Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) protein-derived antioxidant peptides: mechanisms of action and structure-activity relationship in Caco-2 cell models
Yiming Zhou, Xunming She, Zhidong Chen, Yun Wei, Ying Xio, Xioli Zhou,b,*
a School of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai 201418, China
b University Think Tank of Shanghai Municipality, Institute of Beautiful China and Ecological Civilization, Shanghai 201418, China
ABSTRACT
Excessive reactive oxygen species (ROS) can cause oxidative damage and lead to various metabolic disease.Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) is a new kind of protein-rich functional food, the protein in which has been proved to have good antioxidant capacity. In this study, in order to further explore the antioxidant mechanism of Tartary buckwheat protein, 4 peptides (CR-8, LR-8, GK-10 and SR-12) were isolated and identified from it. H2O2 was used to induce oxidative damage to Caco-2 cells to evaluate antioxidant capacity of these peptides. The results of superoxide dismutase (SOD), total antioxidant capacity (T-AOC)and mitochondrial membrane potential etc. showed that these peptides have superior antioxidant capacity.CR-8 has the best antioxidant capacity. In order to further clarify the antioxidant mechanism of CR-8,metabolomics was used to analyze related metabolites and metabolic pathways. The results showed that after CR-8 intervention, the content of metabolites such as L-acetyl carnitine has increased. This indicated that CR-8 can improve the antioxidant capacity of damaged cells by intervening in multiple metabolic pathways. This also revealed the anti-oxidant mechanism of tartary buckwheat protein. In conclusion, it provided a theoretical basis for further studying the activity of tartary buckwheat portein and utilizing buckwheat resources.
Keywords:
Antioxidant protective
Tartary buckwheat peptides
Metabolic mechanim
Caco-2 cells
1. Introduction
Reactive oxygen species (ROS) are oxygen-derived small molecules [1] including hydrogen peroxide and the hydroxyl radical; they are generated from normal metabolic processes. ROS are chemically reactive molecules that have essential functions in living organisms [2]. A moderate increase in ROS can promote cell proliferation and differentiation. ROS at low levels can act as cell signaling molecules by reversibly oxidizing thiol groups and function [3]. However, high levels of ROS can harm cells by nonspecifically damaging macromolecules including DNA, proteins,and lipids [4,5]. ROS can lead to multiple metabolic disease like cardiovascular disease [6], type-2 diabetes [7], Alzheimer’s disease [8], and certain types of cancer [9].
Maintaining ROS homeostasis is crucial for normal cell growth and survival. Cells control ROS levels by balancing ROS generation with ROS elimination via ROS-scavenging systems such as superoxide dismutase, glutathione peroxidase, peroxiredoxins,glutaredoxin, thioredoxin and catalase [5]. Food also contains many biologically active substances that scavenge free radicals [10].Currently, the enzymatic hydrolysis of peptides from proteins has become a research hotspot, including plant proteins and animal proteins. The molecular weight of peptides are between amino acids and proteins and have rich biological activities including antioxidant,cholesterol-lowering, and antibacterial effects [11].
Tartary buckwheat is a functional food and its protein has excellent biological activity. Compared with other proteins, it has a unique amino acid composition [12]. In the previous study, we have isolated 13S globulin from Tartary buckwheat and analyzed its structural characteristics. And we also investigated the protective effect of acidic subunit enzymatic hydrolysates of Tartary buckwheat 13S globulin against oxidative stress induced by H2O2[13]. In this study, to further evaluate the antioxidant effect of these peptides, we used Caco-2 cells to construct an oxidative stress model. At the same time, we used metabolomics to deeply analyze the in fluence of these peptides on related metabolic pathways.
PyMOL is one of the most widely used molecular visualization systems: It is a simple and powerful tool for sequence and structure analysis and homology modeling [14] and is suitable for drug development [15] including the evaluation of binding sites and interaction forces, protein preparation, and molecular mechanics [16].
Metabolomics can analyze the changes in the body’s metabolites under the effects of endogenous or exogenous stimuli and interference such as diseases and drug treatments [17]. It can analyze changes in cell endogenous metabolites, find biomarkers that can reflect the state of the body, and help people understand related metabolic pathways [18]. Currently, liquid chromatography mass spectrometry (LC-MS), gas chromatography mass spectrometry (GC-MS)and capillary electrophoresis mass spectrometry (CE-LC) are among the most commonly used tools in chromatographic analysis of the metabonomics [19]. This experiment used high performance liquid chromatography mass spectrometry (HPLC-MS) to analyze the changes in the metabolism of Caco-2 cells after the intervention of Tartary buckwheat peptides to evaluate the effect of Tartary buckwheat peptides on the cells.
2. Materials and methods
2.1 Materials
The human colon adenocarcinoma Caco-2 cell line was obtained from Chinese Academy of Sciences. Dulbecco’s Modified Eagle Medium (DMEM), nonessential amino acid (NEAA),Hanks’ balanced salt solution (HBSS) were products of Gibco Life Technologies. Malondialdehyde (MDA) determination kit,catalase (CAT) determination kit, superoxide dismutase (SOD)determination kit and total antioxidant capacity (T-AOC)determination kit were purchased from the Beyotime Institute of Biotechnology (Shanghai, China). Synthesized peptides,namely, CTGFVAVR, LRGENDQR, GFIVQAQDLK, and SFFLAGQSQQGR, with purities higher than 98% were provided by GL Biochem Ltd. (Shanghai, China). All chemicals were of chromatographic or analytical quality and purchased from Sinopharm Chemical Reagent (Shanghai, China) unless mentioned otherwise.
2.2 Structure and property analysis of Tartary buckwheat peptides
The identified amino acid sequences were input into PyMOL to simulate the three-dimensional structure of Tartary buckwheat peptides. Then the relationship between structure and antioxidant function was analyzed.
