Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
Xitong Zhang, Lin Du, Weimin Zhang, Mi Yang, Li Chen, Chen Hou,*, Jianke Li,*
a College of Food Engineering and Nutritional Science, Shaanxi Normal University, Xi’an 710119, China
b University Key Laboratory of Food Processing Byproducts for Advanced Development and High Value Utilization, Xi’an 710119, China
Keywords:
Metabolic syndrome
Insulin resistance
Insulin signaling pathway
PPARγ
Pomegranate peel polyphenols
A B S T R A C T
Insulin resistance (IR) has been considered to be an important causative factor of metabolic syndrome (MetS).The present study investigated whether pomegranate peel polyphenols (PPPs) could prevent the development of MetS by improving IR in rats. Male Sprague-Dawley (SD) rats were fed high fat diet (HFD) to induce MetS and supplemented with different dosages of PPPs for 12 weeks. The results showed that HFD-induced insulin resistant rats had disordered metabolism of blood glucose, blood lipid, and terrible muscle fiber morphology when compared with normal diet-fed rats, but PPPs treatment at a dosage of 300 mg/kg·day significantly reversed these negative effects. Moreover, in skeletal muscle tissue of insulin resistant rats,PPPs treatments significantly increased the protein expressions of insulin receptor (InsR) and phosphorylated insulin receptor substrate 1 (IRS-1), stimulated peroxisome proliferator activated receptor gamma (PPARγ)and phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT/PKB) signaling pathway, and aggrandized the protein levels of phosphorylated glycogen synthase kinase-3β (GSK-3β) and glucose transporter 4 (GLUT4).Our results suggest that PPPs possess of the beneficial effects on alleviating IR by enhancing insulin sensitivity and regulating glucose metabolism.
1. Introduction
Metabolic syndrome (MetS) is a complex metabolic disorder that results from the increasing prevalence of obesity, which includes abnormal adipose deposition and function, dyslipidemia, and hyperglycemia [1]. Insulin resistance (IR) has been considered to be an important causative factor of MetS and the main pathologic characteristic, as well as the most accepted theory explaining the pathophysiology of the disease [2-4]. Many evidences have also suggested that IR has a direct epidemiological connection with developing disturbance of carbohydrate metabolism including type 2 diabetes mellitus (T2DM) or impaired glucose tolerance [5,6].
IR is also a metabolic disorder characterized by reduced sensitivity to the action of insulin on tissues and efficiency of insulin signaling for blood glucose regulation. Insulin is the hormone responsible for the maintenance of normal blood glucose level by promoting glucose consumption process while inhibiting glucose production ones and exerts a broad spectrum of anabolic effects in multiple tissues, such as pancreas, liver, skeletal muscle, adipose,brain, and so on. In normal condition, when blood glucose goes up,insulin is synthesized and secreted from pancreatic islet cell to other tissues which have insulin signaling pathway. Consequently, insulin binds to the InsR on the cytomembrane and initiates insulin signal transduction, promoting uptake and utilization of glucose in tissues [7].However, the development of IR inhibits insulin’s ability to regulate blood glucose, leading to increase the risk of MetS. The risk of metabolic abnormality such as obesity and high blood glucose caused by a high-fat diet increases year by year. Regulation of IR is one of the important approaches to reduce the risk of diet-induced metabolic disorder in daily life.
Pomegranate (Punica granatumL.) is a popular fruit originated from the Middle East and now widely cultivated and consumed throughout the world. It is well known that it is a dietary source of diverse bioactive phytochemicals [8]. One study has reported that pomegranate peel extract has higher concentrations of total polyphenols, flavonoids, and tannins than pomegranate juice or seeds [9].As a rich source of polyphenols, several studies have shown that pomegranate has anti-oxidant [10], anti-inflammatory [11,12], antidiabetic [13], anti-obesity [14,15], protecting liver effects [16]in vivoandin vitro. According to these reports, we speculate that pomegranate peel polyphenols (PPPs) may have a potential effect on improving IR through enhancing insulin sensitivity. Recently,studies on the effect of alleviating IR have mostly focused on liver and adipose tissue, but less on skeletal muscle. In fact, skeletal muscle is one of the major tissues involved in the regulation of glucose and glycogen metabolism [17]and the bulk of the glycogen needed in body is synthesized and stored in skeletal muscle [18]. Rosiglitazone,as the PPARγ agonist, is an insulin-sensitive drug that have been used to treat IR and ameliorate skeletal muscle IR by correcting glucose metabolism [19-22]. Therefore, we select rosiglitazone as the reference to investigate whether PPPs can also reduce IR by improving sensitivity of skeletal muscle on insulin.
