GPP (composition of Ganoderma lucidum polysaccharides and Polyporus umbellatus polysaccharides) protects against DSS-induced murine colitis by enhancing immune function and regulating intestinal flora
Liyun Li, Ynnn Guo, Qing Hung, Xiojin Shi, Qingqing Liu,Fng Wng, Qingfei Liu, Kng Yu, Zho Wng,*
a MOE Key Laboratory of Protein Science, School of Pharmaceutical Sciences, Tsinghua University, Beijing 100084, China
b Tsinghua-Peking Center for Life Sciences, Tsinghua University, Beijing 100084, China
c Tianjin Research Institute for Advanced Equipment, Tianjin 300300, China
d Department of Clinical Nutrition and Department of Health Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing 100730, China
Keywords:
Ganoderma lucidum polysaccharides
Polyporus umbellatus polysaccharides
Immune function
Intestinal flora
Colitis
A B S T R A C T
Previous study have demonstrated that a compound composed of water-soluable Ganoderma lucidum polysaccharides (GLP) and Polyporus umbellatus polysaccharides (PUP) in a ratio of 3 : 1 named GPP enhances innate immune function in mice through enhancing the function of macrophage cells and activity of natural killer (NK) cells. Here in our research, we further investigated the effect of GPP on the diversity and composition of intestinal flora, and explored its effect on colitis model mice. The immunoregulatory verification experiments of GPP were conducted in both normal and DSS-induced mice model. Our research showed that GPP increased the diversity of intestinal microorganisms in mice with the extension of administration time. Daily GPP intake attenuated DSS-induced colon injury, protected the splenic lymphocyte proliferation ability, enhanced the serum hemolysin synthesis, and increased peripheral phagocytes and NK cell activity in model mice. Comparisons of the predominant gene pathways of the bacterial microbiota showed that DNA repair and recombination, base mismatch repair pathways was stronger in GPP-treatment group than in control group, indicating the possible molecular mechanisms of immune function regulation.Our study showed that GPP regulated immune function in both health and colitis model, and had a positive effect on maintaining intestinal flora homeostasis.
1. Introduction
Ganoderma lucidumandPolyporus umbellatusare rare edible and medicinal fungi that have been used to treat diseases and regulate body balance in China for more than 2 000 years [1-3].According to the theory of traditional Chinese medicine, the combination ofG. lucidumandP. umbellatusare of great significance since they had the characteristic of supplement without causing stagnation.G. lucidumhad the activity to strengthen body resistance and consolidate the constitution of patients (in traditional Chinese called Fuzheng Guben) [4], whileP. umbellatuscould induce diuresis and excrete dampness [5].G. lucidumandP. umbellatuscontains a variety of active components, among which researchers most interested in the polysaccharides. Polysaccharides from edible mushrooms (EMPs) are a class of natural macromolecule polymers that have a wide range of biological activities and relatively low toxicity [6,7]. The different biological functions of EMPs include anti-tumor, anti-oxidant, regulating gut microbiota and immunoregulatory activities [8]. BothG. lucidumpolysaccharides(GLP) andP. umbellatuspolysaccharides (PUP) exhibited immunoregulation effect, which regulated the function of a variety of immune cells, such as macrophages, T cells, B cells, dendritic cells and NK cells [3,9-11]. In combination with chemotherapy drugs, both GLP and PUP can enhance drug efficacy and reduce toxic side effect [12,13].
Previous studies showed that GPP, a combination of GLP and PUP in a ratio of 3 : 1, enhanced the function of macrophages by enhancing the phagocytic ability, the production of nitric oxide (NO),the mRNA expression level of inducible nitric oxide synthase (iNOS)and tumor necrosis factor-α (TNF-α). GPP was slightly better than GLP but was significantly better than PUP in a certain concentration range in enhancing macrophage functions, indicating that the two polysaccharides GLP and PUP can synergistically enhance the phagocytosis function of macrophages in a certain ratio and concentration. Moreover, GPP significantly improved macrophage phagocytic function and natural killer (NK) cells activity after being administered to mice at a dose of 0, 36, 120, 360 mg/kg BW orally for 30 days, demonstrating that GPP is capable of enhancing innate immune function in mice [14]. The study demonstrated the potential of GPP develop into immunomodulation dietary supplement.However, as edible and medicinal fungus, the molecular mechanism about how GPP affect the immune system and immune cells, and whether GPP has immune modulating function on disease model remains unknown. Gut microbiota is an important constituent part of intestinal microecosystem, it is extremely complicated and dynamic.More than 1011CFU/g of microbes were found in colon, where they complete their colonization. The host provides nutrients for gut microbiota, and gut microbiota in return maintains the intestinal endothelial barrier, immune homeostasis, and protects the host from the threat of pathogenic bacteria [15,16]. By metabolizing nutrients in the diet, gut microbiota provides large quantities of metabolites to human body. As a result, diet are considered to be critical in the regulation of gut microbiota and host health factors [17].
