EEK from ovalbumin has been shown XO inhibitory activity, ex vivo and in vivo experimentation are needed to further verify these results and explain the mechanism in the future.
Con flicts of interest
There are no con flicts of interest to declare.
Acknowledgement
This paper was supported by Beijing Advanced Innovation Center for Food Nutrition and Human Health (20181036).
- 食品科学与人类健康(英文)的其它文章
- A review on mechanisms of action of bioactive peptides against glucose intolerance and insulin resistance
- Adropin as an indicator of T2DM and its complications
- Isolation and characterization of novel peptides from fermented products of Lactobacillus for ulcerative colitis prevention and treatment
- The immunity-promoting activity of porcine placenta in mice as an immunomodulator for functional foods
- Nutritional properties of Europen eel (Anguilla anguilla) bone peptide-calcium and its apoptosis effect on Caco-2 cells
- A comprehensive method to explore inhibitory kinetics and mechanisms of an anticoagulant peptide derived from Crassostrea gigas