2. Methods
2.1 Materials
Pomegranate fruits were obtained from Lintong, Shaanxi province of China. The methods of extraction and purification PPPs were established in our laboratory. Ethanol-ultrasonic extraction was used for polyphenol extraction and the main active ingredients of PPPs were detected by high performance liquid chromatography (HPLC) [23,24].The total polyphenols content of pomegranate peel was(80.07 ± 0.87)% and punicalagin was (38.90 ± 0.93)%. The chromatogram of PPPs displayed main compositions is shown in Fig. 1.
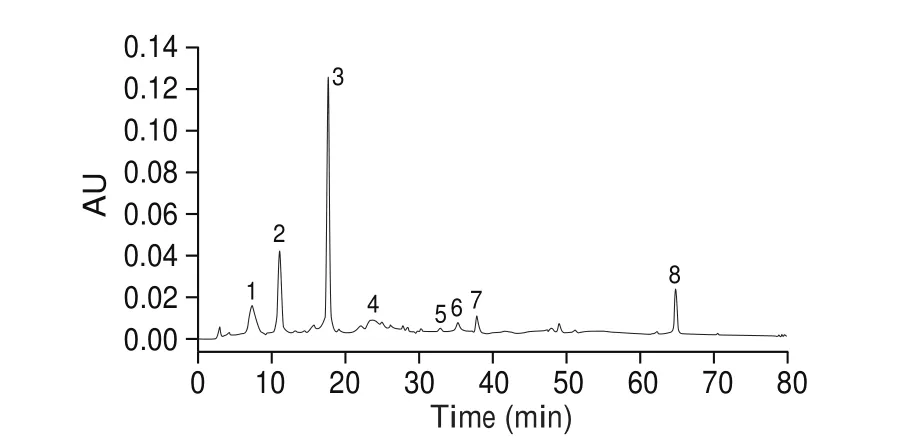
Fig. 1 HPLC chromatogram of PPPs. Peaks: (1) gallic acid,(2) punicalagin-α, (3) punicalagin-β, (4) catechin, (5) chlorogenic acid, (6)caffeic acid, (7) epicatechin, (8) ellagic acid. HPLC conditions: column,Agilent Zorbax SB-C18 column (4.6 × 250 mm, 5 μm); mobile phase: (A)deionized glacial acetic acid (99:1, V/V, pH 3.0), (B) methanol; gradient program:5%–44% B (0-70 min), 44%–44% (70-80 min); flow rate: 1.0 mL/min;detection wavelength: 280 nm; temperature: 30 °C; injection volume: 20 μL.
Antibodies against glyceraldehyde-3-phosphate dehydrogenase(GAPDH) (Order No. D110016), Tubulin-β(Order No. D110015),and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG(Order No. D110058) were purchased from Sangon Biotech (Shanghai,China). Antibodies against insulin receptor substrate 1 (IRS-1, Code No. ab40777), p-IRS-1 (Tyr896) (Code No. ab46800), insulin receptor(InsR, Code No. ab227831), protein kinase B (AKT/PKB, Code No. ab179463), peroxisome proliferator activated receptor gamma(PPARγ, Code No. ab272718), glucose transporter 1 (GLUT1, Code No. ab115730), glucose transporter 4 (GLUT4, Code No. ab216661),and sodium potassium ATPase (Na+/K+-ATPase, Code No. ab76020)were purchased from Abcam (Cambridge, MA, USA). Antibodies against p-AKT (Ser473) (Code No. #4060), phosphoinositide 3-kinase(PI3K, Code No. #4249), glycogen synthase kinase-3β (GSK-3β,Code No. #12456), and p-GSK-3β (Ser9) (Code No. #5538) were obtained from Cell Signaling Technology (Beverly, MA, USA).Glycogen assay kit (Code No. BC0345) was purchased from Solarbio Science and Technology (Beijing, China).
2.2 Animals and experimental treatment
Male Sprague-Dawley (SD) rats with a body mass of (200 ± 20) g purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) were chosen for our experiment. Rats were housed in an internal flawless animal room with a temperaturecontrolled facility ((23 ± 2) °C, (55 ± 10)% relative humidity) and a 12 h light/dark cycle. All animals were allowed access to chow and water freely throughout the period of the experimentation.
