Green tea, epigallocatechin gallate and the prevention of Alzheimer’s disease: clinical evidence
Klaus W. Lange*, Katharina M. Lange, Yukiko Nakamura
Department of Experimental Psychology, University of Regensburg, Regensburg 93040, Germany
Keywords:
Green tea
Epigallocatechin gallate
Alzheimer’s disease
Neurodegeneration
Prevention
A B S T R A C T
Given its increasing global prevalence, Alzheimer’s disease (AD) has become a major public health challenge worldwide. The symptomatic treatments available for AD have shown no significant efficacy, and no disease-modifying interventions are capable of slowing the progression of the disorder. The potential of lifestyle-related factors, including diet, is increasingly recognized as an important consideration in the primary prevention of AD. Numerous mechanisms potentially underlying neuroprotective effects of bioactive components contained in tea, such as (-)-epigallocatechin-3-gallate, as well as their preventive efficacy against AD, have been elucidated in preclinical studies. However, in contrast to the abundance of mechanistic findings in animals, clinical results demonstrating efficacy in humans are scarce. While epidemiological studies have provided some evidence indicating that green tea consumption is associated with a reduced risk of age-related cognitive decline and AD, a causal relationship cannot be established on the basis of these observations.The clinical evidence regarding preventive or therapeutic effects of green tea and its bioactive components is unsatisfactory. A role of green tea in the prevention of AD cannot be recommended until well-designed,randomized, placebo-controlled clinical trials using standardized formulations con firm the purported beneficial effects of green tea.
1. Introduction
Alzheimer’s disease (AD) is characterized clinically by dementia and a global impairment of cognition, including memory, executive,language and other functions. The disorder is associated with a marked decline in activities of daily life [1,2], with symptoms normally appearing late in life following a long preclinical period of several decades. The neuropathological hallmarks of the progressive neurodegeneration in AD are extracellular senile plaques, which contain misfolded amyloid-β(Aβ) peptides, and intracellular neuro fibrillary tangles, which are accumulated hyperphosphorylated tau protein forms [3,4]. Major pathophysiological mechanisms in the complex pathogenesis of AD include oxidative stress and chronic neuroinflammation. The aggregation of Aβcan lead to the generation of large quantities of free radicals, such as active oxygen and nitrogen species, causing oxidative stress and thereby accelerating neuronal dysfunction and ultimately leading to neuronal death [3,5]. The pharmacotherapy of AD includes the use of acetylcholinesterase inhibitors andN-methyl-D-aspartate receptor antagonists. However,these drugs show no significant efficacy in AD, and no treatments capable of effectively improving cognitive function or retarding the progressive neurodegenerative process are currently available [2].Given its increasing prevalence, AD has become a major global challenge for both healthcare systems and entire societies [6]. The prevention of AD is therefore a major public health challenge [7]requiring the development of novel interventions capable of delaying the onset of the disease.
AD does not appear to be an inevitable consequence of aging.In addition to a genetic susceptibility, numerous lifestyle factors,such as obesity, diabetes, hypertension, smoking, “unhealthy” diet,cognitive and physical inactivity, low educational attainment and depression may increase the risk of AD [7-9]. The elimination of modifiable lifestyle factors has been estimated to decrease the incidence of dementia by at least 30% [10]. Recent reports have associated a decreasing incidence and prevalence of dementia in high-income countries with previous investment in vascular health and education at population level [11-13]. This finding highlights the potential value of modifications in lifestyle-related factors in the primary prevention of AD [14,15]. Diet, in particular, is receiving increasing recognition as an important modifiable factor, since it can affect alterations of brain and behavior related to aging and disease [16,17]. Dietary approaches to AD include the Mediterranean diet [18,19], ketogenic diets [20]and certain medical foods [21,22].The potential therapeutic benefits of various food bioactives, such as antioxidants, probiotics and polyunsaturated fatty acids, on mental and neurodegenerative disorders have been investigated [23-34].Numerous natural polyphenols, such as flavonoids, appear to have the potential to modulate the neuropathological changes and cognitive impairment associated with AD through various mechanisms,including the modulation of oxidation and inflammation as well as of Aβmetabolism, catabolism and oligomerization [35]. Furthermore,prospective studies in humans have found an association between high levels of dietary flavonoid consumption and a lower risk of AD [36-38].