2.3 Cell culture and treatments
Caco-2 cells were obtained from the Chinese Academy of Sciences. All cells were routinely cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS). The cytoprotective effect of peptides against H2O2-induced oxidative damage was evaluated in parallel experiments. Caco-2 cells were incubated with synthesized peptides as indicated above followed by stimulation with 600 μmol/L H2O2for 4 h. To determine intracellular enzymatic viability, Caco-2 cells (5 × 104viable cells/well) were seeded onto 96-well black, clear-bottom plates and allowed to attach for 24 h.The cells were then stimulated with 600 μmol/L H2O2for an extra 4 h followed by incubation with synthesized peptides (500 μmol/L).According to the above processing method, the cells were divided into three main groups. They were control group which referred to the Caco-2 cells without treatment. The model group referred to Caco-2 cells under oxidative stress which were induced by H2O2.The intervention group referred to the Caco-2 cells under oxidative stressed which were intervened by Tartary buckwheat peptides. The intervention group were further divided into the CR-8 group, LR-8 group, GK-10 group and SR-12 group.
2.4 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
After incubation, the medium was removed and then 100 μL of serum-free medium containing MTT solution at afinal concentration of 5 mg/mL was added to each well. Cells were then incubated for another 4 h in a humidified atmosphere of 95% air with 5% CO2at 37 °C. Then, the medium was removed and 100 mL of DMSO was added into each well to dissolve the purple formazan crystals.Finally, the absorbance of each well was measured at 490 nm using a microplate reader. Cell viability was expressed as the percentage of the non-treated control, which was set to 100% [20].
2.5 The measurement of apoptosis and necrosis
Referred to Ormerod et al. [21] for measurement of apoptosis and necrosis with some adjustment. The cells to be tested were washed and centrifuged, and mixed with fluorescent dyes Hoechst 33342 and Propidium Iodide (PI) in at a volume of 1:1; they were then added to the cells for staining at 4 °C. The cells were collected by enzymatic digestion after the intervention, and a double staining method was used to determine the degradation and necrosis of the cells. The dark wavelength was used for 20–30 min. Flow cytometry analysis included two channels red and blue; there were 5 000 events.
2.6 Effect of Tartary buckwheat peptides on cell growth cycle
To judge the effect of Tartary buckwheat antioxidant peptides on cell oxidative stress, cells (2 × 105) were collected after the intervention. The Caco-2 cells were digested and blown evenly from a 12-well plate, washed with PBS, and centrifuged for 5 min. Then 70% precooled ethanol was added and fixed at 4 °C for overnight. And then, the cells were centrifuged to remove the supernatant and added 500 μL RNase A (50 μg/mL) at 37 °C for 30 min to digest the RNA in the cells. After PBS washing and adding PI dye (50 μg/mL), the cells were stained at 4 °C for 30 min.
2.7 The measurement of cells membrane potential
After digesting and collecting the cells, we added 1 mL of JC-1 staining working solution and mixed well. The sample was incubated for 20 min at 37 °C in a cell incubator. After incubation,the supernatant was aspirated and washed twice with JC-1 staining buffer; 2 mL of cell culture solution was added mixed well, and the Caco-2 cells were observed with through an inverted fluorescence microscope.
2.8 Determination of T-AOC, CAT, SOD, and MDA in cells
Caco-2 cells were cultured in 24-well plates. After the cells adhered to the wall and spread over the bottom, the samples were interfered for 24 h. Then cells were then collected and crushed with an ultrasonic pulverizer. The T-AOC, MDA, SOD, and CAT activity were evaluated using an intracellular determination kit. The relevant tests were carried out in accordance with the instructions provided by the kit.
2.9 Data processing and metabolites identification of metabolomics
2.9.1 Sample preparation
The intracellular metabolites were extracted according refer to Li et al. [22]. Samples were reconstituted in 200 μL MeOH/H2O (85:15,V/V); 100 μL was diluted to 50% (V/V) MeOH in water for non-targeted metabolomics study followed by 30 μL of sample pooled together from each participant as a quality control (QC) sample. The other 100 μL was used for targeted metabolomics. All samples were detected within 24 h of reconstitution.
2.9.2 HPLC-MS conditional selection
Referred to Ma et al. [23] for chromatographic separation and massed spectrometry detection conditions with some adjustment.HPLC-MS was used in this experiment and the related conditions were optimized.
The optimal conditions of HPLC were set as followed: the metabolite was fractionated using a C18column with a flow rate of 0.3 mL/min. Mobile phase A was 0.1% formic acid in HPLC water,mobile phase B was 0.1% formic acid in ACN and used 40 °C as column temperature. The solvent gradient changed according to the following conditions: 0–2 min, 95% A; 2−12 min, 95%−5% A;12−15 min, 5% A; 15−17 min, 5% A.
The optimal conditions of MS were set as followed: positive ion mode, heater temperature: 300 °C; capillary temperature: 350 °C;electrospray voltage: 3.0 kV; exhaust gas flow rate: 1 arb; sheath gas flow rate: 45 arb; auxiliary gas flow rate: 15 arb; S-Lens RF Level,30%. Negative ion mode: heater temperature: 300 °C; capillary temperature: 350 °C; electrospray voltage: 3.2 kV; exhaust gas flow rate: 1 arb; sheath gas flow rate: 45 arb; auxiliary gas flow rate: 15 arb;S-Lens RF Level, 60%.
2.10 Statistical analysis
Data were expressed as means ± standard deviation (SD). All of the statistical analyses were carried out using SPSS 20.0 software(SPSS Inc., Chicago, IL, USA). Multiple group comparisons were performed using a one-way analysis of variance (ANOVA). The level of statistical significance was set at P < 0.05.
The mass spectrometry data were converted into GDF format by Chroma TOF software and imported into SIMCA-P software,version 11.0 for principal component analysis (PCA), partial least-squares discrimination analysis (PLS-DA), orthogonal partial leastsquares-discrimination analysis (OPLS-DA) and other multivariate statistical analysis.