After one week of acclimatization to the environment, 50 SD rats were randomly divided into 6 groups. Group I: normal diet group (ND,n= 8), Group II: high fat diet group (HFD,n= 10), Group III: high fat diet with low dose (150 mg/kg·BW·day of PPPs group (L-PPPs,n= 8), Group IV: high fat diet with high dose (300 mg/kg·BW·day) of PPPs group (H-PPPs,n= 8), Group V: high fat diet with rosiglitazone(1 mg/kg·BW·day) group (RSG,n= 8), Group VI: normal diet with high dose (300 mg/kg·BW·day) of PPPs group (ND+H-PPPs,n= 8).PPPs and rosiglitazone were dissolved in distilled water and administered by a metallic gavage needle once daily. The ND group and ND+H-PPPs group received a normal diet (ND: 10% of total calories from fat, D12450J, FBSH Biotechnology Inc., Shanghai,China), while the other four groups were fed with high fat diet (HFD:45% of total calories from fat, D12451, FBSH Biotechnology Inc.,Shanghai, China) for subsequent 12 weeks. At the end of the 12thweek, rats were fasted overnight and anaesthetized with diethyl ether.Blood samples were collected by venous plexus after anesthesia and then the rats were killed by cervical dislocation following animal ethical guidelines. Serum was separated by centrifugation(3 000 r/min, 10 min). Skeletal muscle was removed, rinsed with PBS, immediate immersed in liquid nitrogen and stored at -80 °C for future use.
All the experimental procedures and methods were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No.85-23, revised 1985) and were approved by the Animal Care and Use Committee of Shaanxi Normal University.
2.3 Biochemical analysis
Fasting blood glucose (FBG) was determined from tail vein every two weeks and body weight was determined every week during the experimental period. To calculate oral glucose tolerance test (OGTT),the rats of each group were given glucose solution (2 g/kg) by oral administration after fasting. The blood glucose concentration (the tip of rat’s tail) was determined using glucometer in 0, 30, 60 and 120 min post glucose administration [25]. According to the manufacturer’s instructions for biochemical analysis, fasting insulin (FINS), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C)were estimated using reagent kits adapted for fully automated Clinical Chemistry Analyser (AU-400, Olympus, Japan). Homeostasis model assessment of IR (HOMA-IR) and homeostasis model assessment of β-cell function (HOMA-β) were calculated using the formulas as described by Matthews et al. [26], and insulin sensitivity index (ISI)was calculated using the formula as described by Katz et al. [27].Value in ND group of HOMA-β was assigned to 100%.
2.4 Histological analysis
For hematoxylin and eosin (H&E) staining, skeletal muscle taken out of the experimental rats was fixed in 10% neutral buffered formalin solution, dehydrated in a graded series of ethanol, and embedded in paraffin. Tissue sections (5 μm thick) were obtained using a microtome and then stained with hematoxylin-eosin (HE).The method of H&E staining was described by Oyenihi et al. [28].The structural alterations of tissue sections were observed under an inverted fluorescence microscope (40 ×) (Leica, Wetzlar, Germany).The fiber width and cross-sectional area of skeletal muscle were measured using software Leica Application Suite Version 4.4.0.
2.5 RNA extraction and real time-polymerase chain reaction(RT-PCR) analysis
Total RNA of skeletal muscle was extracted using RNAiso Plus reagent (TakaRa, Dalian, China) according to the manufacturer’s instructions and reverse transcribed into cDNA using PrimeScript™RT Master Mix (Perfect Real Time) (Takara, Dalian, China). Primer sequences are shown in Table 1. RT-PCR was determined by CFX 96 Real Time PCR Detection System (Biorad Laboratories Inc, Hercules,CA, USA) using the TB Green®Premix Ex Taq™ II (TliRNaseH Plus) (Takara, Dalian, China). The gene expression (using the 2-ΔΔCtmethod) of each sample was analyzed in duplicates and normalized against the internal control gene. Levels in ND group rats were arbitrarily assigned a value of 1.

Table 1Related primers information for RT-PCR.
2.6 Western blot analysis
Total protein was extracted from skeletal muscle of the experimental rats. Skeletal muscles were lysed in radioimmunoprecipitation assay (RIPA) buffer, incubated on ice for 15 min, and followed by high-speed centrifugation (12 000 ×g, 4 °C for 15 min). Subsequently, protein concentration was measured using BCA protein assay. Protein samples were separated by electrophoresis on sodium dodecyl sulfate polyacrylamide gel electrophoresis(SDS-PAGE), and then transferred to a polyvinylidene difluoride(PVDF) membrane. After being blocked with 5% milk or bovine serum albumin for 1 h, the membranes were incubated with the chosen primary antibodies (dilution ratio 1:1 000) overnight at 4 °C with gentle shaking. After washing with Tris buffered saline with Tween-20 (TBST) three times (5 min each time), the membranes were further incubated in HRP-conjugated IgG antibody (dilution ratio 1:8 000) for 1-2 h at room temperature. Finally, the obtained blots were washed with TBST three times (8 min each time), and the expression was shown by an enhanced chemiluminescense detection kit (Beyotime Biotechnology, Beijing, China). The immunoblotting was visualized with ChemiDocXRS (Bio-Rad, Hercules, CA), and the blot densities were quantified by scanning densitometry using a Bio-Image Analysis System (Bio-Rad, Hercules, CA).
