Evaluation of silymarin extract from Silybum marianum in mice:anti-fatigue activity
Luming Jia, Fei Zhao*
College of Food Science and Engineering, Dalian Ocean University, Dalian 116023, China
Keywords:
Silybum marianum
Silymarin
Anti-fatigue
Exhaustion exercise
A B S T R A C T
Silymarin has been used for centuries for its hepatoprotective properties. The specific objective of this study was to evaluate the anti-fatigue properties of silymarin. The silymarin was administered orally at doses of 50,100, and 200 mg/kg for 4 weeks; the fatigue level and exercise performance were evaluated using exhaustive swimming time and pole-climbing time, as well as levels of plasma lactate, ammonia, glucose, creatine kinase(CK), serum urea nitrogen (SUN), blood lactic acid (BLA), muscle glycogen (MG), and liver glycogen (LG)contents after an intensive swimming session. The results demonstrated that silymarin treatment decreased the BLA and SUN levels while increased the LG and MG levels. In addition, silymarin decreased plasma lactate and ammonia levels and CK activity after swimming test, this is related to the mechanism that increases energy storage (as glycogen) and release (as blood glucose), and decreases plasma levels of lactate, ammonia,and CK. The observation of the skeletal muscle structures of mice also con firmed that skeletal muscles became more damaged in the control group compared with the silymarin-treated mice after prolonged endurance exercise. Therefore, it is reasonable to infer that silymarin may bear potential pharmacological effects in combating fatigue.
1. Introduction
Silymarin is obtained from the plantSilybum marianum(milk thistle). It has been widely used for centuries for its hepatoprotective properties, as well as its ability to resist toxic liver damage, hepatitis,and cirrhosis [1-4]. Besides its antioxidant effects, silymarin has also been proven as an excellent anti-tumor promoting agent [5-8], and has additionally been linked to skin cancer prevention. Silymarin consists primarily of a mixture of active flavonolid isomers: silychristin,silydianin, and two groups of diastereoisomers, namely silybins A and B (Sb A, Sb B), and isosilybins A and B (ISb A, ISb B) [9-11].In recent years, the antioxidative, anti-inflammatory and anti-cancer activities of the isomers of silymarin were elucidated byin vitroandin vivostudies. However, there has been little quantitative analysis of the possible anti-fatigue function of silymarin [12,13].
Fatigue is de fined as physical and/or mental tiredness resulting in negative impacts on exercise efficiency, work performance, family life, and social relationships [14]. Physical fatigue can be accompanied by a deterioration in functional performance [15]. There are at least two mechanisms that are accountable for the occurrence of physical fatigue: oxidative stress and energy exhaustion [16]. Intensive exercise can lead to the accumulation of excess free radicals, resulting in tissue damage. Exhaustion theory suggests that energy depletion and the build-up of an excess metabolite can lead to fatigue [17]. Physical fatigue is a biological phenomenon that appears with bodily stress or exhaustive exercises, which reduces the physical endurance capacity [18].It has been demonstrated that intense exercise leads to increased generation of free radical thus inducing oxidative stress, damaging the cell membranes and other cellular components. While fatigue is usually a consequence of physical exertion, it can also be a symptom of a physical disorder, which is referred to as chronic fatigue when persisting for longer than six months. This type of fatigue is in fact an outcome of various human systemic and neurological disorders; and the latter is often associated with aging, Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis, and depression [19,20].Antioxidative supplements such as flavonoids have a significant impact in alleviating fatigue by preventing the oxidation of inter- or intra-cellular substrates [21]. Flavonoid is a natural component of plants and has a broad range of pharmacological properties. Indeed,it is well-established that flavonoids can delay and even inhibit oxidative stress by terminating the oxidation chain reaction through the removal of free radicals, chelating metal ions, and modulating various cellular signaling pathways [22]. Therefore, the aim of this paper was to evaluate the potential ergogenic and anti-fatigue effects of silymarin using the previously establishedin vivostudy [23].
Along with the fast pace of modern life and the vast popularity of competitive sports, the need to discover anti-fatigue health foods and medications of natural origin with no unpleasant side effects is urgent. Nevertheless, widely used herbal compounds such as silymarin, which contain rich content offlavonoids, have been shown to have antioxidant activity, meaning that it could be effective against oxidative stress and fatigue. Thus, it would be worth investigating whether silymarin possesses anti-fatigue properties. To this end,the anti-fatigue activity of silymarin was assessedin vivoin mouse models through a weight-loaded forced swim test and pole-climbing test. In addition, to validate the effect of silymarin on the endurance capacity of the mice, the biochemical changes in the serum, liver, and muscle tissues were also evaluated. The results provide the evidential foundation for the development and use of silymarin as a health supplement.
