Comparing the difference in enhancement of kokumi-tasting γ-glutamyl peptides on basic taste via molecular modeling approaches and sensory evaluation
Jun Yng, Jing Guo, Ruijie Mi, Ho Dong, Chun Cui, Xiofng Zeng,*, Weidong Bi,*
a College of Light Industry and Food Technology, Guangdong Provincial Key Laboratory of Lingnan Specialty Food Science and Technology,Academy of Contemporary Agricultural Engineering Innovations, Zhongkai University of Agriculture and Engineering, Guangzhou 510225, China
b Key Laboratory of Green Processing and Intelligent Manufacturing of Lingnan Specialty Food, Ministry of Agriculture, Guangzhou 510225, China
c School of Food Science and Engineering, South China University of Technology, Guangzhou 510640, China
ABSTRACT
γ-Glutamyl peptides can enhance basic taste sensations such as saltiness, sweetness, and umaminess, while the molecular mechanism and the difference in taste enhancement remain elusive. Thus, two complex conformations: taste type 1 receptor 1 (T1R1)-MSG and taste type 1 receptor 2 (T1R2)-sucrose were constructed to form binding receptors. These peptides showed affinity for the two receptors, but a higher affinity scores and more binding amino acid residues for the T1R1-MSG receptor, implying that they may exhibit a higher umami-enhancing effect. Thereinto, γ-glutamyl alanine (γ-EA) displayed the highest affinity for the two receptors through mobilizing multiple amino acid residues to form hydrophobic and hydrogen bonds, indicating it had the highest enhancement for umaminess and sweetness among these peptides. Sensory evaluation demonstrated the enhancement of γ-EA on umaminess was superior to that of sweetness. Generally,γ-glutamyl peptides could enhance basic taste sensation via activating taste receptor, and exhibited a highest umami-enhancing effect.
Keywords:
γ-Glutamyl peptides
Taste-enhancing effect
Molecular mechanism
Taste type 1 receptor 1
Taste type 1 receptor 2
1. Introduction
Taste enhancers are usually substances that are tasteless, but enhance the taste intensity of sugar, monosodium glutamate (MSG),or salt. With the increase in public health concerns worldwide, these substances are attracting increasing attention in the food industry because of their special properties. For example, non-caloric sweet taste enhancers have been used to partially replace sugar in a range of foods and beverages to address health concerns, such as obesity and diabetes [1,2]. Salt taste enhancers are increasingly preferred by consumers and merchants because they not only reduce the use of sodium salt to reduce the risk of cardiovascular disease but also maintain tastiness [3]. Therefore, the development of taste enhancers has received extensive attention, especially the natural ones.
Many natural substances have been identified as taste enhancers,but in general, a single sequence can only enhance one taste, for example, Met-Gly, a salt taste enhancer, only enhances saltiness perception [3]; SE-1, a sweet taste enhancer, only enhances sweetness perception [1,2]; while guanosine-5’-monophosphate (GMP)and inosine-5’-monophosphate (IMP) enhance umaminess perception [4,5]. Unlike these substances, a class of kokumi-taste γ-glutamyl peptides have been found to enhance a variety of basic taste sensations, such as saltiness of salt, sweetness of sucrose,and umaminess of MSG [6-14]. These γ-glutamyl peptides that are tasteless in aqueous solution can enhance the umami taste of MSG via the activation of taste type 1 receptor 3 (T1R3) in the presence of MSG [14], and they exhibit kokumi taste property by activating calcium-sensing receptors [7,8,15]. However, the mechanism of their enhancement of sweetness and saltiness and the differences in the mechanism of enhancing basic tastes have not been studied so far.
It is known that sweetness and umaminess are mediated, in large part, by heterodimeric G protein-coupled receptors (GPCRs): taste type 1 receptor 1 (T1R1)-T1R3 and taste type 1 receptor 2 (T1R2)-T1R3,respectively [4,16,17]. The T1R1-T1R3 and T1R2-T1R3 heterodimers are sensitive to umami substances such as Glu, Asp, IMP, GMP, and umami peptides [18-20], and sweet substances such as saccharin,aspartame, and cyclamate [1,2,21], respectively. It is worth noting that these heterodimers are also sensitive to taste enhancers, for example,IMP exhibits umami-enhancing effect through an interaction with the T1R1 receptor [5,18]; umami peptides exhibit umami-enhancing effects through an interaction with the T1R1-T1R3 receptor [22,23].Small-molecule sweet taste enhancers are well known as positive allosteric modulators of T1R2-T1R3 and could be used as a substitute for sugar in food and beverages to reduce the calories in consumer products [1,2]. Our previous study also confirmed that γ-glutamyl peptides, with no basic taste, can enhance the basic taste like sweetness, saltiness, and umaminess perception via activating the calcium-sensing receptor [8,10] and/or an interaction with T1R3 in the presence of MSG [14]. However, the interactions of γ-glutamyl peptides with T1R1/T1R2 taste receptors remain elusive.