2.7 Statistical analysis
All values are shown as mean ± SEM. A one-way analysis of variance (ANOVA) followed by a least significantdifference posthocanalysis was applied to assess the statistical significance of the differences between the study groups (SPSS 13.0). The differences were considered statistically significant whenP< 0.05.
3. Results
3.1 Effects of PPPs on body weight and OGTT of HFD rats
The body weight of rats only in high fat diet feeding group were significantly increased compared to those of treatment groups and normal diet group during the experimental period (Figs. 2A and 2B).At the end of 12 weeks treatment, the body weight of HFD group was 19.4% higher than that of ND group (Fig. 2B). At the same time,the body weight in L-PPPs group and H-PPPs group decreased by 12.4% and 15.8% respectively compared with HFD group, and there were significant differences atP< 0.01 andP< 0.001. There was no significant difference between ND+H-PPPs group and ND group in body weight.

Fig. 2 Effects of PPPs on body weight and OGTT in rats fed with HFD. (A) Representative image of increasing body weight during 12 weeks. (B) Representative statistical analysis of the final body weight of 12th week. Statistical analysis of OGTT (C) and AUC of the OGTT (D). Values are the mean ± SEM (n ≥ 6). ^P < 0.05,^^P < 0.01, ^^^ P < 0.001 compared with ND group; *P < 0.05, **P < 0.01, ***P < 0.001 compared with HFD group.
In OGTT, the highest blood glucose levels in all treatment groups appeared at 60 min after glucose gavage. After glucose stimulation, the glucose concentration (Fig. 2C) and area under curve (AUC, Fig. 2D) of OGTT in HFD group were significantly higher than those in ND group. The glucose tolerance of H-PPPs group was generally stronger than that of L-PPPs group, and the AUC of blood glucose concentration (Fig. 2D) in H-PPPs group was significantly reduced (P< 0.05). RSG also increased the glucose tolerance and significantly decreased the AUC of blood glucose concentration in HFD rats after glucose gavage. There was no significant difference in glucose tolerance between ND group and ND+H-PPPs group (P< 0.05).
3.2 Effects of PPPs on blood lipids and metabolic characteristics in HFD rats
After 12 weeks of feeding, the levels of TC, TG, LDL-C in HFD group were significantly higher than those in control group (P< 0.05)and HDL-C was significantly reduced (P< 0.001). The contents of serum TC, TG and LDL-C were significantly regulated in different degrees after PPPs treatment. There seemed to be no obviously change in HDL-C and LDL-C of serum after 300 mg/(kg·day) PPPs supplement, but H-PPPs significantly lowered the ratio of LDL-C/HDL-C. The reasons for the differences in HDL level and the ratio of LDL-C/HDL-C between ND group and ND+H-PPPs group still need further study. These results are shown in Table 2.

Table 2The effects of PPPs on blood lipids in HFD rats.
As shown in Table 3, compared with ND rats, the levels of FBG and FINS in serum of rats fed with HFD were significantly increased(P< 0.001 andP< 0.01, respectively), but H-PPPs treatment effectively inhibited the increase. In HFD fed rats, ISI was reduced, HOMA-IR was increased, and the function of islet β-cell was severely impaired.The results showed that H-PPPs treatment could significantly improve these disordered states. In addition, there were no significant differences in these factors between ND+H-PPPs group and ND group.

Table 3The effects of PPPs on metabolic characteristics in HFD rats.
3.3 Effects of PPPs on histological examination of skeletal muscle in HFD rats
The representative sections of histological examinations of skeletal muscle (gastrocnemius) in all experimental groups are shown in Fig. 3. The connective tissue width between longitudinal skeletal muscle fibers was significantly reduced by 25.7% (P< 0.05) in HFD group versus in ND group. In addition, skeletal muscle fiber crosssections also showed a huge decrease (reduced by 71.7%) in fiber area in HFD rats versus in ND rats. Skeletal muscle fibers of high-fat diet rats had smaller fiber area, wider fiber space, and more irregular polygonal fiber shape versus normal diet rats. However, longitudinal and cross sections offibers after PPPs treatment seemed to show an amelioration of the damages induced by high-fat diet. Compared with HFD group, fiber thickness appeared to be incrassated, fiber space seemed to be closer and fiber shape was restored to be regular polygonal shape in these groups. The levels of both longitudinal sections and cross sections in L-PPPs rats increased by 21.2% and 54.5% compared to HFD rats, but these were not statistically significant. Nevertheless, longitudinal skeletal muscle fiber thickness and fiber area of cross-section significantly increased by 57.2% and 152.1% in H-PPPs rats respectively (P< 0.001) compared with the values in ND rats. These results suggest that PPPs effectively attenuate HFD-induced skeletal muscle injuries. The results of ND+H-PPPs group seem to show that high conversation of PPPs(300 mg/kg·day) have a certain degree of influence on the fiber area of cross-section in ND group rats.
