Lunasin protease inhibitor concentrate decreases pro-in flammatory cytokines and improves histopathological markers in dextran sodium sulfate-induced ulcerative colitis
Andre Nieto-Veloz, Zhihong Wng, Qixin Zhong, Doris D’Souz,Hri B. Krishnn, Vermont P. Di,*
a Department of Food Science, University of Tennessee, Knoxville 37996, USA
b Agricultural Research Service, USDA, Columbia 65211, USA
ABSTRACT
Lunasin protease inhibitor concentrate (LPIC) is a novel combination of soy bioactive peptide lunasin, Kunitz and Bowman-Birk protease inhibitors. The reported anti-inflammatory and anticancer properties of each one of them suggest LPIC as a promising candidate for the treatment of in flammatory-related diseases. Our objective was to assess the in vivo anti-in flammatory properties of LPIC. First, an in vitro test was performed in lipopolysaccharide (LPS)-activated RAW264.7 murine macrophages by measuring the production of nitric oxide (NO), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α) as in flammatory markers. For the in vivo model, ulcerative colitis (UC) was induced in mice via oral administration of dextran sodium sulfate(DSS). LPIC treatment was performed via daily intraperitoneal injection of 50 mg/kg body weight. Body weight, visible blood in stool and stool consistency were scored daily as macroscopic indicators of disease progression. Occult blood was evaluated by the presence of hemoglobin in stool every third day. Colon length, caecum weight, colonic myeloperoxidase activity (MPO), presence of pro-inflammatory cytokines in blood and colon, changes in the architecture, and expression of inducible nitric oxide synthase (iNOS) in colonic tissue were evaluated. In vitro, LPIC induced production of NO and maintained cytokine levels in comparison to activated untreated macrophages. In vivo, LPIC increased colonic bleeding and did not improve macroscopic markers of the disease, but reduced colonic IL-1β and IL-6, decreased systemic circulation of TNF-α, attenuated neutrophils infiltration and iNOS expression in colonic tissue, and diminished the damage in colonic architecture. Our results suggest that combinations of peptides in LPIC may counteract the antiin flammatory properties in vitro; while in vivo, LPIC can significantly reduce the histopathological damage,hence is a possible therapeutic strategy to attenuate UC.
Keywords:
Bio-peptides
Cytokines
In flammation
Protease inhibitor
Ulcerative colitis
1. Introduction
Plant-derived bioactive peptides are of major interest due to their ability to modulate biological and physiological functions,thus providing health benefits for the consumers. The particular involvement of serine protease enzymes with inflammation,homeostasis, and cancer progression, has made serine protease inhibitor (SPI) peptides a focus of attention in research as feasible therapeutic strategies [1].
Soybean-derived peptides have demonstrated several biological properties against different pathological conditions such as hypertension, diabetes, in flammation, and cancer [2]. Bowman-Birk inhibitor (BBI) and Kunitz trypsin inhibitor (KTI) are two soybeanderived SPI peptides that can inhibit trypsin- and chymotrypsin-like protease activity [3,4]. KTI and BBI have been demonstrated to modulate inflammation by inhibiting reactive oxygen species and prostaglandin production in activated macrophages, prevent ulceratitis, act as a therapeutic agent against gastrointestinal cancer,and exert antiviral activity [5].
Lunasin is a 43 amino acid peptide from soybeans with demonstrated antioxidant, anti-inflammatory, and anticancer properties [6]. In lipopolysaccharide (LPS)-activated murine macrophage, lunasin has been reported to significantly reduce reactive oxygen species and production of pro-inflammatory cytokines interleukin-6 (IL-6), IL-1β and tumor necrosis factor α (TNF-α),prostraglandin E2, and nitric oxide (NO) [6-8]. Lunasin protease inhibitor concentrate (LPIC) has been isolated from soybeans, is highly enriched with lunasin, BBI, and KTI, and has demonstrated in vitro ability to decrease the expression of pro-inflammatory cytokines in activated human macrophage [9]. Considering the probable synergistic interplay of the three peptides, we hypothesized that LPIC may exert anti-inflammatory activity with potential application against in flammation mediated diseases.
Ulcerative colitis (UC) is a condition of chronic in flammation in the large intestine that begins in the rectum and continuously proceeds to the proximal colon as a result of a dysregulated inflammatory response of the immune system [10]. UC is known to increase the risk of developing colorectal cancer [11]. In many cases, conventional treatments for UC can achieve and maintain remission, however,there are side effects and failures of a significant number of patients to respond to some drugs [12]. The need for alternative treatments for UC and the potential anti-in flammatory properties of LPIC create an opportunity that deserves to be explored. Our goal was to evaluate the anti-in flammatory activity of LPIC using an in vivo model of UC.