2. Materials and methods
2.1 Materials
Experimental animals: healthy Kunming mice 80, single-sex male, SPF grade, weighing between 18 and 22 g, were provided by the Liaoning longevity biotechnology companies (Liaoning, China).SPF level rat food was provided by the Experimental Animal Center of Shenyang Agricultural University.
2.2 Silymarin extraction from S. marianum
After rolling and peeling, theS. marianumfruit was mixed with water; the seed coat was isolated by winnowing, then collected,dried, and crushed. Then the powder was passed through a 40-mesh sieve and set aside. To extract the silymarin, 1 kg of dried powder from theS. marianumseed coat was mixed with 2 000 mL petroleum ether (boiling range 60–90 °C), skimmed for 8 h, filtered, and then defatted for 4 h with petroleum ether. 500 g of dry weight, nonfat,S.marianumseed coat powder was dissolved in 100 mL of 50% ethanol in a beaker, then placed in a water bath with a constant temperature of 35 °C and the pH adjusted to 5.0. The solution was sonicated with an ultrasonic output power of 200 W for the duration of the extraction,then solution was subjected to 3 U/g of the cellulase enzyme for 1 h;following this, the enzyme was inactivated by incubation at 85 °C for 20 mins. Subsequently, the solution was processed through vacuum suction filtration, and further purified by the HPD-600 macroporous resin purification column, concentrated under reduced pressure, and then freeze-dried to obtain the silymarin powder, which was later dissolved in distilled water and aliquoted into vials at a concentration of 1.0 mg/mL for use in animal testing. The purity achieved was greater than 90%.
2.3 Experimental animals
Eight-week-old male mice (18–22 g) were housed at a room temperature of (23 ± 1) °C with cycles of dark and light in an equal ratio (lights on from 06:00 to 18:00), and they were on an ad libitum feeding. Mice were treated in compliance with the current law and the Guiding Principles for the Care and Use of Laboratory Animals approved by the Animal Ethics Committee of China.
2.4 Experimental design
The 80 mice were randomly divided into 4 groups of 20 mice each. Silymarin was given by gavage to the mice at doses of 0, 50,100, and 200 mg/kg, the four groups were designated accordingly as the negative control group (CG), the silymarin low-dose group(silymarin-LG), the middle-dose group (silymarin-MG), and the high-dose group (silymarin-HG). The same volume of distilled water was given to mice in the CG. Silymarin was administered orally using an atraumatic feeding needle once per day at 08:00 for 4 weeks. Changes of the bodyweight of the mice were checked and recorded during the initial, intermediate, and terminal stages of the test. The climbing swimming capacities, as well as the corresponding biochemical parameters, were also documented, including serum urea nitrogen (SUN), BLA, LG, muscle glycogen (MG), and blood biochemical variables.
2.5 Weight-loaded swimming test and pole-climbing test
After 28 days, 10 mice from each group underwent a weightloaded swimming test. The procedure used in this experiment was similar to that described by Mansuri et al. [24]. 30 min after the last gavage administration, the mice were individually placed in a swimming pool that was 25 cm in diameter and 30 cm high, at (25 ± 1) °C,in which the mice could only support themselves by standing on the bottom. A tin wire (7% of the mice’s weight) was loaded onto the bottom of their tail. The swimming period was standardized as the time spent by the mouse floating in the water while struggling and paddling until exhaustion. The benchmark for exhaustion is when they failed to rise to the water surface to breathe within 10.At the end of the experiment, the mice were removed from the water, dried with paper towels, and placed back in their home cages.The time taken to the point of exhaustion was used as the index of the forced swimming capacity.
Similarly, 10 mice from each group were tested for endurance using a one-meter long vertical climbing pole rack placed in a pool about 30 cm deep with water temperature kept at 25 °C. The test was started at 30 min after the last gavage administration when the mice were placed onto an iron rod, as the mouse muscle fatigues and weakens, it will cease climbing and fall in the pool, the time taken was recorded. At the end of the experiment, the poleclimbing time reached until exhaustion was used as the index of the forced climbing capacity.