A review of the mechanisms of action and neuroprotective effects of bioactive agents contained in tea concluded that these compounds might be of value in the prevention of neurodegeneration [39].Several lines of evidence suggest that tea consumption may decrease the incidence of AD [40]. In particular, the polyphenolic flavonoid (–)-epigallocatechin-3-gallate (EGCG) appears to show neuroprotective activity in preclinical models and may have clinical effects in AD [40]. EGCG accounts for 50%–80% of total tea catechins [41], and a cup of tea brewed with 2.5 g of green tea leaves contains approximately 130–180 mg of EGCG [42]. The present short review summarizes the clinical evidence in support of preventive efficacy of tea and EGCG in AD and the difficulty translating animal findings on EGCG to AD in humans.
2. Evidence from animal studies
Experimental models, the majority of which are animal models,are used as a research tool in the investigation of the pathogenesis and pathophysiology of AD as well as the preclinical development of novel therapeutics. Major models are transgenic mice overexpressing mutated human genes that lead to the formation of amyloid plaques [43,44]. Other less commonly used models include zebrafish [45]and invertebrates, such asDrosophila melanogasterandCaenorhabditis elegans[46-48].
Various findings using animal models of AD have suggested neuroprotective effects of green tea polyphenols. In transgenic mice with an overexpression of Aβ, the administration of EGCG has been found to significantly decrease Aβdeposition and oxidative stress,to modulate tau pathology and to reduce cognitive impairment,particularly in spatial memory tasks [49,50]. Mechanisms of action of EGCG include free radical scavenging, anti-inflammatory and ironchelating activities as well as effects on various other intracellular molecular targets [51]. The preclinical findings of neuroprotective effects of EGCG in Alzheimer models have recently been reviewed in more detail [52]. However, the translational utility of many animal models of AD in examining the potential of novel therapeutics has been questioned [53].
The criteria commonly used for the evaluation of animal models are met to different degrees in Alzheimer models. Face validity(similar pathology in models and humans) is satis fied in most animal models, while construct validity (same pathomechanisms in models and humans) and predictive validity (translation offindings in models to humans) remain problematic [54,55]. The frequent failure to translate successful therapy from preclinical to human studies in AD research [56]may be the result of too limited a mirroring of human pathology in animal models [57]. For example, cognitive impairment occurs at different stages of the pathogenetic process in rodent models compared to humans, with impairment of cognitive functions present in animals prior to or at the onset of plaque development and in humans many decades following the development of plaques [58].Studies administering a combination of nutritional supplements and thereby targeting a range of pathophysiological mechanisms involved in age-related cognitive decline and neurodegeneration may be of more value than trials of single tea compounds.
3. Evidence from human studies
3.1 Observational studies
While some observational studies have reported an inverse association of tea consumption with the risk of AD, the paucity of high-quality epidemiological studies leaves the potential of green tea in the prevention of dementia in humans unclear.
A 5.7-year prospective study investigated the association between green tea intake and incident dementia [59]. The study cohort comprised 13 645 individuals aged 65 years or older (6 030 men,7 615 women, mean age ± standard deviation (73.8 ± 5.9) years),and the follow-up rate was almost 99%. Information on the daily intake of green tea was collected using a questionnaire, and data on incident dementia were retrieved from a public insurance database.More frequent consumption of green tea was associated with a lower risk of incident dementia (hazard ratio for daily intake of ≥ 5 cups versus < 1 cup: 0.73; 95% con fidence interval: 0.61-0.87) [59].Non-significant associations were found between incident dementia and consumption of black tea or oolong tea. Limitations of the study included an assessment of green tea consumption only at baseline, the absence of a clinical diagnosis, and the lack of both an evaluation of the causes of dementia and a comprehensive assessment of confounding factors. Nevertheless, the study found a statistically significant inverse association between green tea intake and incident dementia, suggesting a preventive effect of green tea against dementia.
A recently published systematic review of observational studies investigating the association of green tea consumption and dementia identified five cross-sectional and three longitudinal studies, including the above study [60]. Three cross-sectional studies and one cohort study supported beneficial effects of green tea, one cross-sectional and one longitudinal study found partial positive effects, and the remaining studies reported no significant association of green tea with any kind of cognitive impairment (Table 1). These results suggest that green tea consumption might reduce the risk of cognitive decline and AD.However, more robust evidence in support of this hypothesis is needed.