3. Results
3.1 Structure and properties of Tartary buckwheat peptides
The 4 peptides consist of 8–12 amino acids and have a molecular weight of about 800–1 400 Da. The properties of amino acids can determine the activity of peptides [24], and 4 peptides have been identified as containing amino acids with antioxidant capacity.Therefore, these 4 peptides may have antioxidant properties, but the antioxidant activity needs to be further verified. These Tartary buckwheat peptides all have random coils and β-sheets, and some peptides have α-helices. These structures greatly affect the properties of the peptides. The sequence of antioxidant peptides included a high hydrophobic moiety. This moiety can scavenge lipid-derived radicals due to interaction with lipid molecules [25]. Small peptides were extraordinarily potent in preventing cell death due to pro-oxidants [26].The peptides contained 27 amino acids (3 037.49 Da) and showed antioxidant activity that positively correlated with concentration; there was no antimicrobial activity. The small size and hydrophobic amino residues contributed to the high activity [24,27] (Fig. 1).
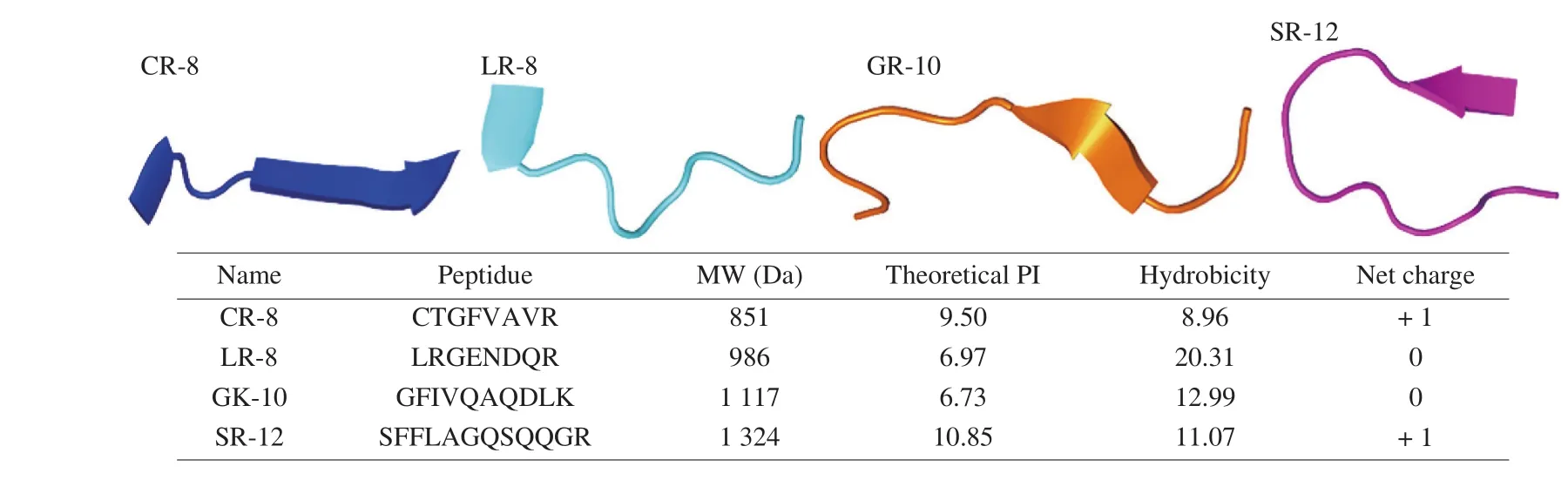
Fig. 1 Three-dimensional simulated structure of Tartary buckwheat peptides.
Theoretical isoelectric point, hydrophobicity, and net charge of each peptide was calculated using ExPASyf (http://ExPASyf.com).
3.2 Determination of cell viability and oxidative damage model
3.2.1 Cell viability
The toxicity of Tartary buckwheat peptides to cell were evaluated including the protective effect of Tartary buckwheat peptides on oxidative damage to Caco-2 cells. The results were shown in Fig. 2.The cell viability measured by the MTT method showed that at a concentration of 500 μg/mL, the cell viability of Tartary buckwheat peptides intervention was not significantly different from that of the control group. Cells can neither show inhibitory viability nor promote cell growth, i.e., Tartary buckwheat peptides do not impact oxidation.The peptides hydrolyzed by alkaline protease were relatively safe for the cell event at 500 μg/mL.
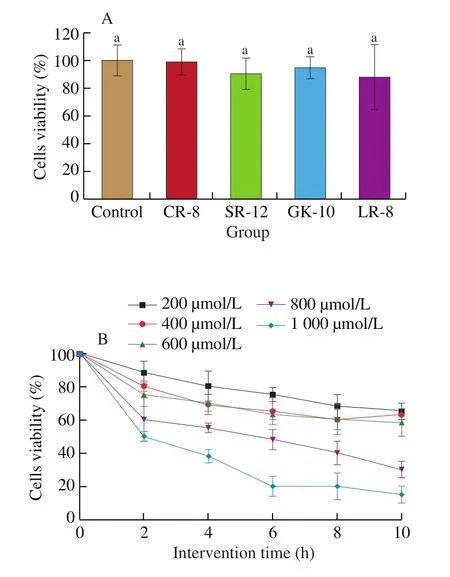
Fig. 2 Construction of cell viability and cell damage model. (A) The effect of Tartary buckwheat peptides content on cell viability. (B) The effect of different concentrations of H2O2 and intervention time on Caco-2 cells.Different lowercase letters indicate significant differences (P < 0.05).
3.2.2 Construction of oxidative damage model
H2O2can induce oxidative stress and damage to cells. In order to determine the amount of H2O2used and the intervention time.Under the conditions of different concentration gradients of H2O2and intervention time, a cell viability of about 50% was determined as the best intervention condition. The experimental results were shown in the Fig. 2. The survival rate of Caco-2 cells was related to the concentration of H2O2and the intervention time. With the extension of the intervention time, the cell viability showed a downward trend.After the intervention for 4 h, the cell viability under different H2O2concentrations tended to be flat. When the intervention concentration of H2O2was 800 μmol/L, the cell viability could reach 55% relative to the control group. Therefore, the best result was when the intervention time was 4 h, the H2O2concentration was 800 μmol/L.