2.6 Determination of LG, MG, SUN and BLA
After 28 days, the other 10 mice from each group were taken out for analyses of LG, MG, and blood biochemical parameters SUN and BLA. 30 min after the last gavage administration of silymarin, the mice were forced to swim (weight-unloaded) for 90 min. After the mice were allowed to rest for 60 min, the blood samples of the mice were collected in heparinized tubes by removing the left eyeball.After adding the anticoagulant to the blood, plasma was prepared by centrifugation at 1 000 r/min and 4 °C for 10 min. After the blood has spontaneously coagulated, serum is prepared by centrifugation at 1 000 r/min and 4 °C for 15 min. The blood plasma was used to determine the concentration of BLA and the serum was used to test for SUN. After the blood sampling, the mice were immediately dissected and the livers and the skeletal muscle were harvested, frozen in liquid nitrogen, and kept at −80 °C until the analysis of glycogen concentration was performed. The concentrations of BLA, SUN, LG,and MG were determined following the recommended procedures provided by the manufacturer of the kit used.
首先,会计信息化的发展,要求企业能够与时俱进,跟随时代的发展,企业需要去了解财务管理信息化的模式,一旦企业财务管理信息处理不当或者系统故障,企业会引发后期一系列的问题,面临极大的经营风险。
2.7 Determination of blood biochemical variables
The effects of silymarin on plasma lactate, ammonia, glucose levels, and creatine kinase (CK) activity were evaluated after exercise.After 1 h of the last administration, a 15 min swimming test was performed without weight. Blood samples were immediately collected from the submandibular duct of pre-treated mice after swimming exercise. The plasma was produced by centrifugation at 1 500 ×g,4 °C for 10 min. Biochemical analyses were performed with the use of an automatic analyzer.
After the mice were fed and swimming on the last day of the end of the test week, the blood physiological and biochemical indexes of the mice were measured according to the instructions of the kit. The physiological and biochemical indexes include aspartate aminotransferase (AST), alanine aminotransferase (ALT), and serum alkaline phosphatase (ALP), lactate dehydrogenase (LDH), albumin,total bilirubin (TBIL), total serum protein (TP), creatinine, uric acid(UA), total serum cholesterol (TC), triglycerides (TG).
2.8 Histological staining of tissues
Different tissues were collected and fixed in 10% formalin. They were dissected transversely or longitudinally to obtain skeletal muscle cross-sections. Tissues were then embedded in paraffin and cut into 4 μm thick slices for morphological and pathological evaluations.Tissue sections were stained with H&E and examined using a light microscope equipped with a CCD camera (BX-51, Olympus, Tokyo,Japan) by a clinical pathologist.
2.9 Statistical analysis
All data are represented as the mean ± standard error (SE) in the tables and the standard errors are symbolized by vertical bars.Differences between groups were determined by analysis of variance and Student’st-test.P-value of less than 0.05 was considered significant and considered very significant if less than 0.01.
3. Results and discussion
3.1 Body weight, skeletal muscle mass, and other metabolism-related organ weights
Morphological data from each experimental group are summarized in Table 1. There was no significant difference in initial body weights among the control, silymarin-LG, silymarin-MG, and silymarin-HG groups. An increase in body weight induced by silymarin was dependent on food intake. In addition, the trend analysis also displayed significant increases in tissue weights of the liver, muscles, and kidneys as dosages of silymarin treatment were raised. The relative tissue weight (%) is a measure of different tissue weights adjusted for the individual body weight, and there were no significant changes found in the liver, skeletal muscle (gastrocnemius and soleus muscles), and kidney among the groups.
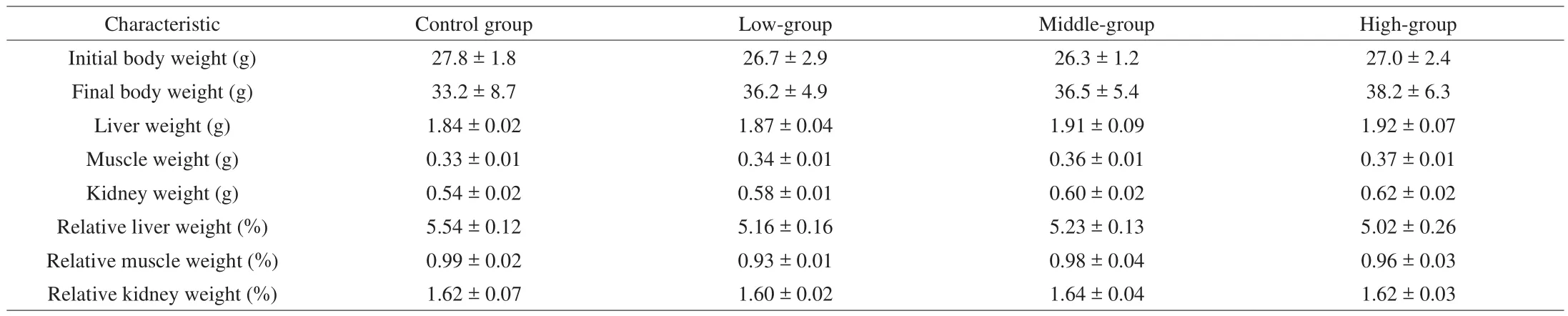
Table 1General characteristics of the experimental groups.