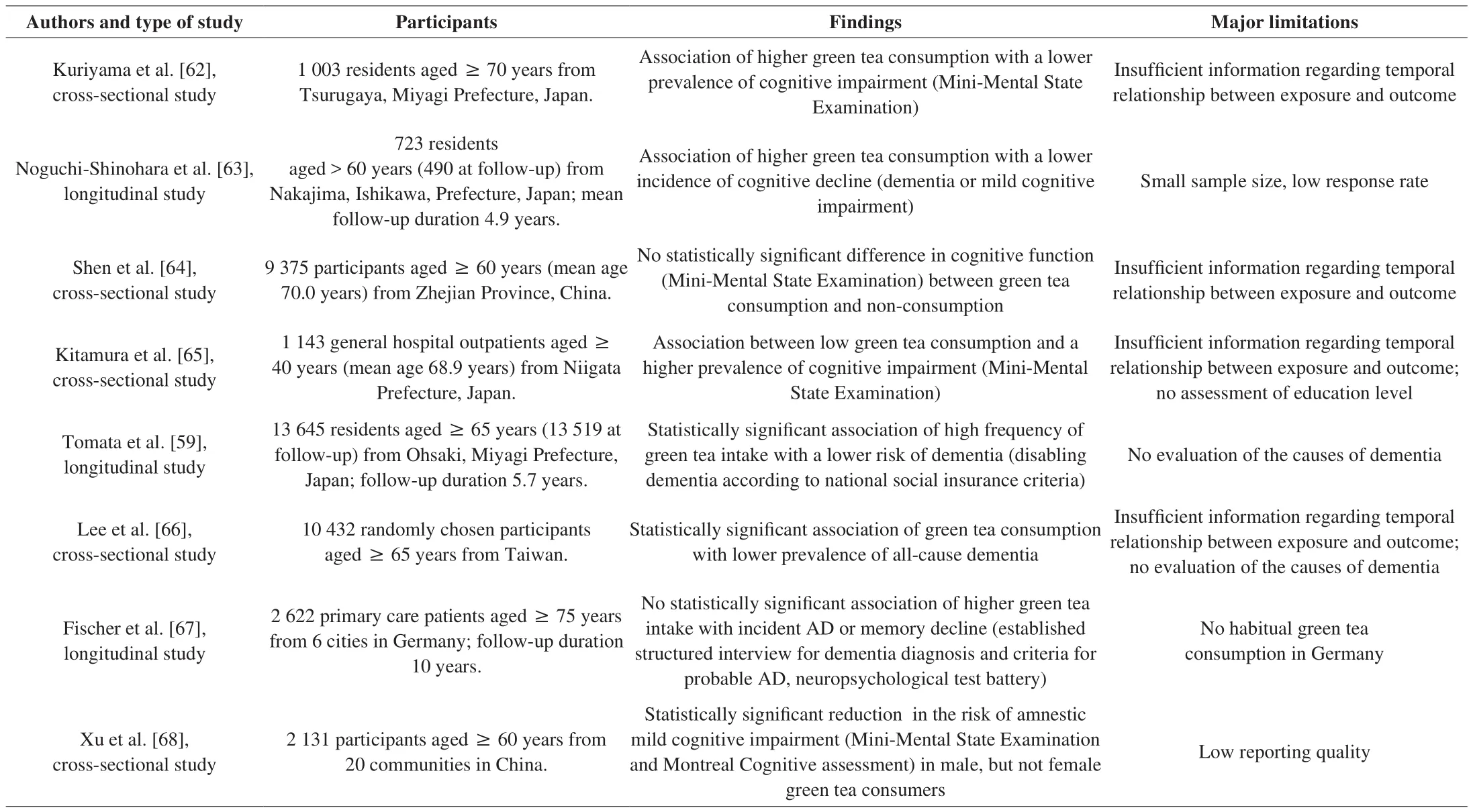
Table 1Findings of cross-sectional and longitudinal studies on tea and cognitive impairment or dementia.
The observational studies on tea consumption and cognitive impairment are subject to confounding and selection bias, since unmeasured or unmeasurable factors may affect the effects and cannot be accounted for by statistical methods [61]. Even when adjusting for confounding factors, the potential influence of unmeasured confounders and residual confounding cannot be excluded completely.Positive health effects of tea, as assessed in observational studies,may be influenced by many other known or unknown characteristics and lifestyle factors among the cohort investigated. Tea consumption may be related to social and health-related lifestyle factors that could confer some of the protective effects. In addition, reverse causality might bias the association between cognition and intake of tea, with a medical condition leading to a change in the intake of tea. Thus,observational studies cannot establish causal relationships between intervention and outcome.