Therefore, in this study, the molecular mechanisms of the sweetor umami-enhancing effect of γ-glutamyl peptides were investigated by analyzing the interactions between T1R1 and T1R2.
2. Material and methods
2.1 Reagents
γ-Glutamyl alanine (γ-EA) was obtained from the Peptide Biological Technology Co., Ltd. (Nanjing, China). A commercial soy sauce and blank chicken broth stock powder were purchased from a local supermarket in Guangzhou, China.
2.2 Homology modeling and molecular docking of taste receptors with γ-glutamyl peptides in the presence of MSG or sucrose
Homology modeling was performed to establish the 3-dimensional (3D)structure of T1R1 and T1R2 taste receptors using the Discovery Studio/Modeler 9.20 protocol, based on the method of Yang et al. [14].The gene sequences of hT1R1 and hT1R2 were obtained from UniProt (https://www.uniprot.org/uniprot/) and the metabotropic glutamate (Glu) receptor was used as the template (PDB ID: 1EWK).The model-single script in the protocol was executed to calculate the score of GA341, molpdf, and DOPE, and the optimal structure, with a larger GA341, a smaller molpdf, and a more negative DOPE score,was selected.
To compare the differences in the taste-enhancing effect of γ-glutamyl peptides, receptor complexes of basic taste substance (MSG or sucrose) and taste receptor (T1R1 or T1R2) were constructedfirst,following the method of Yang et al. [14]. Specifically, sucrose was docked into T1R2 to construct the T1R2-sucrose receptor complex,and MSG was docked into T1R1 to construct the T1R1-MSG receptor complex using the DS/PyMOL protocol. Subsequently, molecular docking was performed to evaluate the affinity between these ligands and the two receptor complexes using SYBYL-X 2.1/Sur flex dock. The molecular docking scores and binding amino acid residues were recorded.
2.3 Molecular dynamics (MD) simulation of γ-EV-receptor complexes
MD simulations of γ-EA-T1R1-MSG and γ-EA-T1R2-sucrose complexes were performed using the Amber 12 procedure following the method of Yang et al. [14]. For the two complexes, 17 177 and 0, and 16 760 and 17 of water molecules and Na+ions were added, respectively. The molecular mechanics generalized born surface area (MM/GBSA) method in the Amber Tools suite was used to calculate the binding energies of each complex. The formula is as follows:
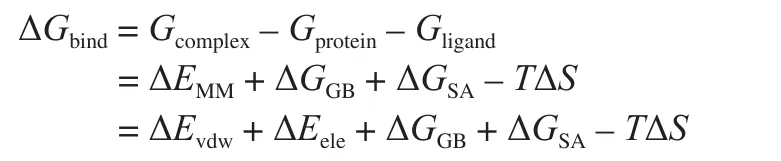
where Gcomplex, Gprotein, and Gligandrepresent the total Gibbs free energy of the complex of peptides-T1R3-MSG, the Gibbs free energy of the protein (T1R3-MSG), and the ligands (peptides),respectively. The MM in the MM/GBSA method represents the binding energy (ΔEMM) of the interaction between protein receptors and ligands in the vacuum state, mainly including van der Waals forces(ΔEvdw) and electrostatic interactions (ΔEele). The GBSA in the MM/GBSA method represents the solvent water molecule’s in fluence on the interaction between the receptor protein and ligands. The effect of the solvent water molecule includes polar contribution (ΔGGB) and nonpolar contribution (ΔGSA); TΔS was the configurational entropy at (T) temperature.
LIGPLOTs were generated for γ-EV-receptor complexes for the computational determination of hydrogen bonds and hydrophobic interactions [24].
2.4 Evaluation of taste-enhancing characteristic of γ-EA
The taste-enhancing characteristic of γ-EA was verified by mixing γ-EA (final concentration: 2 mmol/L, pH 6.5, (23 ± 2) °C) with either the blank chicken broth or soy sauce solutions, thereinto, blank chicken broth was made from moderately dilution of commercially available chicken stock powder, boiling for 1 min and then cooling to (23 ± 2) °C, and soy sauce solution was prepared via diluting to 10-fold with boiled water (at room temperature). Sensory characteristics such as saltiness, sourness, sweetness, umaminess and kokumi properties like mouthfulness and continuity were measured.10 mL of each solution was tasted for 20 s prior to swallowing in a sensory room at (25 ± 2) °C. 15 panelists (8 males and 7 females,aged 23–32 years) who had gained similar sensory training were asked to evaluate these solutions using a 5-point intensity scale (0, not detectable; 5, strongly detectable). The taste scores of soy sauce (SS),blank chicken broth (BCB) before and after adding 2 mmol/L of γ-EA were recorded.