3.2 Silymarin prolonged the exhaustive swimming time and pole-climbing time
Fatigue is re flected through a decrease in exercise, which refers to the exercise capacity of an animal as assessed by their endurance and workload completed during the exercise period. Exercise tolerance can be assessed accurately using a tolerance test, such as the weightloaded forced swimming test and pole-climbing test in this study.The length of time of the two parameters measured by these tests indicate the degree of fatigue [25]. Accordingly, the swimming time and the pole-climbing time until exhaustion of mice were quantified to inspect the fatigue-inhibiting effect of silymarin. The results indicated a significant increase in the swimming and climbing capacity after administration of silymarin for 28 days compared with that of the CG (Table 2). A closer look at Table 2 revealed that the increased percentage of time until exhaustion of each treatment group (silymarin-LG, silymarin-MG, and silymarin-HG) were 37.5%,473.0%, and 274.9%, respectively for the swimming time; and 68.6%,152.4%, and 108.6%, respectively for the climbing time. Thus, it is justifiable to conclude that silymarin had meaningful positive effects on increasing the exercise endurance of all treated mice in this experiment, most evidently in the silymarin-MG (100 mg/kg,P< 0.01) and the silymarin-HG (200 mg/kg,P< 0.05) groups.These results clearly indicated that silymarin could extend the exercise time until exhaustion in mice, highlighting the antifatigue property of silymarin. This result conformed to a previous study with silymarin supplements. These data indicate that selective concentrations of silymarin may contribute differently to physiological activities, and a 100 mg/kg dose may be the optimal amount for enhancing endurance capacity.
3.3 Silymarin decreased SUN and BLA in the blood
Blood urea nitrogen (BUN) is derived through the metabolism of dietary and tissue turnover proteins in the liver which releases amino acids into the blood and transports it to the kidney for excretion.Prolonged exercise significantly increases the level of serum BUN,referred to as serum urea nitrogen (SUN), thus, the SUN concentration can be used as a measurement of physical activity and serves as a blood biochemical parameter related to fatigue [26].
After the swimming exercise, the changes of SUN were measured and described above, and the data for all the groups are presented in Fig. 1A. The results revealed that after exercise, the SUN level of all the treated groups was significantly lower than that of the control group (P< 0.05). In particular, the decrease of the SUN level was most prominent in the middle-dose group compared to the control group (P< 0.01). Thus, it is plausible that silymarin exerts its antifatigue effect by reducing the level of SUN.