2. Materials and methods
2.1 Extraction of LPIC
LPIC was obtained via aqueous-ethanol extraction and calcium precipitation from soybeans as previously described [9]. LPIC was lyophilized, dialyzed against elhylene diamine tetraacetic acid(EDTA) and purified via DE-52 anion exchange chromatography.Thefinal product had immunoreactivity against lunasin, BBI and KTI antibodies. The three proteins accounted for 80% of the total proteins.
2.2 Effect of LPIC over in vitro in flammatory markers
Murine RAW264.7 macrophages were cultured in a regular humidified 5% CO2incubator at 37 °C, in 10% fetal bovine serum and 1% penicillin/streptomycin supplemented DMEM media, and 2.5 × 104cells/well were seeded in 96-well plates and allowed to attach overnight. Adherent cells were exposed to LPIC (0–2.5 mg/mL)for 8 h and then challenged with 1 μg/mL LPS for 16 h. The supernatant was collected for analysis NO and pro-inflammatory cytokines IL-6 and TNF-α, via Griess reagent assay and ELISA(BioLegend, San Diego, CA, USA), respectively, according to the protocol provided by supplier. Cell viability was assessed by MTS assay (Promega, Madison, WI, USA) following manufacturer’s instructions.
2.3 Induction of colitis and administration of LPIC in animal model
All protocols for the animal experiments followed the National Institute of Health guide for the care and use of laboratory animals(NIH Publications No. 8023, revised 1978), and were approved by the Institutional Animal Care and Use Committee of the University of Tennessee (Approval Protocol #2591-0418). Twenty C57BL/6 7-week old male mice (Jackson Laboratories, Harbor, ME, USA) were housed under standard controlled conditions and free access to water and chow diet provided ad libitum.
Dextran sulfate sodium (DSS) was used to induce UC. Mice were randomly divided into control (n = 6), DSS (n = 8), and DSS+LPIC(n = 6) groups. To simulate periods of relapse and recovery experienced by inflammatory bowel disease (IBD) patients [13],groups were treated as follows: the DSS groups were administered with 3% DSS (MW = 36–50 kDa, MP Biomedicals, Santa Ana, CA,USA) in drinking water in 2 phases of 5 days each, with a recovery period of 6 days in between. The control groups received normal drinking water. The LPIC+DSS groups were administered daily via intraperitoneal injection with 50 mg LPIC/(kg·bw) dissolved in 100 μL sterile water, starting at day 4 after DSS initiation, and until the end of the study, while DSS and control groups were injected with sterile water. After 15 days of treatment, blood was collected by cardiac puncture from isoflurane-anesthetized mice followed by cervical dislocation.
2.4 Macroscopic assessment of colitis
Disease activity index (DAI) was determined daily by scoring change in body weight, visible blood in stool or anus, and stool consistency. Every third day, stool was collected from the cages and assessed for occult blood via hemoglobin content as previously described [14]. The gastrointestinal tract was harvested. Caecum weight and colon length were recorded. After washing with PBS, the colon was longitudinally dissected into two pieces: one piece was fixed in 10% buffered formalin for histological analysis, while the other piece was frozen in liquid nitrogen for further analysis.
2.5 Histological analysis
Four µm thick paraffin embedded colon tissue sections were used to evaluate changes in colon architecture by hematoxylin and eosin(H&E) staining and to detect inducible nitric oxidase synthase (iNOS)via immunostaining with iNOS primary antibody (ProteinTech,Chicago, IL, USA) and Pierce®peroxidase detection kit (Thermo Scientific, Walthman, MA, USA) following the manufacturer’s protocol. iNOS quantification was performed with ImageJ software.
2.6 Myeloperoxidase activity (MPO) and pro-inflammatory cytokines
Colonic extracts were prepared and MPO measured as previously described [14]. Colonic extracts and blood serum were used to quantify pro-inflammatory cytokines IL-6, TNF-α, and IL-1β using ELISA kits (BioLegend, San Diego, CA, USA), following manufacturer’s protocol.
2.7 Statistical analysis
Statgraphics Centurion®software was used to establish significant differences (P < 0.05) among treatments via analysis of variance followed by Tukey post hoc test. All experiments were performed in triplicate.