2.5 Statistical analysis
All experiments and analyses were performed in triplicate, the data were averaged, and values presented as means ± SD. Analysis of variance and significant difference tests were performed by a one-way ANOVA using SPSS 16.0 statistical software.
3. Results and discussion
3.1 Conformations of T1R1-MSG and T1R2-sucrose receptor complexes
Homology modeling of sweet and umami taste receptors was mainly carried out to model the extracellular ligand-binding region of taste receptors using the Venus flytrap (VFT) module region in mGluR1 as templates [23,25]. Then, the ligands: MSG and sucrose were docked into T1R1 and T1R2, respectively using the PyMOL protocol to form the T1R1-MSG and T1R2-sucrose receptor complexes, respectively (Fig. 1), which are consistent with those in literature [5,19,26]. In particular, in the T1R1-MSG complex, theα-carboxylate group of MSG interacted with the carbonyl oxygen of Ser-300 and the -NH groups of Arg-277 and Thr-149. The -NH group of MSG interacted with the carbonyl oxygen of Ala-170 and Asp-147. The γ-carboxylateof MSG was bound to the carbonyl oxygen of Ala-170. These orthosteric binding sites, including Asp-147,Thr-149, and Arg-277, were also present in the binding site between Glu and T1R1 [5] and between MSG and IMP to T1R1 [16]. In contrast, theα-carboxylate of Glu showed a stronger affinity to the orthosteric binding sites of T1R1, which was mostly consistent with those reported [5,14,19]. In the T1R2-sucrose complex, there were 6 key residues (Ser-40, Asp-142, Ser-144, Ser-165, Asp-278, and Ser-303) in and proximal to the binding pocket of the sweet taste receptor that are important for ligand recognition and activity of sucrose. These orthosteric binding sites, including Ser-40, Asp-142,Ser-144, Ser-165, and Asp-278, were also present in the binding site between aspartame and T1R2 [21].
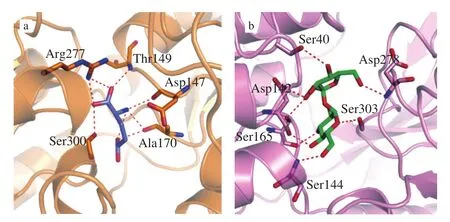
Fig. 1 Dominant conformation of binding of MSG with T1R1 (a) and binding of sucrose with T1R2 (b).
3.2 Molecular docking between γ-glutamyl peptides and receptor complexes
The SYBYL-X 2.1/Surflex dock protocol was used to evaluate the affinity of different γ-glutamyl peptides to receptor complexes to elucidate their taste-enhancing effects. The higher the score, the higher the affinity of ligands for the complexes. The molecular docking scores of γ-glutamyl peptide with T1R1-MSG and T1R2-sucrose receptor complexes were 5.569 2–8.857 8 and 4.129 7–6.441 4,respectively (Table 1). The results showed that the affinity of these peptides to the T1R1-MSG receptor was higher than that of the T1R2-sucrose receptor, implying that the umami-enhancing effect of these peptides was higher than that of the sweet-enhancing effect.Furthermore, the molecular docking scores of γ-glutamyl peptide with T1R3-MSG (5.866 3–9.425 3) were higher than those with T1R1-MSG [14], implying that these peptides had the best affinity for T1R3 among the three receptors.