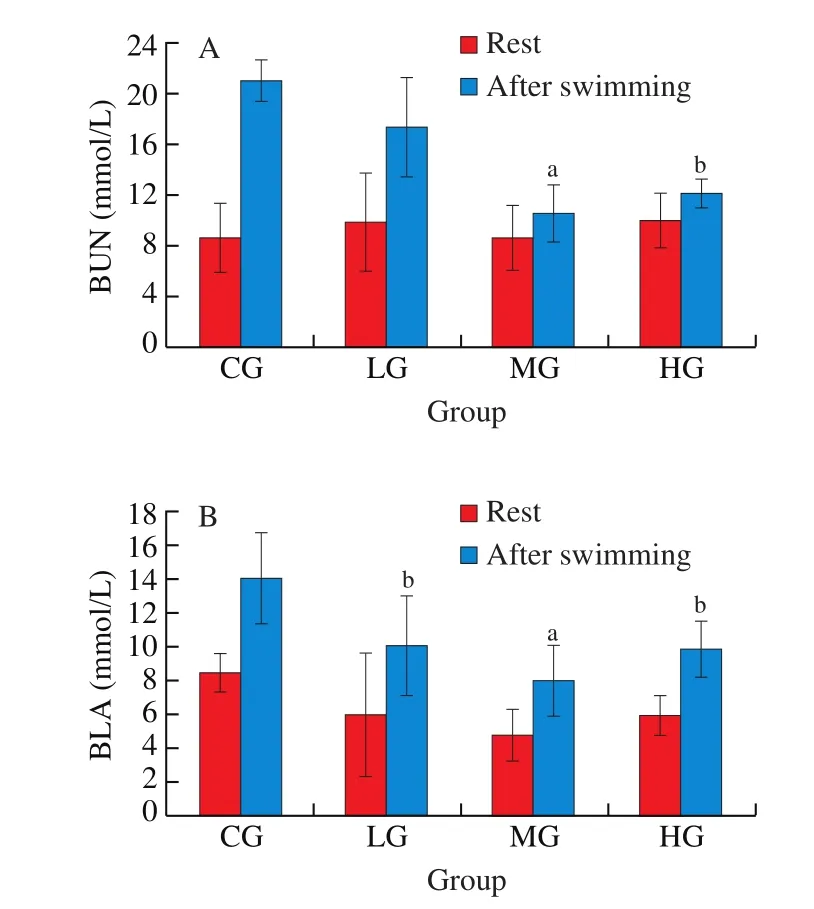
Fig. 1 Effect of silymarin on BUN (A) and BLA (B) in mice. Compared with control-group, the letter a indicates a significant difference at P < 0.01, the letter b indicates a significant difference at P < 0.05.
Prolonged strenuous exercise depletes oxygen and accelerates glycolysis in the body, inducing the production of large amounts of lactic acid and its ionic lactate form, thereby triggering a process that eventually reduces calcium ion reabsorption and the subsequent release-a major factor contributing to fatigue [27]. Therefore, blood lactate level is another significant indicator of fatigue within human bodies. Accordingly, after the swimming exercise, the levels of BLA were measured and the data for all the groups are presented in Fig. 1B. The results showed that after the exercise the production of lactic acid in all the treated groups was significantly reduced compared to the control group (P< 0.05), and this reduction is most obvious in the silymarin-MG group compared with those in the CG(P< 0.01). Therefore, the data indicated that silymarin can in fact reduce lactic acid generation, which may represent another pathway whereby this milk thistle extract can delay the onset of fatigue or speed up the recovery from it.
3.4 Silymarin increased LG and MG
In contrast to short high-intensity exercises in which muscle cell glycogen serves as an immediate source of glucose for muscle cells, in prolonged endurance exercises, glycogen from all sources is consumed, but notably LG and other energy sources such as fat and proteins are drained, which is often accompanied by extreme fatigue.There is a large amount of published studies advocating that exercise leads to physical exhaustion and MG depletion, which always occur simultaneously. With the increasing consumption of glucose by muscle cells, the human body would increase the LG metabolism in order to maintain blood glucose levels. Hence, glycogen is a crucial source of energy for muscle tissue, as it is responsible for increasing blood glucose levels when they are depleted during prolonged endurance exercise. In summary, LG and MG concentrations are sensitive indicators re flecting the degree of fatigue.
The effect of silymarin on the levels of MG and LG in treated mice after exercise is shown in Fig. 2. After exercises, the levels of MG of all the silymarin treatment groups were higher than that of the control group (P< 0.05); with a higher increase in muscle glycogen content in the silymarin-MG group compared with those in the CG (P< 0.01). Similarly, after exercises, the level of LG of all the silymarin treatment groups were higher than that of control group (P< 0.05); with the increase being more significant in the silymarin-MG group (P< 0.01). Therefore, the antifatigue property in silymarin could be mediated through a pathway involving an increased level of MG and LG.

Fig. 2 Effect of silymarin on MG (A) and LG (B) in mice. Compared with control-group, the letter a indicates a significant difference at P < 0.01, the letter b indicates a significant difference at P < 0.05.
3.5 Effect of silymarin on plasma lactate, ammonia, glucose,and CK Levels after exercise
Biochemical variables, including lactate, ammonia, glucose, and CK, are important indicators of muscle fatigue after exercise [28].Before exercise, no statistically significant differences were found between these four biomarkers among four groups (Fig. 3A-D). After an acute swimming exercise challenge, the levels of lactate, ammonia,glucose, and CK in the control group were significantly increased,when compared with basal status as illustrated in the Fig. 3.