3.2 Intervention studies
Intervention studies using randomized controlled trials are needed to establish a cause-and-effect relationship between an intervention and a health outcome. However, intervention studies assessing the preventive or symptomatic efficacy of green tea in agerelated cognitive decline or AD are scarce. A small randomized,double-blind, placebo-controlled study found beneficial effects of combined green tea extract andL-theanine on cognition in individuals with mild cognitive impairment [69]. Another 12-month doubleblind, randomized controlled study examined the effects of green tea intake on cognitive dysfunction [70]. Twenty-nine female and four male nursing home residents (mean age ± standard deviation:(84.8 ± 9.3) years) with cognitive dysfunction (with a score below 28, mean ± standard deviation: 15.8 ± 5.4, on the Japanese version of Mini-Mental State Examination) were randomly assigned to a green tea (2 g/day powder containing 220.2 mg of catechins) or placebo powder group. The daily intake of green tea powder was equivalent to 2-4 cups of brewed tea [71]. Following 12 months of green tea intake, changes in the Mini-Mental State Examination did not differ significantly from participants in the placebo group [70],indicating no effects of green tea on cognitive function. However,limitations of the study include a small sample size, lack of psychiatric assessment or more sophisticated neuropsychological testing and a short follow-up duration. In summary, further highquality, long-term controlled trials are required to examine the efficacy of green tea and its components.
4. Future directions
The observational findings of an association of incident dementia with the intake of green tea, but not oolong tea or black tea [59],suggests that the effect of tea consumption may be attributable to specific components contained in green tea. EGCG levels, for example, have been found to be highest in green tea compared to oolong tea and black tea [72]. In general, the effects of tea and EGCG on AD in humans appear to be less favorable than those found in preclinical models. This discrepancy may be due to a variety of confounding factors in clinical studies, such as age, sex, genetic polymorphisms, intestinal microbiota, alcohol consumption, smoking or other lifestyle factors [73-75]. In addition, different clinical populations, such as individuals with mild cognitive impairment, AD and dementia not further classified, were included in the available studies [59]. Moreover, dose and time of tea or EGCG administration as well as different catechin metabolism between animals and humans need to be considered [76].
In addition to possible preventive effects of tea consumption,therapeutic benefits of green tea have been proposed. A systematic literature review reported beneficial effects of green tea on memory and attention functions, with improved cognition not being linked to any single tea component [77]. However, whether the positive effects of tea or EGCG on cognitive functions, as reported in healthy people(e.g. [78,79]), can also be found in individuals with preclinical or clinical dementia is unknown. The level of tea intake and optimum dose of EGCG potentially providing beneficial effects in AD remain to be clarified. Furthermore, the poor bioavailablity of tea polyphenols, including EGCG, needs to be considered [80,81].
In addition to EGCG, other compounds contained in green tea,such asL-theanine, caffeine and thea flavins, may also have preventive effects against neurodegeneration and cognitive decline [39,82].Furthermore, interactive effects of nutrients on brain health may be more significant than those of single agents. In the prospective Oregon Brain Aging Study, various plasma biomarkers related to diet were analyzed in older participants (mean age 87 years) [83]. This analysis yielded three distinct patterns relevant for age-related cognitive functioning and magnetic resonance imaging (MRI) measures. Two of these patterns were positively associated with cognitive and MRI outcomes: (1) a pattern high in plasma vitamins B, C, D and E, and (2)a pattern high inω-3 fatty acids. In contrast, the third pattern, which was high in trans-fatty acids, was associated with worse cognitive performance and a greater reduction in brain volume [83]. These findings emphasize the role of nutritional patterns in brain health, and the role of tea in age-related cognitive decline should be investigated in combination with other dietary factors.
Studies attempting to test disease-modifying approaches in AD face the problem that neuropathological alterations begin to develop many years prior to the appearance of clinical symptoms. Major challenges of AD prevention trials include the need for large samples of community-dwelling people, long follow-up durations, an optimum time window for intervention and clinically relevant outcomes [84].The identification of populations at increased risk of AD on the basis of age and genetic or clinical factors will be important, as will the need to administer preventive treatments to cognitively unimpaired elderly people before the disease is fully expressed.
5. Conclusion
Preclinical studies have shed light on numerous mechanisms through which the bioactive components contained in tea, such as EGCG, may exert anti-amyloid effects and thereby protect against AD. However, in contrast to numerous mechanistic findings in animals, few clinical results in humans have demonstrated the efficacy of these compounds. While epidemiological studies have provided some evidence indicating that green tea consumption is associated with a reduced risk of age-related cognitive decline and AD, a causal relationship cannot be founded on these observations. In summary, the clinical evidence regarding preventive or therapeutic effects of green tea and its bioactive components is unsatisfactory. The inconsistent findings of epidemiological studies require further evidence derived from well-designed clinical interventions. A recommendation for the use of tea in the prevention of AD cannot be made on the basis of currently available evidence. Well-designed, randomized, placebocontrolled clinical trials using standardized compounds and dosages are required to con firm the purported benefits of green tea in AD.
Conflict of interest
The authors declare that there is no actual or potential conflict of interest.
- 食品科学与人类健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