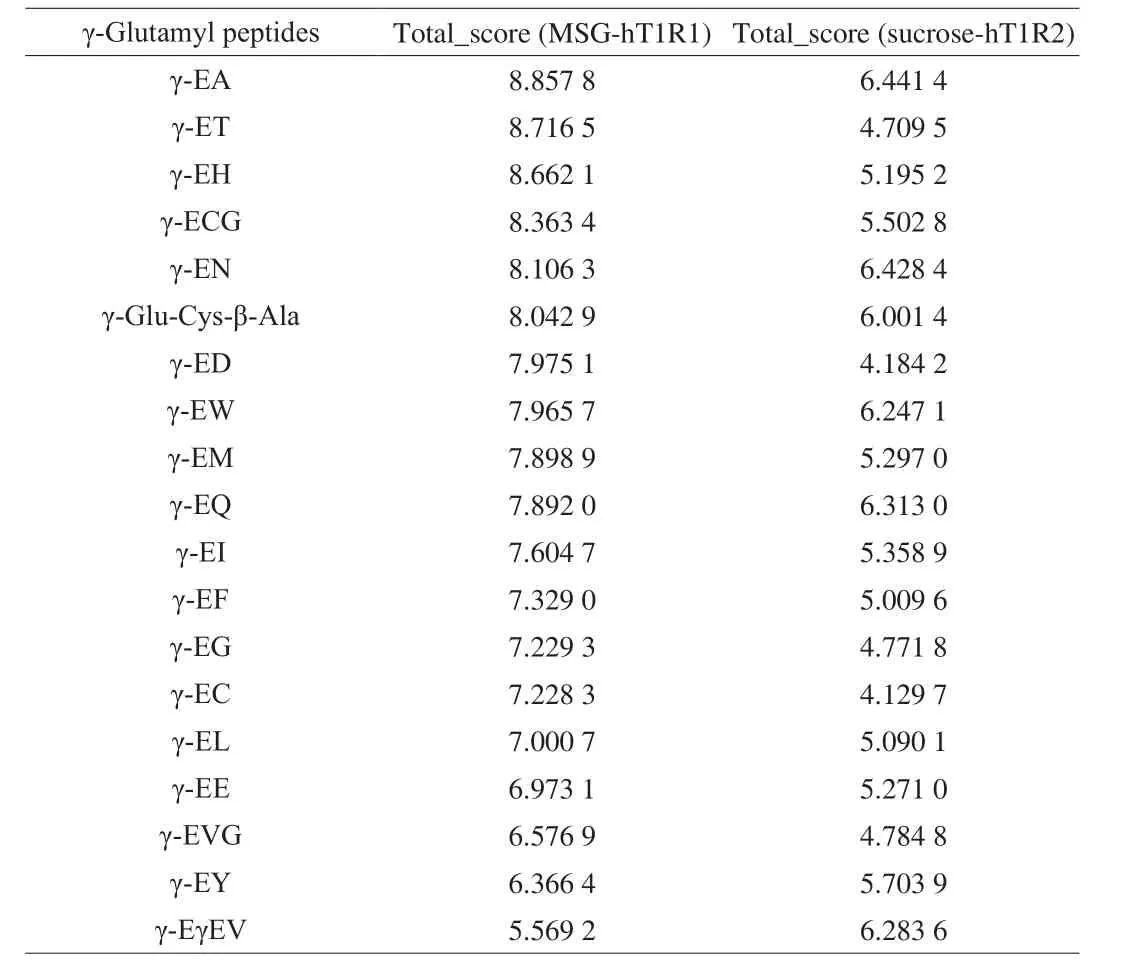
Table 1Scores of molecules docking of γ-glutamyl peptides with hT1R1 and hT1R2 after addition of MSG and sucrose.
A total of 24 amino acid residues from T1R1 and 26 residues from T1R2 were mobilized as binding amino acid sites to participate in the interactions of ligand-T1R1-MSG and ligand-T1R2-sucrose receptor complexes, respectively (Tables 2 and 3). Each ligand γ-glutamyl peptide can interact with 14–19 amino acid residues from T1R1 in the ligand-T1R1-MSG complex and 9–18 amino acid residues from T1R2 in the ligand-T1R2-sucrose receptor complex, respectively. The number of binding sites of each γ-glutamyl peptide was close to that of other tastants such as aspartame, sucrose, sweet proteins,L-Glu,and 5’-ribonucleotides [4,5,17,23]. In addition, it was obvious that each ligand interacted with different groups of amino acid residues.Overall, there were 10 identical amino acid residues (Phe-274,Ser-275, Leu-305, Ser-385, Asn-69, His-71, Leu-75, Trp-303,Ala-304, and Trp-454) from T1R1 that were simultaneously mobilized to participate in the ligand-T1R1-MSG interaction, while only 3 identical residues (Tyr-103, Glu-145, and Asp-213) were mobilized in the ligand-T1R2-sucrose interaction, indicating that these amino acid residues were the key participants in ligand-receptor interactions.Moreover, it is worth mentioning that γ-glutamyl peptides strongly interacts with both Glu-binding and sucrose-binding sites in their respective binding complex models. This phenomenon also occurred in the interactions between Glu and umami enhancers such as umami peptides and γ-glutamyl peptides [14,23], suggesting that there is a certain interaction between taste enhancer and taste substance in the receptor protein binding area.