The muscle produces a large quantity of lactate when it obtains enough energy from anaerobic glycolysis during high-intensity exercise. The increased lactate level further reduces pH value,which could induce various biochemical and physiological side effects, including glycolysis, phosphofructokinase and calcium ion release, through muscular contraction [29]. Lactate levels in the CG, silymarin-LG, silymarin-MG, and silymarin-HG groups were(7.2 ± 1.1), (5.9 ± 0.9), (5.2 ± 0.8), and (5.4 ± 0.5) mmol/L, respectively(Fig. 3A), demonstrating a significant reduction with silymarin treatments by 18.1% (P< 0.01), 27.8% (P< 0.01), and 25.0%(P< 0.01) respectively, than CG.
It has been long established that ammonia, the metabolite of protein and amino acid, is associated with fatigue. An increase in ammonia level in response to exercise can be managed by the use of glutamine and/or carbohydrates that interfere with ammonia metabolism [30]. The increase in ammonia level is related to both peripheral and central fatigue during exercise. Plasma ammonia levels in the CG, silymarin-LG, silymarin-MG, and silymarin-HG groups were (184 ± 23), (130 ± 19), (101 ± 20), and (128 ± 15) μmol/L,respectively. Silymarin treatments significantly lowered the ammonia concentration by 29.3%–45.1% compared with the CG treatment(P< 0.000 1) (Fig. 3B).
During exercise, the initial energy supply comes from the breakdown of glycogen, later intense exercise calls for circulating glucose released by the liver [31]. Therefore, the blood glucose level is an important index for performance maintenance during exercise.The plasma glucose levels in the CG, silymarin-LG, silymarin-MG,and silymarin-HG groups were (133 ± 11), (170 ± 11), (183 ± 12),and (177 ± 14) mg/dL, respectively; and was significantly higher by 27.8% (P< 0.01), 37.6% (P< 0.01), and 33.1% (P< 0.01),respectively, compared to CG (Fig. 3C).
Plasma level of creatine kinase (CK) is a clinical biomarker for muscle damage, muscular dystrophy, severe muscle breakdown, myocardial infarction, autoimmune myositides, and acute renal failure [32].The CK activity in the CG, silymarin-LG, silymarin-MG, and silymarin-HG groups were (360 ± 15), (255 ± 20), (226 ± 22), and(249 ± 23) U/L, respectively (Fig. 3D). The activity levels were significantly lowered by 29.2% (P= 0.017), 37.2% (P= 0.003), and 30.8% (P= 0.012) with silymarin-LG, silymarin-MG, and silymarin-HG, respectively. Therefore, silymarin supplements should ameliorate skeletal muscle injury induced by intense exercises.
The trend analysis revealed the silymarin treatment did not have a significant dose-dependently effect.
3.6 Effect of silymarin on the clinical biochemistry tests
The clinical biochemistry values of the control and treatment group were measured at the end of the 28-day oral feeding trial.As shown in Table 3, there were no major side effects in the liver profile (AST, ALT and albumin), bile duct function (ALP and total bilirubin), cardiac profile (LDH), muscular function (CK), renal profile (creatinine and uric acid), nutritional status (total protein), and lipid levels (TC and TG) among the control and treatment groups.Therefore, the acute toxicity study of silymarin at 50, 100, and 200 mg/kg administered orally to mice did not cause any death or acute adverse effect during the clinical observation period or mortality. The results suggested that silymarin supplements should be safe for all test animal subjects.