3.3 Effects of Tartary buckwheat peptides on Caco-2 cells
3.3.1 Analysis of cell apoptosis and necrosis
The apoptosis and necrosis of cells can be distinguished by flow cytometry (Fig. 3A). The apoptosis and necrosis rate of the cells treated with H2O2increased significantly, which indicated that the cells were damaged and gradually necrotized under the effect of H2O2.Long term oxidative stress state would lead to irreversible damage of most organelles: ROS damaged double-stranded DNA and caused apoptosis [28]. The intervention of Tartary buckwheat peptides can effectively reduce the proportion of apoptotic cells, which showed that Tartary buckwheat peptides could act on the early apoptotic state of cells and improve the viability of cells; however, this could not reach the level of normal cells, i.e., the control group, in which the effect of CR-8 peptide was the best, and that of GK-10 was second.Compared with the cells in the early stage of apoptosis, Tartary buckwheat peptides had no obvious effect on the cells in the late stage of necrosis.
3.3.2 Analysis of cell cycle
The effect of Tartary buckwheat peptides on the Caco-2 cell cycle were shown in Fig. 3B. Compared with the control group, the Caco-2 cells in the G1 phase of the model group were significantly reduced.This was due to the concentration of the nucleus and fragmentation of the DNA, resulting in a decrease in the fluorescence intensity of the cell DNA. H2O2would induce oxidative fragmentation of DNA,which would eventually lead to cell death [29]. At the same time, the proportion of cells in the S phase increases, indicating that the cells were blocked at this stage. After the intervention of Tartary buckwheat peptides, the proportion of cells in the G1 phase increased, and the S phase decreased. These changes suggested that the cells resumed their normal growth cycle. The smaller molecular weight meant that the peptides could more easily penetrate the cell membrane.
3.3.3 Mitochondrial membrane potential
Under oxidative stress, the mitochondrial membrane potential decreases [30]. After the intervention of Tartary buckwheat peptides,the mitochondrial membrane potential of most cells was significantly improved (Fig. 3C). The cell mitochondrial viability after CR-8 intervention was the highest. The cells were covered with clear red fluorescence. SR-12 had the worst effect, and the red fluorescence distribution was scattered and weak. Changes in mitochondrial viability affected mitochondrial dynamics leading to a loss in membrane potential, increased production of reactive oxygen species,and depletion of ATP. This led to severely impaired mitochondrial function, mitochondrial dysfunction-mediated apoptosis [31].Buckwheat peptides could significantly improve the vitality of damaged mitochondria and gradually restore the cells to normal.
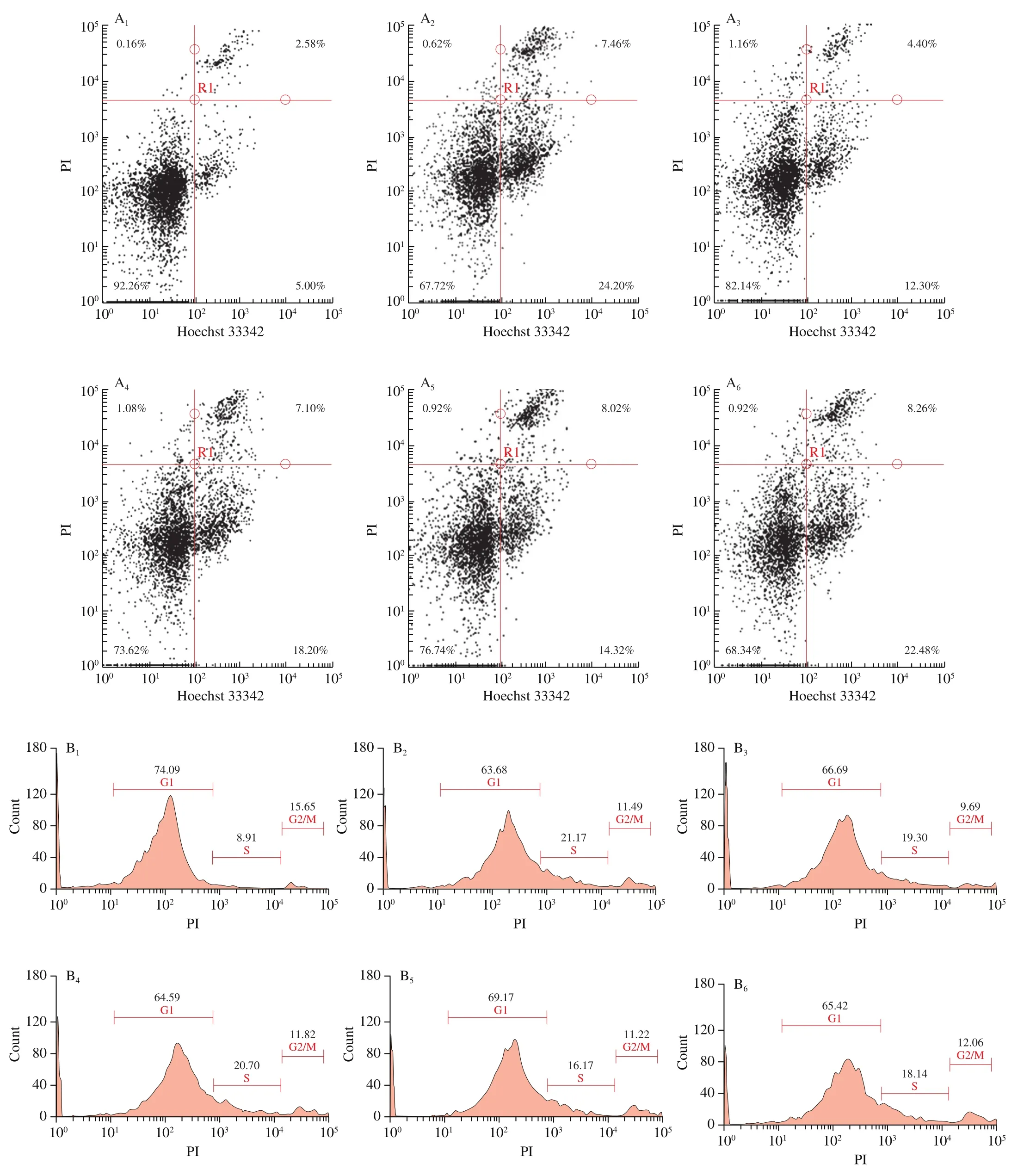
Fig 3 Effects of Tartary buckwheat polypeptide on Caco-2 cells. (A) The effects of Tartary buckwheat peptides on apoptosis and necrosis rate of Caco-2 cells under oxidative stress. It shows the distribution of apoptosis and necrosis of different groups of Caco-2 cells. Numbers adjacent to the outline indicate the percentage of cells in each area. (B) The effects of Tartary buckwheat peptides on Caco-2 cell cycle. Numbers indicate the percentage of cells in each stage.(C) The effects of Tartary buckwheat peptides on mitochondrial membrane potential of Caco-2 cells. The distribution of red fluorescence represents the viability of cell mitochondria. (1) Control group; (2) H2O2 treated group; (3–6) Tartary buckwheat peptides (CR-8, LR-8, GK-10, SR-12) treated before the same H2O2 stimulation. In thefigures, red circles were used to circle the area where the membrane potential changes more obviously.