3. Results and discussion
3.1 LPIC promoted nitric oxide production and maintained cytokines levels in murine macrophage in vitro
The inflammatory response of LPIC pre-treated murine macrophage to LPS was evaluated. Fig. 1 presents the effect of LPIC pre-treatment over viability, production of NO, and pro-in flammatory cytokines IL-6 and TNF-α in presence or absence of LPS stimuli. The viability was not significantly affected by LPIC, while NO production significantly increased upon LPIC treatment even in the absence of LPS stimulation, indicating that LPIC itself promotes NO production independently from LPS.
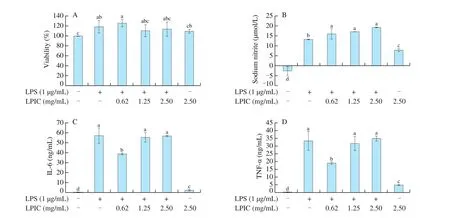
Fig. 1 (A) Changes on viability, (B) NO production, and pro-in flammatory cytokines (C) IL-6 and (D) TNF-α in activated (LPS+) and non-activates (LPS-) murine macrophage upon in vitro exposure to LPIC. Results are presented as mean ± SEM. Different letters indicate significant differences (P < 0.05) among treatments.
Physiological NO is synthesized by three NOS isoforms. iNOS produces large amounts of NO with cytotoxic effect, acting as a defense mechanism triggered by the recognition of stimulating antigens such as LPS [15]. Neuronal NOS and endothelial NOS(eNOS) are calcium dependent and produce low levels of NO [15].Calcium-independent activation, via protein kinase B (AKT) or AMP-activated protein kinase (AMPK) mediated phosphorylation of eNOS has been also reported [16,17]. eNOS has been detected at the mRNA and protein levels in rodent and human macrophages [18]. Lunasin has been demonstrated to promote maturation of dendritic cells with the consequent upregulation of co-stimulatory molecules, cytokines(IL-6 and IL-1β) and chemokines [19], and to synergistically induce, when combined with IL-2 or IL-12, interferon γ (IFN-γ) and granzyme B in natural killer cells, favoring their cytotoxicity [20].Hence, the immunomodulatory properties that mediate lunasin activation of innate immunity in the macrophage is a potential explanation for significantly increased levels of NO observed. Upon doubling concentrations of LPIC the insignificant increase in NO could be attributed to the fact that high levels of NO play an autoregulatory role by suppressing iNOS-NO produced via nuclear factor κB (NF-κB) pathway [18,21,22]. The extra NO produced in addition to the large amount produced via LPS/NF-κB/iNOS induced a feedback response that consequently decreased iNOS transcription maintaining regulated NO levels.
Production of pro-inflammatory cytokines TNF-α and IL-6 followed the same trend and exhibited an interesting behavior. A high concentration of LPIC, under the absence of LPS stimulation,promoted cytokines release; however, the concentration of both of the molecules upon LPS activation significantly decreased upon treatment with a low concentration of LPIC, but remained the same as LPS-treated macrophages at higher concentrations. These trends suggest that the anti-inflammatory properties may have played a predominant role when LPIC was used at 0.62 mg/mL. At higher LPIC concentrations, the immunomodulatory effect of lunasin could become significant and induce downstream effects that counteracted the anti-in flammatory effects. Our in vitro results suggest that LPIC may act as a double-edged sword by exhibiting anti-inflammatory properties mediated by BBI and KTI, and possible immunomodulatory properties mediated by lunasin.
3.2 LPIC did not improve macroscopic markers of colitis in vivo
Abdominal pain, malaise, weight loss, fever, and fatigue account for the most common symptoms of UC, with bloody diarrhea being the main indicator of the severity of the disease during an in flammation flare [12]. Body weight of the control group remained relatively constant, while DSS treated groups exhibited about 7% loss by day 7 (Fig. 2A). Upon DSS removal, both groups totally recovered their body weight by day 10, but LPIC group lost weight at a faster rate than DSS as soon as the second DSS challenge started and the relative body weight remained lower than the other groups. Similarly,occult blood (Fig. 2B) reached a maximum on day 6, and returned to a minimum on day 9, with LPIC group levels significantly higher than the DSS group.