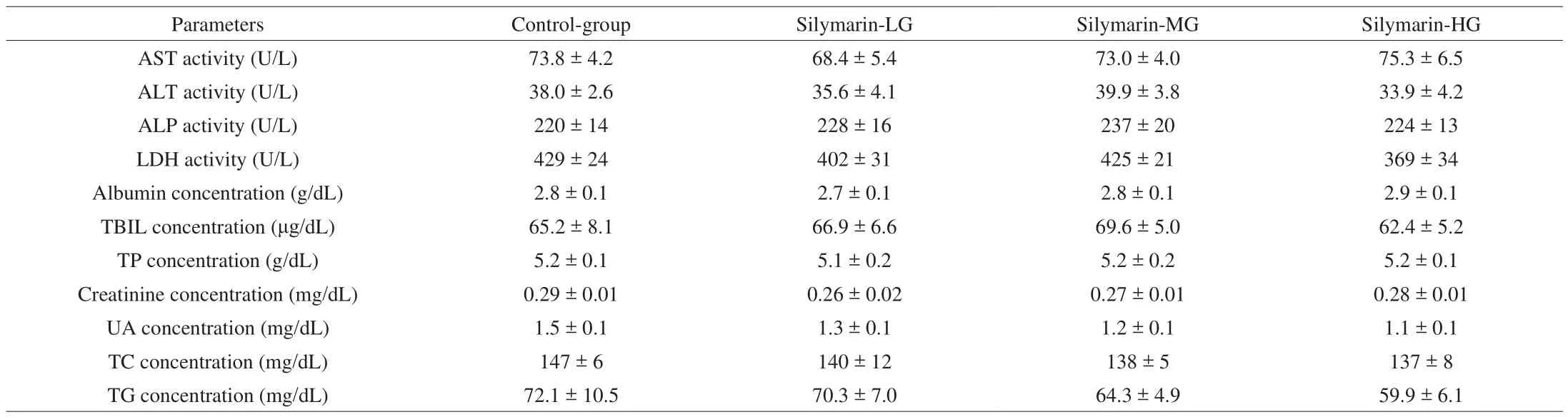
Table 3Biochemical analysis of the silymarin treatment groups at the end of experiment.
3.7 Silymarin protective effect on endurance exercise
Observation of the appearances of the structure of skeletal muscle of mice under paraffin confirmed skeletal muscle damage arise after prolonged endurance exercise. as shown in Fig. 4. Different structural impairment of the control mice are visible, including widened spacing, muscle fiber breakage, increased tubular structure expansion, and mitochondrial damage. On the other hand, the damage in the skeletal muscles of mice treated with silymarin was milder and included mild muscle pitch, slight widening, a small amount of individual mitochondria vacuoles, and no significant swelling. The cell increases mitochondrial biogenesis. Incidentally,an increase in the number of skeletal muscle mitochondria, referred to as mitochondrial biogenesis, was observed, which usually occurs in cells to overcome a bioenergetics deficit or to compensate for an increased energy requirement [33]. Since mitochondria are the major energy-producing organelles for oxidative phosphorylation metabolism, they are fundamental for endurance sports, particularly when glycogen, glucose, free fatty acids, and other energy compounds all react within the mitochondria and generate ATPs. Therefore,the mitochondrial number and energy metabolism, and severity of mitochondrial damage, may re flect the fatigue status of the body. The results illustrated that silymarin has a protective effect and reduces the damage of skeletal muscle structure caused by prolonged endurance exercise. Silymarin bears antioxidant effects that prevent muscle cell damage and reduce free radical attack against cell membranes and other vulnerable targets, which may be the theoretical basis for its protective function. The results of this present study also suggested that the silymarin supplement had no toxic effects on major organs such as the skeletal muscles according to histopathological examinations.
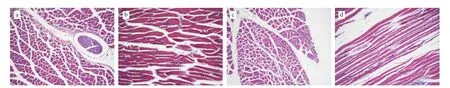
Fig. 4 Histological examination of skeletal muscle of mice stained with H&E dye (10 × 40). a: Cross-section of the control group; b: Longitudinal section of the control group; c: Cross-section of the exercise group; d: Longitudinal section of the exercise group; e: Cross-section of the silymarin-HG; f: Longitudinal section of the silymarin-HG; g: Cross-section of the silymarin-MG; h: Longitudinal section of the silymarin-MG; i: Cross-section of the silymarin-LG; j: Longitudinal section of the silymarin-LG.