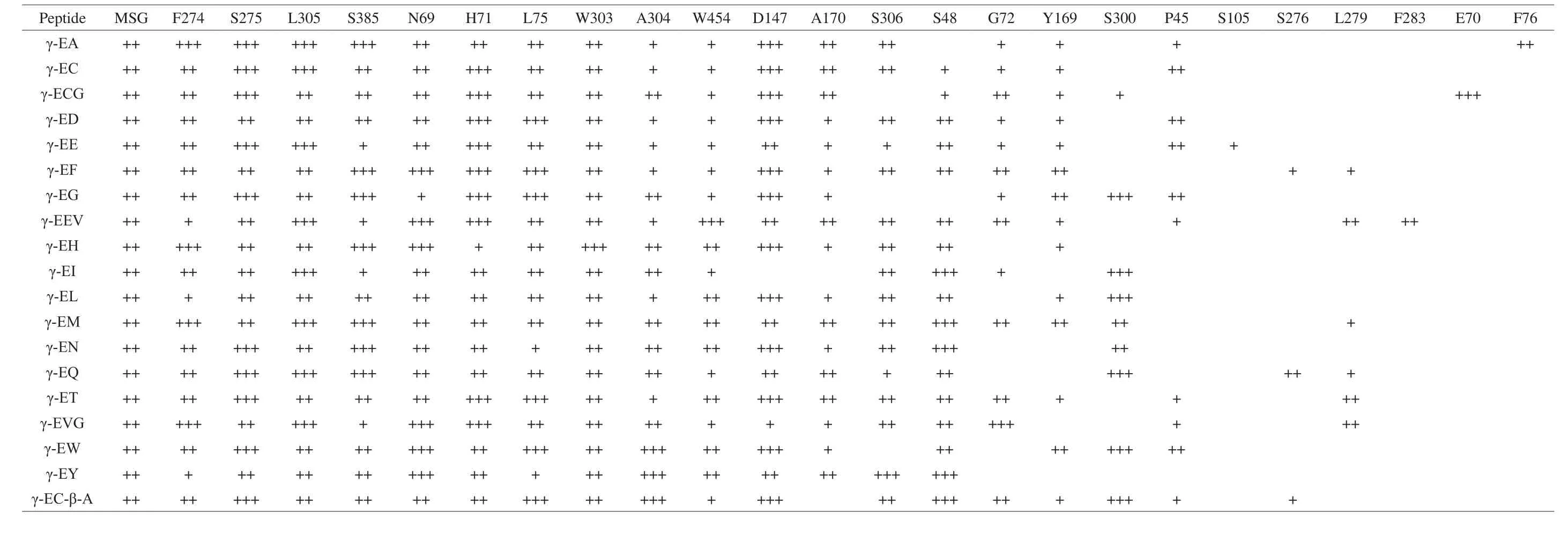
Table 2The interaction of γ-glutamyl peptides with the amino acid residues in hT1R1molecule after addition of MSG.
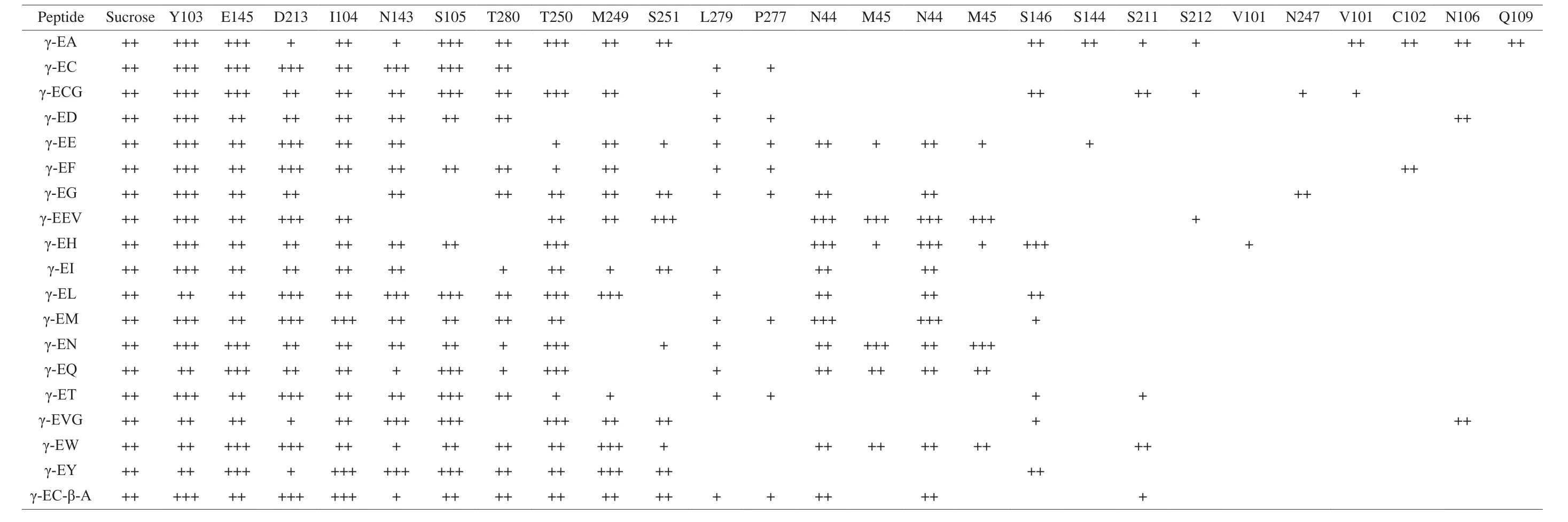
Table 3The interaction of γ-glutamyl peptides with the amino acid residues in hT1R2molecule after addition of sucrose-hT1R2.