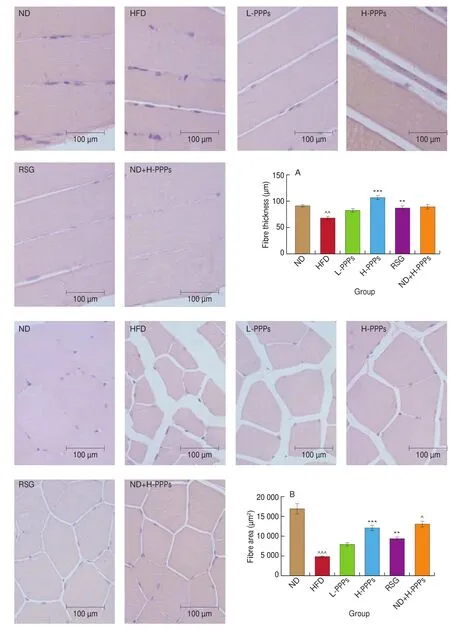
Fig. 3 Representative photomicrographs of haematoxylin-eosin stained longitudinal sections and cross-sections of skeletal muscle in rats fed with HFD.Magnification, 40 ×, Scale bars, 100 μm. Statistical analysis of longitudinal sections (A) and cross-sections (B) of skeletal muscle. Data is presented as mean ± SEM (n ≥ 6). ^P < 0.05, ^^P < 0.01, ^^^P < 0.001 compared with ND group; *P < 0.05, **P < 0.01, ***P < 0.001 compared with HFD group.
3.4 Effects of PPPs on InsR and IRS-1 gene/protein expression
There were significant differences inInsR(Fig. 4C) andIRS-1(Fig. 4E) gene content between HFD group rats and ND group rats(P< 0.01 andP< 0.05). H-PPPs treatment could significantly(P< 0.01) restore the decrease both ofInsRandIRS-1gene expression. L-PPPs treatment also improved the HFD-induced the reduction ofInsRandIRS-1gene, but the effects were not obvious.WB results showed that the protein expression levels of InsR (Figs.4A and 4D) and p-IRS-1 (Tyr896)/IRS-1 (Figs. 4A and 4F) in HFD group were significantly lower than those in ND group (P< 0.01), but the reduction could be significantly inhibited by two dosages of PPPs in various degrees. Furthermore, the protein ratio of p-IRS-1 (Tyr896)/IRS-1 in L-PPPs treatment was higher than that of H-PPPs treatment.The results indicate that PPPs can increase the phosphorylation level of IRS-1 protein inhibited by HFD in rats.

Fig. 4 Effects of PPPs on InsR, IRS-1, PPARγ, PI3K and AKT gene/protein expression in rats fed with HFD. Images of western blotting for InsR (A) using Na+/K+-ATPase as the loading control, p-IRS-1 (Tyr896) and IRS-1 using GAPDH as the loading control; PPARγ, PI3K, p-AKT (Ser473) and AKT (B) using GAPDH as the loading control. Statistical analysis of InsR gene expression (C), InsR protein expression (D), IRS-1 gene expression (E), p-IRS-1 (Tyr896)/IRS-1 protein expression ratio (F), PPARγ gene expression (G), PPARγ protein expression (H), PI3K gene expression (I), PI3K protein expression (J), AKT gene expression (K), and p-AKT (Ser473)/AKT protein expression ratio (L). Values are the mean ± SEM (n = 3). ^P < 0.05, ^^P < 0.01, ^^^P < 0.001 compared with ND group; *P < 0.05, **P < 0.01, ***P < 0.001 compared with HFD group.

Fig. 4 (Continued)
3.5 Effects of PPPs on PPARγ, PI3K and AKT gene/protein expression
As shown in Fig. 4, the gene expressions ofPPARγ(Fig. 4G),PI3K(Fig. 4I) andAKT(Fig.4K) were huge decreases in rats fed with HFD compared with those fed with ND (P< 0.01,P< 0.05 andP< 0.001, respectively). PPPs treatments could improve the expressions of these genes at different significant levels and the effects of H-PPPs were better than L-PPPs. And then, we assessed the protein expressions of PPARγ, PI3K and AKT (Fig. 4B). We found that HFD resulted in a significant decrease in the content of PPARγ(P< 0.001) and the ratio of p-AKT (Ser 473)/AKT (P< 0.001)compared with ND group, but the decrease of PI3K had no significance. Two dosages PPPs could efficiently stop the decline of these three proteins and the improvements of PPPs were dosedependent. RSG also significantly increased the expressions of these three proteins in both gene level and protein level in HFD rats. These results are shown in Figs. 4H, 4J and 4K. These results suggest that PPPs and RSG have similar action mode in promoting the activation of PPARγ/PI3K/AKT signaling pathway.