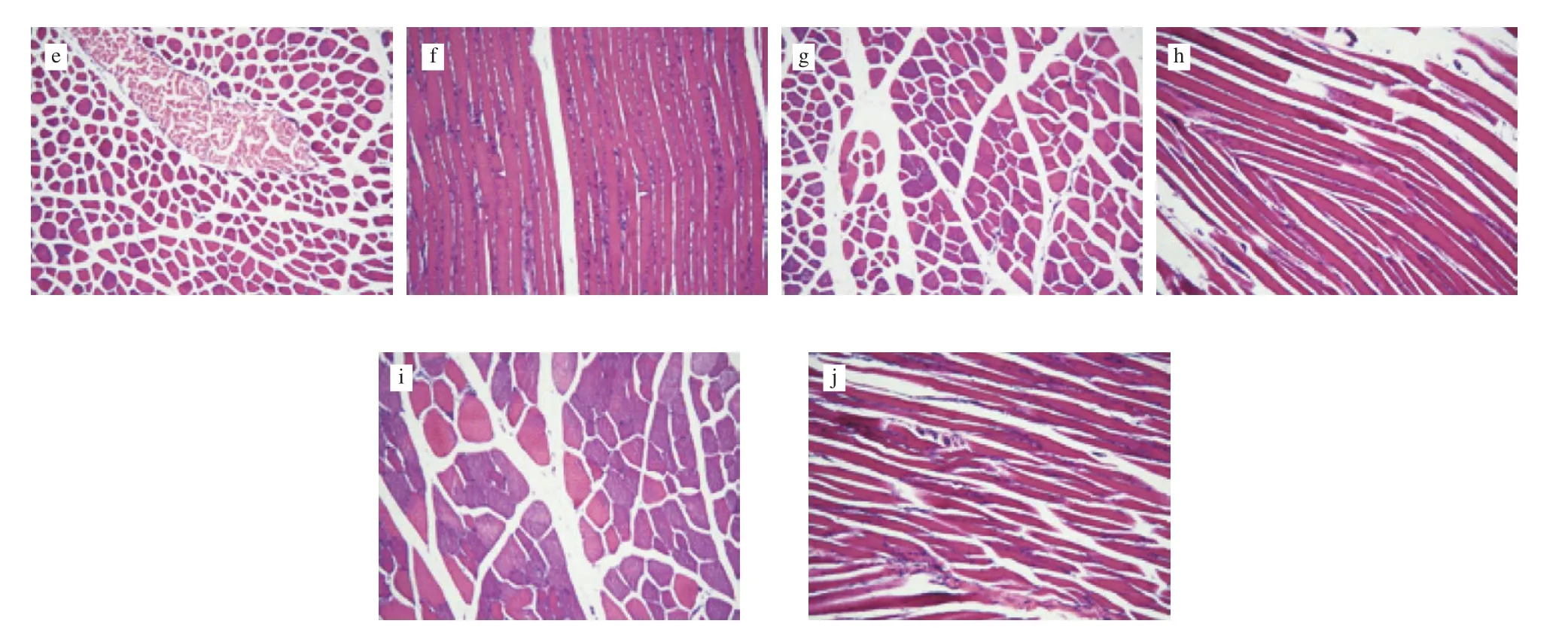
Fig. 4 (Continued)
4. Conclusion
Past literature has established the anti-inflammation, antioxidation, anti-carcinogenic, and anti-angiogenesis properties of silymarin. However, to our best knowledge, to date there has been no report on the testing of bio-efficacy on the anti-fatigue property of silymarinin vivo. In this study, the fatigue-alleviating effects of silymarin were evaluated from various aspects, the findings suggested the following: 1) Exercise training combined with silymarin supplements increased endurance with exercise and increased hepatic and muscle glycogen content; 2) silymarin reduced exercise-induced accumulation of byproducts such as blood lactate and ammonia after intense exercise; and 3) daily silymarin administration for 8 weeks had no toxic effects according to biochemical parameters and histopathological examination. Thus, silymarin may have ergogenic and anti-fatigue functions.
In conclusion, the current study implied that the silymarin extract from milk thistles could increase the swimming time to exhaustion of test animals, increase plasma glucose and muscular and hepatic glycogen levels, and decrease the plasma lactate and ammonia levels [34]. This finding, while preliminary, suggests that silymarin has anti-fatigue property and can elevate exercise performance. Although the exact bioactive phyto-compounds and exact mechanisms remain to be elucidated, this study provides science-based evidence to support that silymarin could be a promising anti-fatigue agent and an ergogenic aid.
- 食品科学与人类健康(英文)的其它文章
- Dietary bioactives and essential oils of lemon and lime fruits
- Green tea, epigallocatechin gallate and the prevention of Alzheimer’s disease: clinical evidence
- Simultaneous quantification of 18 bioactive constituents in Ziziphus jujuba fruits by HPLC coupled with a chemometric method
- A systematic study on mycochemical profiles, antioxidant, and anti-inflammatory activities of 30 varieties of Jew’s ear (Auricularia auricula-judae)
- GPP (composition of Ganoderma lucidum polysaccharides and Polyporus umbellatus polysaccharides) protects against DSS-induced murine colitis by enhancing immune function and regulating intestinal flora
- Immunoregulatory polysaccharides from Apocynum venetum L.flowers stimulate phagocytosis and cytokine expression via activating the NF-κB/MAPK signaling pathways in RAW264.7 cells