Generally, it was obvious that more binding sites from T1R1 were mobilized to participate in the interaction of γ-glutamyl peptides with umami taste receptor, suggesting that the difference in the score of molecular docking may be due to the difference in the number of binding sites and that γ-glutamyl peptides have a better effect on umami enhancement than on sweet enhancement. In fact,reported that some γ-glutamyl peptides such as γ-[Glu](n>1)-Val and γ-[Glu](n>1)-Met were less effective at enhancing sweetness than umaminess [12]. Additionally, the order of affinity of these 20 peptides for T1R1 differed from that for T1R2. Among these peptides,γ-EA showed the highest affinity for both complexes, suggesting that it may exhibit the highest umami- and sweet-enhancing effect.
3.3 Molecular dynamic (MD) simulation between γ-EA and receptor complexes
The above results indicated that γ-EA had the highest affinity for T1R1 and T1R2 in the presence of MSG and sucrose. Therefore, MD simulations of γ-EA-T1R1-MSG and γ-EA-T1R2-sucrose complexes were performed to further compare the difference of γ-EA in taste enhancement by analyzing the stability of dynamics, binding free energy, and binding sites.
Dynamic stability was calculated using the root-mean-square deviation (RMSD) (Fig. 2). The two complexes would achieve dynamic equilibrium within 6 ns, and the average RMSD value was only about 3.0 Å, indicating that the conformations were in a state of dynamic equilibrium for the negligible shift of the main chain atoms of the receptor protein T1R1/T1R2. There was no significant difference in dynamic stability between the two simulations, and they all showed higher stability than other umami peptides, which took 20 ns to balance out [1].

Fig. 2 The RMSD of the backbone atoms of the during the 10 ns production simulation.
The ΔGbindof γ-EA-T1R1-MSG and γ-EA-T1R2-sucrose complexes were (-26.77 ± 3.04) and (-20.03 ± 2.87) kcal/mol,respectively, indicating that γ-EA showed a higher binding free energies to T1R1 than to T1R2 when there was one basic taste substance (Fig. 3), and was in line with the molecular docking scores (Table 1). Specifically, the main contributor to the total binding free energies between γ-EA and the receptor complex was ΔEvdw. The total electrostatic interaction (ΔEele+ ΔGGB) can be negligible because ΔEeleand ΔGGBwould be offset by each other. Free energy decomposition calculations indicated that Asn-69, His-71, Ser-275, Trp-303,Ala-304, Leu-305, and Ser-306 from the N-terminal VFT domain of T1R1 and Tyr-103, Ile-104, Ser-105, Asn-143, Met-249, Thr-250,and Ser-251 from the γ-EA-T1R2-sucrose VFT domain of T1R2 were the main specific amino acid residues contributing to the total binding free energy, indicating that they may be the key residues in the interaction of the ligand-receptors.
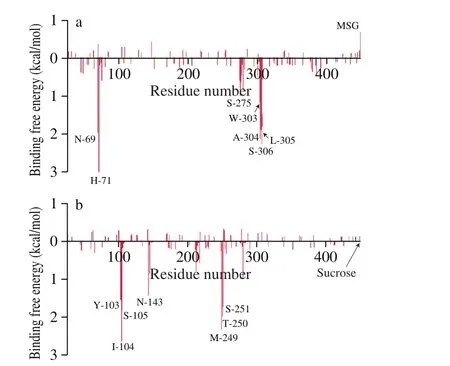
Fig. 3 Binding free energy. The major amino acid residues for binding free energy decomposition of (a) γ-EA-T1R1-MSG and (b) γ-EA-T1R2-sucrose inserted a table of binding free energy calculated in MM/GBSA (kcal/mol).
The binding conformations between the above amino acid residues and γ-EA are shown in Fig. 4. In the γ-EA-T1R1-MSG complex conformation, γ-EA was predicted to form a number of hydrophobic interactions and hydrogen bonding with the “active-site”center of VFT. Hydrogen bonds with γ-EA could be formed, involving different amino acid residues such as His-71, Ser-385, Ser-275, and Ser-306 and various bonding patterns such as via α-COOH and -NH group of γ-glutamyl residue and α-COOH of Ala residue in the γ-EA molecule. Hydrophobic interactions involved Phe-274 and Trp-303 with α-COOH group of γ-glutamyl residue, and Ser-276, Leu-279, Ala-304,and Leu-305 with α-COOH of Ala. Similarly, in the γ-EA-T1R2-sucrose complex conformation, the α-COOH of γ-glutamyl residue in the N-terminal of the γ-EA molecule interacted with Asn-143 and Ser-105 residues of T1R2 through hydrogen bonds, Ile-104, and Ser-105 residues of T1R2 through hydrophobic interactions, respectively. The -NH group of γ-glutamyl residue interacted with Tyr-103 and Ile-104 through hydrophobic interactions. The Ala residue at the C-terminal of the γ-EA molecule showed strong hydrophobic interactions with the Asp-213 and Thr-280 of T1R2. These results are highly consistent with those of molecular docking (Tables 2 and 3).