3.6 Effects of PPPs on muscle glycogen, GSK-3β, GLUT1 and GLUT4
Effects of PPPs on glycogen content in skeletal muscle of highfat diet rats are shown in Fig. 5B. Muscle glycogen of rats fed with HFD significantly decreased by 43.8% (P< 0.001) compared with rats fed with ND, while muscle glycogen of rats treated with H-PPPs significantly increased by 24.2% (P< 0.01) compared with that of rats fed with HFD. The mRNA value and the protein content of GSK-3β which is the key enzyme controlling glycogen synthesis were detected in HFD rats. The results showed that compared with ND group, HFD group had negative effect on gene expression of GSK-3β(Fig. 5C), but the effect hadn’t statistic difference. Moreover, HFD diet group significantly reduced the protein expression ratio of p-GSK-3β (Ser9)/GSK-3β (P< 0.001) versus normal diet group(Fig. 5D), and PPPs treatments could significantly restore the inhibition effect in HFD group (P< 0.01 andP< 0.001). The improvement effects of H-PPPs on skeletal muscle glycogen synthesis were similar to those of RSG.
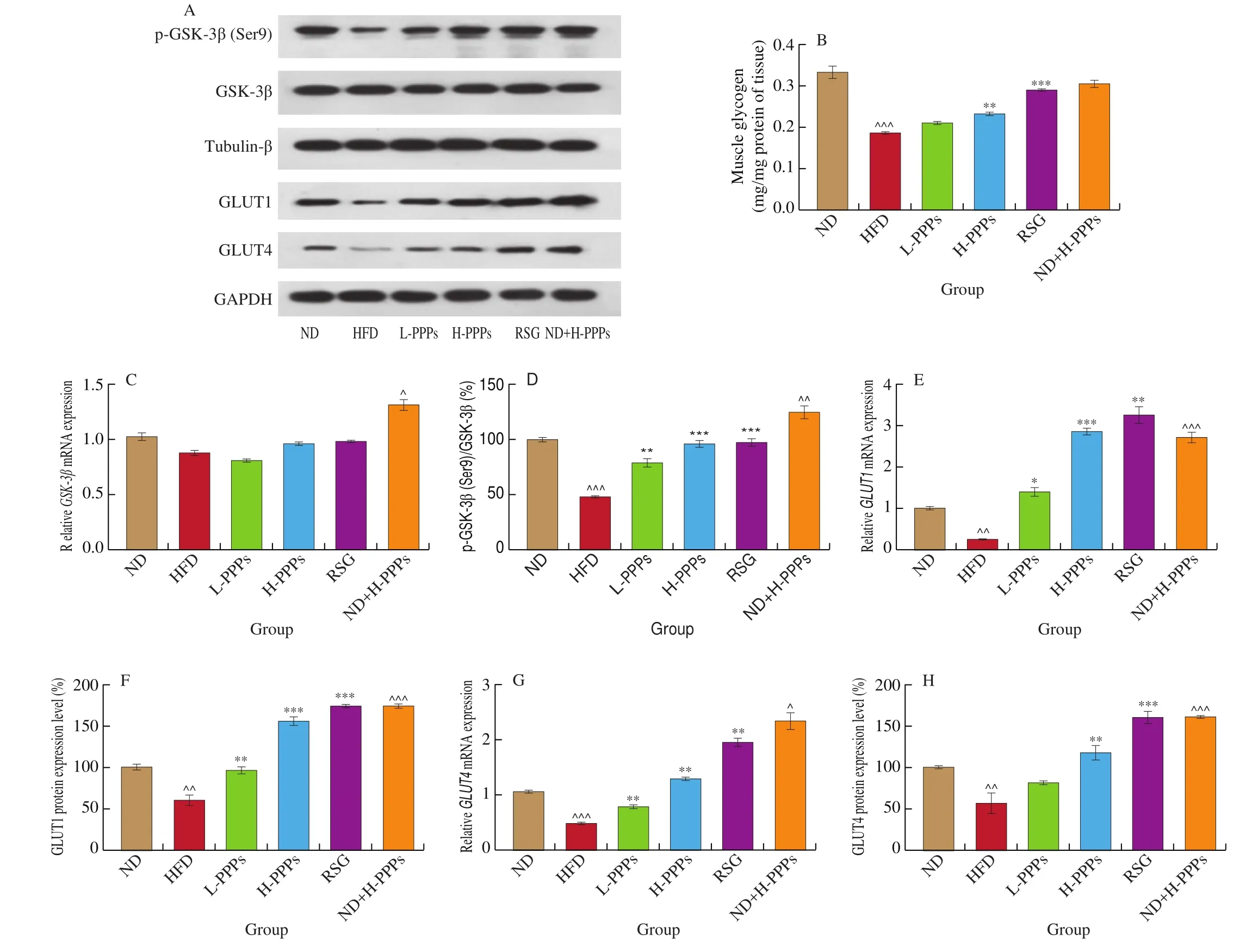
Fig. 5 Effects of PPPs on muscle glycogen, GSK-3β, GLUT1 and GLUT4 gene/protein expression in rats fed with HFD. (A) Representative images of the western blotting for p-GSK-3β (Ser9)/GSK-3β using β-tubulin as the loading control, GLUT1 and GLUT4 using Na+/K+-ATPase as the loading control. Statistical analysis of muscle glycogen (B). Statistical analysis of GSK-3β (C), GLUT1 (E), and GLUT4 (G) gene expression. Statistical analysis of p-GSK-3β (Ser9)/GSK-3β (D),GLUT1 (F), and GLUT4 (H) protein level. Values are the mean ± SEM (n = 3). ^P < 0.05, ^^P < 0.01, ^^^P < 0.001 compared with ND group; *P < 0.05,**P < 0.01, ***P < 0.001 compared with HFD group.
Compared with ND group, HFD group significantly changed the gene expressions ofGLUT1(Fig. 5E) andGLUT4(Fig. 5G)(P< 0.01 andP< 0.001). PPPs treatments interfered with the decrease of GLUT1 and GLUT4, and significantly increased the genes ofGLUT1andGLUT4. Meanwhile, the protein expressions of GLUT1 (Fig. 5F) and GLUT4 (Fig. 5H) in HFD group were significantly inhibited (P< 0.01) versus ND group. PPPs treatments could significantly promote the protein expressions