Fig 3 (Continued)
3.4 Cytoprotective effect of Tartary buckwheat peptides against H2O2-induced oxidative stress in Caco-2 cells
The antioxidant capacity of cells is composed of enzymatic and non-enzymatic systems. The total antioxidant capacity can re flect the health of the cells [32]. The total antioxidant capacity of Caco-2 cells decreased significantly (P < 0.05) under the induction of hydrogen peroxide (Fig. 4A). The total antioxidant capacity of Caco-2 cells could be improved by Tartary buckwheat peptides intervention, and CR-8 had the best effect: it was 51.38% higher than the H2O2group,but 33.53% lower than the control group. The molecular weight and length of Tartary buckwheat peptides could affect its biological activity, but there seemed to be no definite relationship between activity and weight/length. A relatively small molecular weight can more easily act intracellularly with better antioxidant activity.However, the relatively long peptide (SR-12) also had a better effect,which showed that the amino acid composition of peptides had a large in fluence on antioxidant activity.
The CAT activity (Fig. 4B) in the model group decreased by 45.21%, compared with the control group, and H2O2decreased the activity of antioxidant enzymes. All four peptides could significantly increase the activity of CAT enzyme in injured cells. Especially, after the CR-8 intervention, the CAT activity increased by 64.66%, and the activity of SOD (Fig. 4C) was similar to CAT. The activity of the antioxidant enzyme in the CR-8 group increased by 64.66% compared with the model group, but the activity of the antioxidant enzyme in the Tartary buckwheat peptides group was still far from that in the control group.
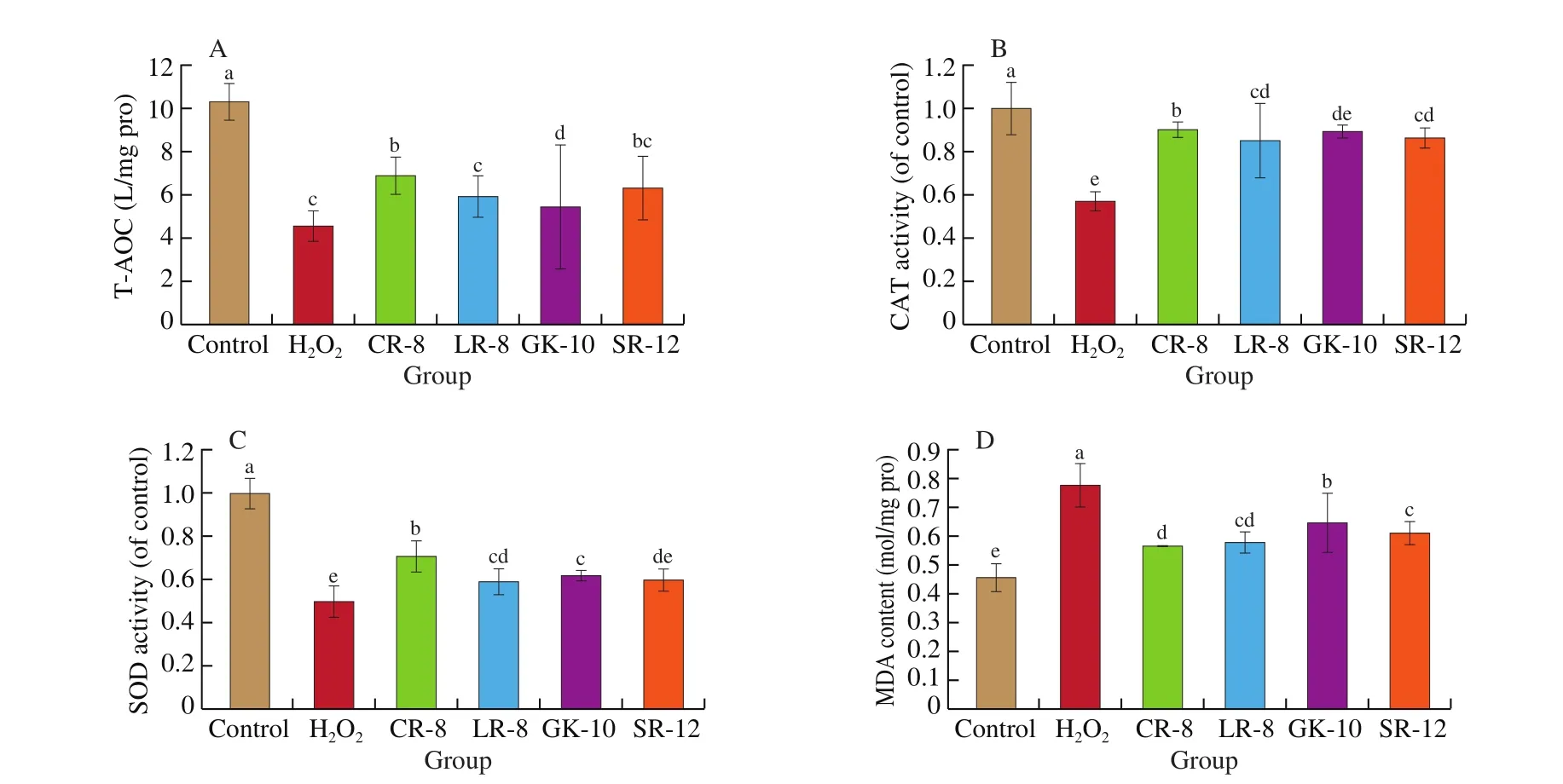
Fig. 4 Cytoprotective effect of Tartary buckwheat peptides against H2O2-induced oxidative stress in Caco-2 cells. The bar graph shows the impact of Tartary buckwheat peptides on different groups from four different aspects. (A) The effect of Tartary buckwheat peptides on the total antioxidant capacity of Caco-2 cells under oxidative stress. (B) Effect of buckwheat peptides on CAT enzyme activity of Caco-2 cells under oxidative stress. (C) Effect of Tartary buckwheat peptides on SOD activity in Caco-2 cells under oxidative stress. (D) The effect of Tartary buckwheat peptides on MDA content in Caco-2 cells under oxidative stress.Different lowercase letters indicate significant differences (P < 0.05).