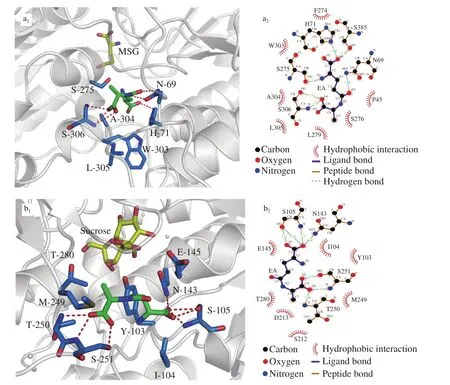
Fig. 4 The structural representations of binding sites between (a1) γ-EA-T1R1-MSG and (b1) γ-EA-T1R2-sucrose, as well as the LIGPLOT profiles of the crystal structure of (a2) γ-EA-T1R1-MSG and (b2) γ-EA-T1R2-sucrose, respectively.
Overall, the results of MD showed that γ-EA-T1R1/T1R2-MSG/sucrose complexes can quickly achieve dynamic equilibrium (within 6 ns),and multiple amino acid residues were mobilized to be involved in the interaction of the two ligand-receptor complexes through hydrophobic interactions or hydrogen bond interactions. Meanwhile, the binding free energy between γ-EA and T1R1 was higher than that of T1R2,which implied that γ-EA might have a better effect on umami enhancement than that on sweet enhancement.
3.4 Validation of taste-enhancing characteristics of γ-EA
The above results illustrate that γ-EA has a strong affinity for T1R1 and T1R2, indicating that it has the potential to increase the intensity of umaminess and sweetness. Previous studies have shown that γ-EA only exhibits sourness and astringency in aqueous solution,but could improve the umaminess perception of monosodium glutamate (MSG) [10,14]. However, the effect of γ-EA on sweetness and saltiness has not been studied, therefore, sensory analysis was used to verify the basic taste-enhancing effect of γ-EA and compare the difference in taste enhancement effect. As shown in Fig. 5,sensory characteristics of the soy sauce (SS) and blank chicken broth (BCB), including mouthfulness, continuity, umaminess, sweetness,saltiness and sourness, before and after adding γ-EA (2 mmol/L)were evaluated. As a result, a significant enhancement (P < 0.05)of the mouthfulness, continuity, umaminess, sweetness, and saltiness of both soy sauce and blank chicken broth solutions was observed. γ-EA had the strongest enhancing effect on mouthfulness and continuity (kokumi taste), and their scores increased by about 0.63 and 0.54, 0.67 and 0.50 in SS and BCB,respectively. Among the basic taste, γ-EA exhibited the strongest enhancement on umaminess, increasing by 0.48 and 0.56 in SS and BCB, respectively. Followed by saltiness and sweetness, whose scores were increased by 0.42 and 0.37, 0.29 and 0.26 in SS and BCB, respectively. These results were consistent with the results of molecular modeling approaches above that the enhancement effect of γ-EA on umami taste was better than that on sweet taste.Moreover, the enhancement effect of γ-EA on salty taste was also consistent with the literature report [12,13]. The results of sensory evaluation and molecular simulation illustrated that γ-EA could enhance the three basic taste sensations (umaminess, sweetness,and saltiness), and the enhancement effect for umaminess was better than that for sweetness.