of GLUT1 and GLUT4 in different significant levels and the promotion effects were dose-dependent. The regulation effect of RSG on the expressions of two GLUTs in both gene level and protein level was better than PPPs treatments. These results suggest that PPPs can promote muscle glycogen synthesis and glucose uptake in skeletal muscle tissue of HFD-induced IR rats.
4. Discussion
The pomegranate peel makes up about 50% of the whole fruit and it is rich in many compounds such as phenolics, flavonoids,ellagitannins, proanthocyanidin compounds, complex polysaccharides,and minerals [29]. The main polyphenol compounds of pomegranate peel are gallic acid, punicalagin (punicalagin-α and punicalagin-β),catechin, chlorogenic acid, epicatechin, ellagic acid, caffeic acid, and other components identified by HPLC in our previous studies [30,31].Punicalagin is a unique and major polyphenol component of pomegranate fruit and peel, which can release ellagic acid upon hydrolysis [23,30]. Ding et al. [32]investigated that ellagic acid could ameliorate oxidative stress and IR via miR-223-mediated keap1-Nrf2 activation in high glucose-induced IR HepG2 cell. Rebollo-Hernanz et al. [33]found that in 3T3-phL1 adipocytes of IR, phosphorylation of InsR significantly increased and phosphorylation of IRS-1 at serine significantly diminished after treatment with coffee silverskin aqueous extract (CSE) and coffee husk aqueous extract (CHE). As the main pure phenolic components of CSE and CHE, chlorogenic acid,gallic acid and caffeic acid also helped to translocate GLUT4 into the cell membrane and significantly enhanced insulin-dependent glucose uptake to reduce IR in 3T3-phL1 adipocytes. Bettaieb et al. [34]investigated the effect of epicatechin on high fructose induced IR in liver and adipose tissue of MetS rats. A daily supplement of 20 mg/kg epicatechin could significantly alleviate IR in high-fructose feeding rats by activating components of the insulin signaling cascade,including p-InsR, p-IRS-1 and p-AKT. These results indicate that the improvement effects of PPPs on HFD-induced IR in skeletal muscle of rats in this study may be the result of the combined effects of multiple polyphenols in PPPs.