The DAI (Fig. 2C) reflects the effect of LPIC over stool consistency and presence of visible blood in each of the two stages of disease onset. During thefirst phase, all of the animals in DSS group presented visible blood in stool but only some of them had a soft consistency, and rectal bleeding was observed in two of them; while,in LPIC group, stool pellet was formed and consistent, with only some animals having blood in the stool, and no rectal bleeding observed.During the second stage instead, the DSS group had some animals with soft stool and visible blood, with one case of rectal bleeding and another of loose stool, while in the LPIC group all the animals had a soft stool with visible blood and one animal with recurrent rectal bleeding. Taken together, occult blood and daily monitoring of stool indicated that LPIC did not alleviate, but increased the bleeding that resulted in the colonic tissue damage induced by DSS. Beyond their bioactive properties, KTI and BBI are widely recognized for their potent trypsin and chymotrypsin inhibitory activities, which have been suggested to act as an effective shield that protects bioactive peptides,such as lunasin, from the proteolytic activity of gastrointestinal proteases [23]. However, no evidence indicates how the protease inhibitory activity of these compounds may alter other physiological processes such as coagulation.
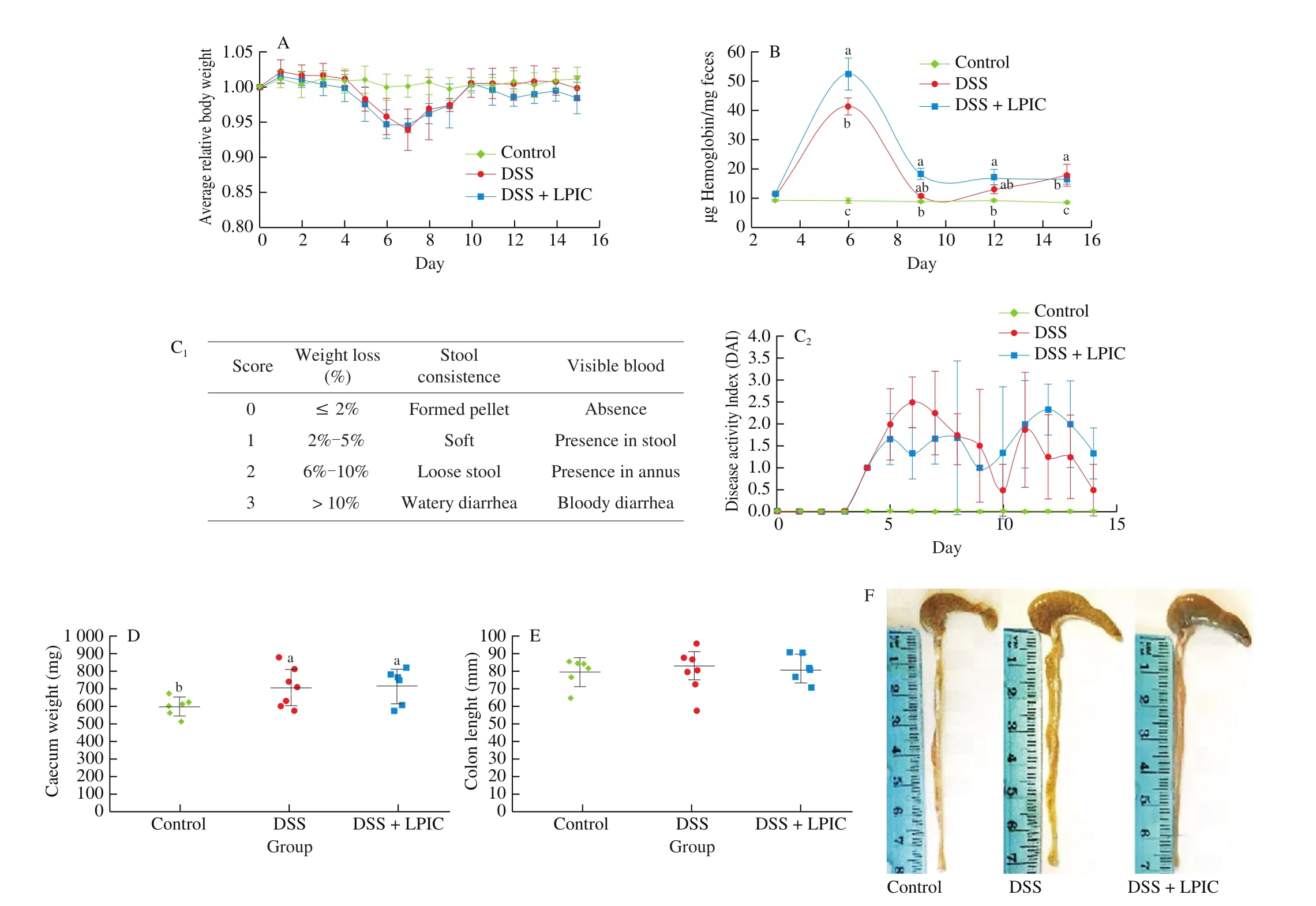
Fig. 2 Macroscopic markers of disease progression in vivo. (A) Relative body weight, (B) occult blood in stool, (C) disease activity index, (D) caecum weight and (E-F) colon length. Results are presented as mean ± SEM. Different letters indicate significant differences (P < 0.05) among groups. DAI = weight loss score +stool consistency score + visible blood score.