The MDA content (Fig. 4 D) in the model group was significantly higher than the control group (47.33%, P < 0.05) perhaps due to the peroxidation of unsaturated fatty acids in the cell biofilm by H2O2, and the production and dissociation of a high concentration of MDA in the cells. This enhanced lipid peroxidation damages the cell membrane structure. Tartary buckwheat peptides could significantly reduce the content of MDA in cells and gradually returned them to the level of MDA in the control group. This showed that the lipid peroxidation in Caco-2 cells had been reduced, and the function of the cell membrane had been restored. This result may be related to the hydrophobicity of peptides, i.e., the peptides with a higher hydrophobicity were more likely to act on the cell membrane composed of lipids to remove accumulated peroxides, prevented the peroxidation of unsaturated fatty acids, and maintained the normal structure and function of the cell membrane.
Based on the above experimental results, Tartary buckwheat peptides could improve cell activity by improving cell mitochondrial activity and avoid early cell apoptosis. In addition, Tartary buckwheat peptides could significantly improve the antioxidant capacity of Caco-2 cells under oxidative stress. However, different Tartary buckwheat peptides had different effects. Among them, CR-8 had the best effect.
3.5 Multivariate statistical analysis of cell metabolomics
In order to further explore the protective mechanism of Tartary buckwheat peptides on Caco-2 cells under oxidative stress. We selected the CR-8 group with the best experimental results as the samples for metabolomics experiments, and conducted in-depth identification and analysis of its metabolites. The differences among the samples from different cell groups were examined using principal components analysis (PCA). After mass spectrometry analysis, the PCA scores of the positive ion mode and the negative ion mode were shown in Fig. 5. In order to further differentiate the data and highlight the differences between groups, the data was analyzed by PLS-DA and OPLS-DA. At the same time, the permutation test was used to verify the reliability of the model with the intercept of the fitted regression line on the Y axis being less than 0.

Fig. 5 The mean PCA, PLS-DA, Replacement verification plot and OPLS-DA of the control, the H2O2 and CR-8 groups. (A) Score graph in negative ion mode.(A1) PCA score plot (R2X = 0.607, Q2 = 0.439). (A2) PLS-DA score plot (R2X = 0.53, R2Y = 0.985, Q2 = 0.931). (A3) Replacement verification plot, intercept of the regression Q2 = -0.260 773. (A4) OPLS-DA score graph. (B) Score graph in positive ion mode. (B1) PCA score plot (R2X = 0.729, Q2 = 0.594). (B2) PLS-DA scoreplot (R2X = 0.729, R2Y = 0.99, Q2 = 0.948). (B3) Replacement verification plot, intercept of the regression Q2 = -0.260 773. (B4) OPLS-DA score graph.
In the negative ion mode, a total of 3 035 metabolites were obtained. Three principal components are obtained by PCA. In the positive ion mode, a total of 1 383 metabolites were obtained, and 5 main components were obtained by PCA. Through PLS-DA, OPLS-DA and Permutation test, we found that the three groups were more distinct in the negative ion mode, while the samples in the group were relatively concentrated. However, the quality control group clustered better in the positive ion mode. However, the experimental group was not obvious, and there was a cross-stacking phenomenon in the control group, model group and the intervention group, indicating that there was a certain similarity in the metabolites in the positive ion mode. It was further confirmed by PLS-DA and OPLS-DA.
Based on the above analysis, although the PCA score chart in the negative ion mode showed more obvious clustering, the cumulative principal component score was lower than that in the positive ion mode,and the modelfit was worse than that in the positive ion mode. Therefore,we chose the metabolites in positive ion mode for further analysis.
The control group and the intervention group were well distinguished, and the model group was more scattered. Compared with the control group, the model group was closer to the intervention group, indicating that the two groups of metabolites detected in the positive ion mode were similar to a certain degree, which may be due to the same metabolic pathways, resulting in similar scatter scores.After H2O2intervention, the cell metabolites were significantly different and the points were relatively scattered, indicating that the metabolites were complex, and the relatively concentrated scatter score chart indicated that the internal clustering was better. This may be different from the pathway of cellular metabolism that peptides participate in. It showed that CR-8 could affect multiple cellular metabolic pathways to a certain extent.