The skeletal muscle tissue plays an important role in the process of glucose metabolic since it is accountable to the uptake of about 80% of glucose in the postprandial state and it expends more than 30% of the total body energy [35]. Therefore, skeletal muscle tissue is the primary target of insulin, which is responsible for the glucose uptake, storage and utilization [36]. Impaired insulin-mediated glucose uptake of skeletal muscle is deemed to be a primary defect in the development of whole-body IR [37]. Earlier studies also have indicated that insulin/PI3K/AKT signal pathway plays an important role in IR [38-41]. Jiang et al. [42]reported that the compound K,which is a final metabolite of panaxadiol ginsenosides from Panax ginseng, could improve HFD/STZ-induced rats IR through promoting the protein expressions of insulin/PI3K/AKT signaling pathway in skeletal muscle tissue. Similar results were obtained in this study.We found that PPPs possess of the ability of controlling body weight,regulating blood glucose and maintaining muscle fiber physical form in high fat diet induced insulin resistant rats. According to the resultsin vivo, we demonstrated the improvement effects of PPPs on IR. We also find that the action modes of PPPs and RSG in insulin resistant skeletal muscle are much alike. Both of them can activate PPARγ,facilitate the expressions of insulin signaling pathway, and improve the expressions of GSK-3β and GLUTs. It suggests that PPPs are capable of increasing skeletal muscle insulin sensitivity, and repairing glucose metabolism in MetS rats by promoting uptake of glucose and synthesis of glycogen.
As a kind of fruit, pomegranate has been widely used since ancient times. Due to this fact, it has been generally regarded as safe for utilization in humans. Numerous toxicity studies of various pomegranate products, such as pomegranate juice, whole pomegranate extract, pomegranate seed oil and other products have been widely reported [43,44]. Patel et al. [45]published a study on the subchronic toxicity of pomegranate polyphenols in 2008. In their study, they used 600 mg/kg·day of whole pomegranate extract (contain 30% punicalagin, 180 mg/kg·day as a maximum dose of daily oral dose for rats. There was no adverse effects in male and female rats fed with pomegranate extract for 90 days. Heilman et al. [46]demonstrated that urolithin A, as the main metabolite of pomegranate polyphenolsin vivo, also had no genotoxic. The polyphenols contents in PPPs used in our study was lower than the dose used in those studies.And ND+H-PPPs group remained a good state, which showed no significant difference in body weight and other metabolic indexes except HDL-C. Therefore, we believe that the maximum dosage of PPPs in our study had no obvious adverse effects and toxicity for rats.
In addition to the above, there is a worth noting result of ND+HPPPs group that PPPs seem to have certain regulating effects on glucose metabolism in normal physiological metabolic rats. The regulating effects are reflected in three aspects. Firstly, PPPs don’t disorder the blood glucose homeostasis in rats. Comparing with ND group, there was no significant difference in OGTT, FBG, FINS, and three indices of HOMA in ND+H-PPPs group. Secondly, PPPs don’t cause the over-expression of InsR and p-IRS-1/IRS-1 proteins, which lead to skeletal muscle tissue always being in a state of high sensitive respond to insulin. Thirdly, PPPs possess the ability of improving the efficiency of uptake and utilization glucose in skeletal muscle tissue.The results showed that PPPs not only significantly activated PPARγ/PI3K/AKT signaling pathway, but also facilitated the downstream GLUTs proteins expression and GSK-3β protein phosphorylation,which can increase the rates of glucose transport and glycogen synthesis in skeletal muscle tissue while the total amount of muscle glycogen remains in equilibrium. Therefore, we believe that daily supplementing PPPs is beneficial to promote the glucose metabolism process in normal rats, especially in rat’s skeletal muscle.
Altogether, PPPs showed beneficial effects on improving glucose metabolism, especially in high fat diet induced IR rats. Enhancing insulin sensitivity of skeletal muscle is one of the major roles of PPPs.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31871801, 32001679), the Science and Technology Research of Shaanxi Province (2020QFY08-03), Forestry Science and Technology Programs of Shaanxi Province (SXLK2020-0213), and Fundamental Research Funds for the Central Universities(GK201604013).
- 食品科学与人类健康(英文)的其它文章
- Dietary bioactives and essential oils of lemon and lime fruits
- Green tea, epigallocatechin gallate and the prevention of Alzheimer’s disease: clinical evidence
- Simultaneous quantification of 18 bioactive constituents in Ziziphus jujuba fruits by HPLC coupled with a chemometric method
- A systematic study on mycochemical profiles, antioxidant, and anti-inflammatory activities of 30 varieties of Jew’s ear (Auricularia auricula-judae)
- GPP (composition of Ganoderma lucidum polysaccharides and Polyporus umbellatus polysaccharides) protects against DSS-induced murine colitis by enhancing immune function and regulating intestinal flora
- Immunoregulatory polysaccharides from Apocynum venetum L.flowers stimulate phagocytosis and cytokine expression via activating the NF-κB/MAPK signaling pathways in RAW264.7 cells