Thrombin, the key effector in the coagulation cascade that regulates the wound healing process [24], is a serine protease with recently demonstrated modulatory activity of the immune response by inducing polarization of macrophage towards the M1 phenotype [25]and by activating the pro-inflammatory cytokine IL-1α [26]. As a peculiar enzyme with chymotrypsin and trypsin-like proteolytic activity [24], thrombin might be susceptible to the inhibitory effect of BBI and KTI. The increased presence of blood in stool upon DSS challenge and administration of LPIC agreed with previous observations on a bitter gourd derived bio-peptide with potent trypsin inhibitory activity [27] that when injected intraperitoneally induced significantly higher presence of occult blood and rectal bleeding than DSS itself [14]. Motta and coworkers [28] recently demonstrated that oral inhibition of luminal thrombin in mice led to gastrointestinal bleeding, with the presence of blood in feces. Hence, it is likely that the bleeding associated with the damage in the intestinal barrier was controlled by the coagulation cascade in the DSS group, allowing appropriate wound healing during the period of DSS cessation. In the LPIC group, the diminished proteolytic activity of thrombin affected the activation of the coagulation process, preventing the adequate healing of injuries and maintaining active bleeding even after the removal of DSS.
Caecum weight (Fig. 2D) significantly increased due to the administration of DSS, suggesting that possible muscle thickening may have occurred as a result of in flammatory processes occurring in this organ. Accumulation of DSS in the caecum, as well as damage in caecal tissue have been previously reported for DSS-induced colitis in rodent models [29-31]. Colon length (Figs. 2E-2F) did not exhibit reduction. The muscular contraction that leads to colon shortening may have occurred after the first onset of the disease; when the highest loss in body weight was achieved, however, the resting period from DSS administration possibly allowed muscular relaxation and the consequent recovery of colon length.
3.3 LPIC decreased release of pro-in flammatory cytokines
Cytokines play a critical role in cellular communication,contributing to maintaining homeostasis and modulation of the immune response and in flammation when cellular or tissue integrity is compromised by injury or infection [32]. Previous reports indicate increased tissue levels of IL-1β and IL-6 in patients with IBD, while in serum, IL-1β was seldom detected and IL-6 was only elevated in Crohn’s disease (CD) but not in UC [12]. In Fig. 3, it can be seen that significant variations in IL-1β and IL-6 only occurred at the local,but not at the systemic level, while changes in TNF-α were only significant in blood. In every case, the DSS challenge promoted a significant increase in the cytokine level that was reversed to the basal level by LPIC administration. The observed cytokine profile indicates that DSS succeeded in the specific development of UC.
Upon DSS administration, impairment of the barrier function of the intestinal mucosa facilitated the translocation of commensal bacteria into the luminal wall, leading to the activation of dendritic cells and macrophages, with the consequent production of large amounts of pro-inflammatory cytokines, probably via the NF-κB pathway.Lunasin, KTI, and BBI have been demonstrated to reduce in flammatory response by blockade of this in flammatory pathway [33-35].
In IBD, cytokines not only drive intestinal in flammation, but are also involved in extra-intestinal disease manifestations such as the release of acute phase proteins by the liver, arthritis, and cachexia [36].Also, the ability of IL-6 and TNF-α to promote epithelial cell proliferation and survival by the induction of signal transducer and activator of transcription 3 (STAT3) and NF-κB anti-apoptotic genes,increased the risk of tumors formation and the consequent development of colitis associated colorectal cancer [11]. Therefore, the ability of LPIC to decrease the expression of pro-in flammatory cytokines may not only have a positive local effect but can also alleviate the peripheral symptoms associated with UC and reduce the risk of cancer.