3.6 Identification of differential metabolites
In this study an S-plot map of OPLS-DA was used to screen the differential metabolites under the conditions of |P(corr)| > 0.5 and|P| > 0.1. We used the FC value (fold change) to observe the change in differential metabolites between groups. The qualitative analysis of metabolites was based on the comparison ofm/zand HMDB. As it showed in Fig. 6, the control group and the model group involved relatively few differential metabolites, while the difference between the model group and the CR-8 group increased significantly. This may indicate that Tartary buckwheat peptide CR-8 can interfere with multiple metabolic pathways to achieve antioxidant effects.
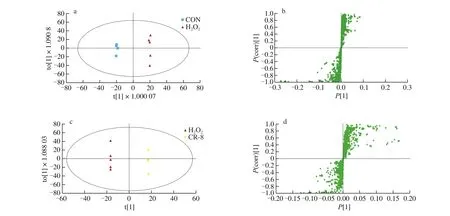
Fig. 6 Metabolic difference analysis between control group, model group and CR-8 group. (a) OPLS-DA score plot of the control group vs. model group.(b) OPLS-DA S-plot of the control group vs. model group. (c) OPLS-DA score plot of the model group vs. CR-8 group. (d) OPLS-DA S-plot of the model group vs. CR-8 group.
The major potential metabolites in the positive ion models were screened in samples of cells (Table 1). The analyzed metabolic differences mainly included: L-acetylcarnitine; L-tryptophan;acetylspermidine; lysophosphatidic acid (20:4); lysophosphatidic acid (20:3); lysophosphatidic acid (16:0); pantothenic acid;glycerophosphoethanolamine; L-histidine; L-alanine. These metabolites involve multiple metabolic pathways. Table 1 showed the logarithm of the ratio of the model group to the control group and the intervention group to the model group (base 2). A positive number means an increase, a negative number means a decrease. Among them, L-histidinol and L-aspartic acid levels increased. It showed that the change of free amino acid content could directly affect cell growth and proliferation, and also confirmed the experimental results of apoptosis and necrosis and cell cycle.
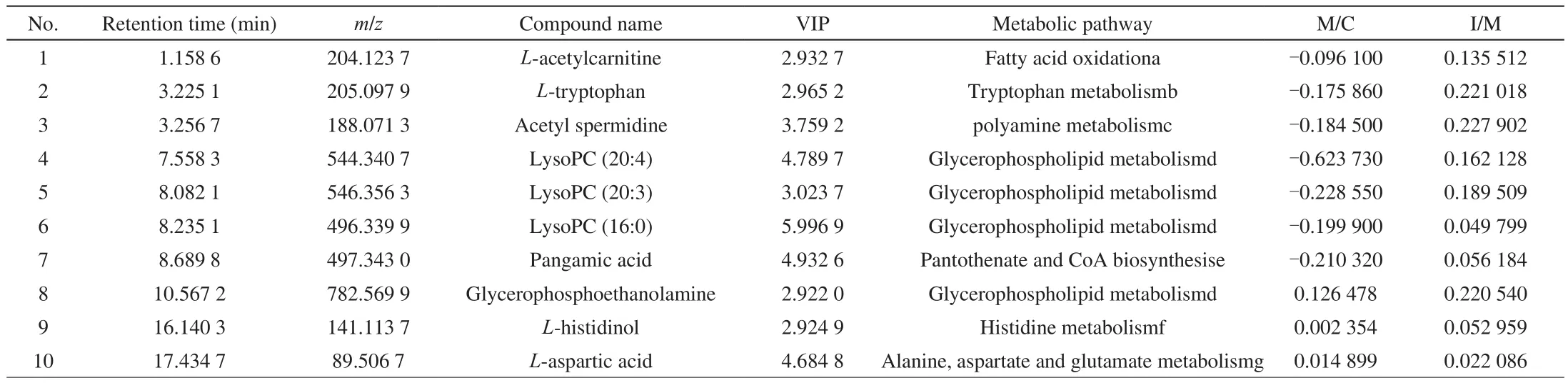
Table 1The effect of CR-8 on the marker differential metabolites and metabolic pathway of Caco-2 cells under oxidative stress.
3.7 Metabolic pathway analysis
Some metabolic pathways have significant effects on the oxidative stress of Caco-2 cells: fatty acid metabolism; glycerol phospholipid metabolism; tryptophan metabolism; pantothenic acid and coenzyme A biosynthesis; histidine metabolism; and alanine, aspartic acid and glutamate metabolism (Fig. 7).
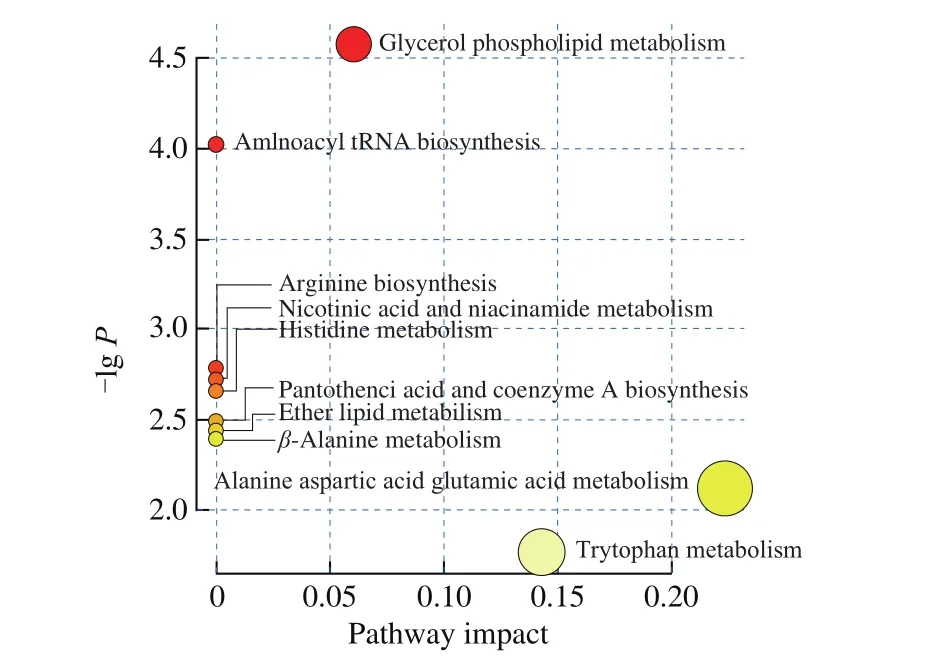
Fig. 7 Tartary buckwheat peptides involved in metabolic pathways.Summary of pathway analysis using MetaboAnalyst 3.5. Each point represents one metabolic pathway; dot size and shade of color positively correlate with the impact of the metabolic pathway.