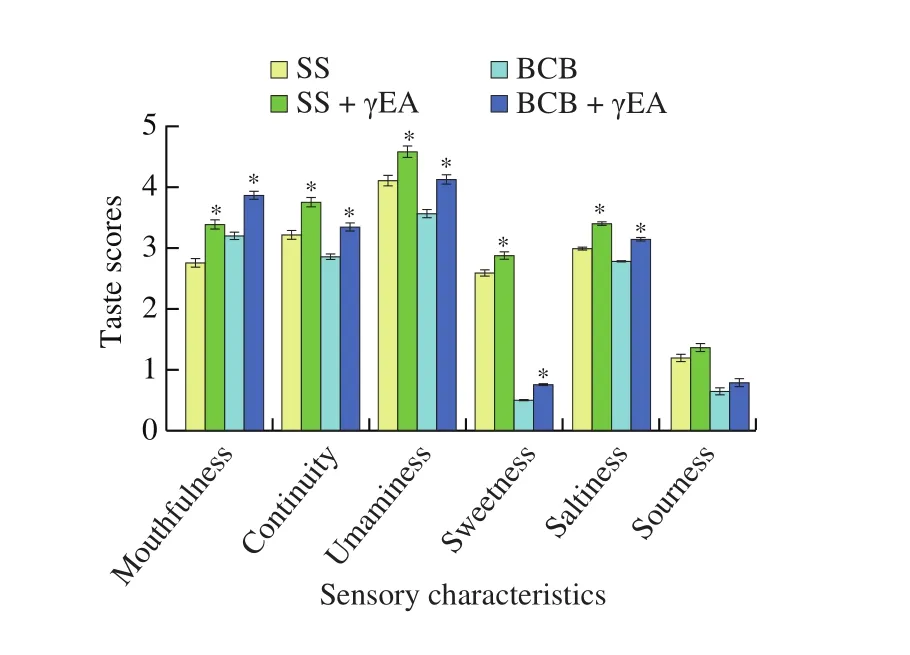
Fig. 5 The SS and BCB before and after adding 2 mmol/L of γ-EA.
4. Conclusion
The difference in kokumi-tasting γ-glutamyl peptides on basic taste-enhancing effects was evaluated via molecular modeling approaches. Two complex conformations were first constructed to form binding receptors, T1R1-MSG and T1R2-sucrose. All the selected γ-glutamyl peptides exhibited affinity for the T1R1-MSG and T1R2-sucrose receptors, with molecular docking scores of 5.569 2–8.857 8 and 4.129 7–6.441 4, respectively. Each ligand γ-glutamyl peptide interacted with 14–19 amino acid residues from T1R1 in the ligand-T1R1-MSG complex and 9–18 amino acid residues from T1R2 in the ligand-T1R2-sucrose receptor complex, respectively. 10 identical amino acid residues (Phe-274, Ser-275, Leu-305, Ser-385,Asn-69, His-71, Leu-75, Trp-303, Ala-304, and Trp-454) from T1R1 were simultaneously mobilized to participate in the ligand-T1R1-MSG interaction, while only 3 identical residues (Tyr-103, Glu-145,Asp-213) were mobilized for the ligand-T1R2-sucrose interaction.Thus, the higher docking scores and more amino acid binding sites in the ligand-T1R1-MSG complex suggested that γ-glutamyl peptides have a better effect on umami enhancement than on sweet enhancement.
Among the selected γ-glutamyl peptides, γ-EA showed the highest affinity for both complexes, indicating that it may have the highest umami-enhancing and sweet-enhancing effects simultaneously.Therefore, MD simulations were performed, and the results showed that γ-EA-T1R1-MSG and γ-EA-T1R2-sucrose complexes can achieve dynamic equilibrium within 6 ns, and the main contributor to the total binding free energies between γ-EA and the receptor complex was ΔEvdw. Hydrogen bonds with γ-EA could be formed and involve different amino acid residues, for example: His-71, Ser-385,Ser-275, and Ser-306 of T1R1 and Asn-143 and Ser-105 residues of T1R2. Hydrophobic interactions involve Phe-274, Trp-303, Ser-276,Leu-279, Ala-304, and Leu-305 of T1R1 and Ile-104, Ser-105, Tyr-103,Asp-213, and Thr-280 residues of T1R2 through hydrophobic interactions, respectively. Sensory evaluation demonstrated that the enhancement of γ-EA on umaminess was superior to that of sweetness. Therefore, these results suggest that the kokumi-tasting γ-glutamyl peptides could enhance basic taste such as umaminess,saltiness, and sweetness, and their enhancement on umaminess was superior to that of sweetness.
Con flict of interest
The authors declare that they have no known competingfinancial interests or personal relationships that could have appeared to in fluence the work reported in this paper.
Acknowledgements
The authors are grateful for thefinancial support by the National Natural Science Foundation of China (31901814), Guangzhou Science and Technology program key project (202104020028), and the Major State Basic Reasearch Development Program Of China(2018YFD0901003).
- 食品科学与人类健康(英文)的其它文章
- A review on mechanisms of action of bioactive peptides against glucose intolerance and insulin resistance
- Adropin as an indicator of T2DM and its complications
- Isolation and characterization of novel peptides from fermented products of Lactobacillus for ulcerative colitis prevention and treatment
- The immunity-promoting activity of porcine placenta in mice as an immunomodulator for functional foods
- Nutritional properties of Europen eel (Anguilla anguilla) bone peptide-calcium and its apoptosis effect on Caco-2 cells
- A comprehensive method to explore inhibitory kinetics and mechanisms of an anticoagulant peptide derived from Crassostrea gigas