3.4 LPIC attenuated histopathological damage in colonic tissue
As can be seen in Fig. 4A top and 4B, DSS administration promoted about 16-fold increase in iNOS expression in the mucosa layer, along the villi but not at the bottom of the crypts, indicating that enterocytes may have an important contribution of NO during inflammation. LPIC administration was able to reduce iNOS expression by about 63%.
Fig. 4A bottom shows alteration of the colonic architecture of animals under DSS. Disruption of the mucosal crypts is mostly associated with damage in the villi due to large zones of erosion of the layer of absorptive cells (white arrows), accompanied by goblet cells depletion. Infiltration of immune cells (black arrows) is mostly limited to the lamina propria and the muscularis mucosae. Recovery of absorptive cells layer, higher presence of goblet cells, and infiltration limited to small regions of the lamina propria near the base of the crypts were observed in the group treated with LPIC.

Fig. 3 Changes in local (A, C, E colonic tissue) and systemic (B, D, F blood serum) distribution of pro-in flammatory cytokines. Results are presented as mean ± SEM.Different letters indicate significant differences (P < 0.05) among groups.
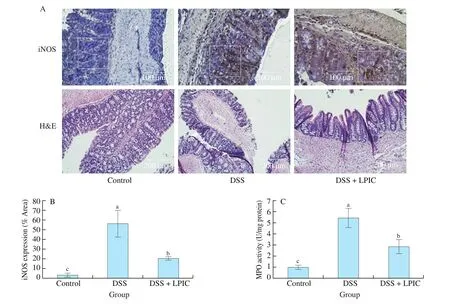
Fig. 4 Histopathological analysis of colonic tissue and in flammatory infiltration. (A, top) iNOS immunostaining micrographs, (A, bottom) colon architecture,(B) iNOS quantification, and (C) MPO activity. White squares represent sections utilized for iNOS quantification. Black and white arrows indicate zones of immune cells infiltration and alteration of crypts architecture, respectively. Results are presented as mean ± SEM. Different letters indicate significant differences(P < 0.05) among groups.
MPO is a peroxidase enzyme present mainly in neutrophils granules that act as a major mediator of pathogen killing by serving as a catalyst in the formation of reactive oxygen intermediates,particularly hypochlorous acid [37]. The 5-fold increase of MPO observed in the DSS group (Fig. 4C) suggests considerable neutrophils infiltration in the colonic tissue. The decreased MPO in the LPIC group corroborated the attenuation of in flammatory mediators, and as such a lower presence of immune cells. These results agreed with the observations in H&E micrographs.
According to our overall results, it is possible that the recognized ability of lunasin to suppress the NF-κB pathway [32], lead to the reduced expression of iNOS and the pro-inflammatory cytokines TNF-α, IL-6, and IL-1β. This biological activity of lunasin could result in a decreased recruitment of immune cells to the colon.Moreover, the inhibitory effect of BBI and KTI exert over proteases involved in the inflammatory process such as cathepsin G and chymase. They are important proteolytic effectors present in immune cells [37,38] and probably ameliorated the impact of the unspecific damage caused by cells such as neutrophils over the colonic tissue and architecture.
4. Conclusion
Our results indicate that the combination of components in LPIC induce the production of NO and maintain the cytokine levels in vitro.In contrast, LPIC significantly decreased histopathological markers of the disease, by reducing neutrophils infiltration, the damage in the colonic architecture, the release of pro-inflammatory cytokines,MPO activity, and iNOS expression in vivo. We have demonstrated that LPIC may be a promising therapeutic strategy to mitigate histopathological damage during UC. The possible interference of the protease inhibitory activity of LPIC with the coagulation process points out potential antithrombotic properties.
Con flicts of interest
The authors declare no con flicts of interest.
Acknowledgement
This work was partially supported by HATCH 1010230 and Hatch Project TEN00487. Ph.D study for thefirst author is partially supported by COLCIENCIAS-FULBRIGHT cohort 2017.
- 食品科学与人类健康(英文)的其它文章
- A review on mechanisms of action of bioactive peptides against glucose intolerance and insulin resistance
- Adropin as an indicator of T2DM and its complications
- Isolation and characterization of novel peptides from fermented products of Lactobacillus for ulcerative colitis prevention and treatment
- The immunity-promoting activity of porcine placenta in mice as an immunomodulator for functional foods
- Nutritional properties of Europen eel (Anguilla anguilla) bone peptide-calcium and its apoptosis effect on Caco-2 cells
- A comprehensive method to explore inhibitory kinetics and mechanisms of an anticoagulant peptide derived from Crassostrea gigas