Among them, glycerol phospholipid metabolism had the highest contribution value, and glycerol phospholipid has an important in fluence on cell membrane function. This also proved that CR-8 can improve Caco-2 cells which under oxidative stress by interfering with glycerol phospholipids and other related metabolic pathways.
4. Discussion
A comparison of the structure of Tartary buckwheat peptides suggest that Val can mprove the CAT activity in the cell when the N-terminal and ASP are at the C-terminal of the peptides these conclusions are consistent with the results whereby hydrophobic amino acids such as Leu, Pro, Phe, and Val play an important role in the antioxidant activity of the peptides [26,33]. ROS involved in cell growth and metabolism [34]. ROS accumulation can damage organelles including mitochondria, endoplasmic reticulum, and cell membrane [35]. Caco-2 cell necrosis during oxidative stress, may be altered by Tartary buckwheat peptides. These peptides affect cells with mild damage but not cells with severe damage.
The peptides sequence has a stronger antioxidant activity at about 8 amino acid [24]. Hydrophilic amino acids also affect antioxidant activity [36]. For example, the total antioxidant capacity of SR-12 is next to that of CR-8, which may be related to the high content of glutamine [37]. However, CR-8 had strong free radical scavenging ability, which was related to the N-terminal cysteine (Cys). Since tryptophan had lost H, it could show stronger oxygen free radical binding ability.
L-acetylcarnitine is mainly involved in fatty acid metabolism [38].Its main function is to transfer fatty acids from cytoplasm to mitochondria for β-oxidation and to store energy in the form of acyl carnitine Disorders of lipid metabolism could cause abnormal TCA cycle intermediate products [39], elevated free fatty acids [40], and changed in amino acid content [41] may directly affect cell growth and proliferation. Compared with the control group, the carnitine content of the model group decreased. This may be caused by H2O2inducing oxidative damage to the cells, which made the cell fatty acid metabolism disorder. After CR-8 intervention, the content of L-acetylcarnitine increased, lipid metabolism gradually returned to normal, and cell oxidative damage was improved. Cell cycle,apoptosis and necrosis experiments can also prove it. In fatty acid metabolism, fatty acid synthase is a key enzyme in the process of fatty acid synthesis, which catalyzes the synthesis of endogenous long fatty acids [42]. The increase in the expression of fatty acid synthase will significantly increase the deposition of triglycerides in the body.
Lysophosphatidic acid (LPC) is involved in glycerol phospholipid metabolism [43]. It is mainly regulated by lysophosphatidylcholine transferase and phospholipase in the blood [44]. The PLA2/LPC pathway is one of the most important metabolic pathways in the metabolism of glycerophospholipids. And the metabolism of glycerophosphatidyl plays an important role in lipid metabolism [45].Compared with the control group, the LPC content of the model group decreased, which was caused by severe oxidative damage.The increase in LPC content in the CR-8 group indicated that the antioxidant capacity of cells and the lipid oxidation situation has been improved. Because PLA2 not only maintains cell membrane phospholipid homeostasis, it also plays an important role in regulating oxidative damage and inflammation under physiological and pathological conditions [37]. This proved that Tartary buckwheat peptides can regulate lipid metabolism to a certain extent, and protect cells under oxidative damage.
The content of alanine, histidine and tryptophan changed, and the amino acid content of the intervention group increased compared with the model group. This may indicate that CR-8 can interfere with the amino acid metabolism pathway of the cell and improve cell viability.The results of cell cycle experiments can also prove this.
Therefore, Tartary buckwheat polypeptide represented by CR-8 can increase the activity of Caco-2 cells under oxidative stress. The main principle is to improve cell viability by improving the state of organelles. Because different kinds of Tartary buckwheat polypeptides have different effects and can only partially restore cells. This may be related to the intervention concentration of Tartary buckwheat polypeptide, so further research is needed.
In conclusion, this study provided new evidence that Tartary buckwheat peptides has an effect on cells with mild damage from ROS. The CR-8 peptide has the best effect and contains a peptides sequence with 8 amino acids. We analyzed the related metabolites of lipid oxidation, and the intervention of Tartary buckwheat peptides CR-8 partially improved lipid metabolism and restored cells to a normal state. At the same time, we analyzed the metabolic pathways related to amino acids, because the Tartary buckwheat peptides participates in the related metabolic pathways. These pathways improve cell viability and promote cell growth. These results may influence cell reparation and affect multiple metabolic diseases.Therefore, this study will provide a research direction for the development of Tartary buckwheat in food science.
Con flicts of interest
The authors confirm that there are no con flicts of interest in this manuscript.
Acknowledgements
The authors thank Shanghai Natural Science Foundation(20ZR1455800), the National Science Foundation of China(31871805), China Agriculture Research System (CARS-08-D2)and Shanghai Municipal Education Commission (Plateau Discipline Construction Program).
- 食品科学与人类健康(英文)的其它文章
- A review on mechanisms of action of bioactive peptides against glucose intolerance and insulin resistance
- Adropin as an indicator of T2DM and its complications
- Isolation and characterization of novel peptides from fermented products of Lactobacillus for ulcerative colitis prevention and treatment
- The immunity-promoting activity of porcine placenta in mice as an immunomodulator for functional foods
- Nutritional properties of Europen eel (Anguilla anguilla) bone peptide-calcium and its apoptosis effect on Caco-2 cells
- A comprehensive method to explore inhibitory kinetics and mechanisms of an anticoagulant peptide derived from Crassostrea gigas