Previous studies showed that certain polysaccharides from traditional Chinese medicine can affect the ecological structure and metabolism of gut microbiota, thus improve the health status of the host [18]. GLP can reduce the ratio of Firmicutes to Bacteroidetes in the gut microbiota of mice fed with high fat diet, maintain the integrity of intestinal barrier, reduce metabolic endotoxemia, and thus reduce the weight and fat accumulation in mice [19]. In a chronic pancreatitis mouse model induced by diethyldithiocarbamate (DDC),supplementation of GLP improved the symptoms of inflammation,altered the composition and diversity of intestinal microbiota,decreased the relative abundance of phylum Bacteroidetes and increased that of phylum Firmictutes. At the genus level, GLP increased the relative abundance of the beneficial bacteria such as Lactobacillales, Roseburia and Lachnospiraceae [20]. Furthermore,GLP can regulate the gut microbiota of normal rats, improve the intestinal barrier function, regulate the intestinal immune function and gut microbiota abundance in rats [21].
Ulcerative colitis (UC) is a type of inflammatory bowel disease(IBD), mainly characterized by chronic intestinal mucosal damage caused by recurrent IBD in susceptible populations [22,23]. A widely accepted view of the etiology and pathogenesis of UC is that the development of the disease is accompanied by the change or disorder in the function of immune response, mucosal barriers, and metabolic balance in intestinal epithelium [24]. DSS-induced colitis is a wellestablished animal model for the study of colon inflammation, which has similar clinical symptoms and histological changes to IBD patients [25,26].
In this study, we mainly investigated the effect of GPP on immune system and the structure of gut microbiota in both normal and DSS-induced colitis model mice, and the possible molecular mechanism behind them. Our research found that GPP increased the diversity of intestinal microorganisms in normal mice.The administration time of GPP has significant influence on the species distribution of gut microbiota compared with the dosage of administration in normal mice.
In DSS-induced mice model, daily GPP intake attenuated DSS-induced colon injury, including decreasing the disease activity index(DAI) score and reducing the histological injury of colon. GPP treatment in DSS-induced mice also protected the splenic lymphocyte proliferation ability, enhanced the serum hemolysin synthesis,increased peripheral phagocytes and NK cell activity in colitis model mice, indicating that GPP promotes immune function in both normal and colitis model mice. Comparisons of the predominant gene pathways of the bacterial microbiota in different groups showed that DNA repair and recombination, base mismatch repair pathways is stronger in GPP treatment group, suggesting its protective function.Taken together, these findings revealed that GPP regulates immune function in both normal and colitis model and has a positive effect on maintaining intestinal flora homeostasis.
2. Materials and methods
2.1 Materials
GLP and PUP sheep were purchased from Yuanye Biotechnology(Shanghai, China). Lipopolysaccharide (LPS), NO assay kits, Neutral Red Cell Proliferation, Cytotoxicology Assay Kits were obtained from Beyotime Biotechnology (Shanghai, China).
2.2 Animals and induction of colitis
Six to eight-week-old ICR mice weighing about 17–22 g were purchased from the laboratory animal center of Tsinghua University(Beijing, China) and were housed in cages with free access to food(Keao Xieli Feed Co., Ltd, Beijing) and water in a room with an ambient temperature of (22 ± 2) °C and a 12 h light/dark cycle.All animal experiments were conducted according to the relevant guidelines and regulations and with the approval of the Institutional Ethical Committee of China. All the animal experiments are followed by the ARRIVE reporting guidelines [27].
The immunoregulatory verification experiments of GPP were divided into two parts, in normal animals and in DSS-induced mice model. In the normal animal treatment part, a total of 192 experimental animals were randomly divided into 4 batches of 48 each. The animals were divided into solvent control group, low dose group (treated with 0.036 g/kg BW GPP per day), medium dose group(treated with 0.12 g/kg BW GPP per day) and high dose group (treated with 0.36 g/kg BW GPP per day), with 12 animals in each group.In the DSS-induced mice model parts, a total of 240 experimental animals were randomly divided into 4 batches of 60 each. The animals were divided into solvent control group, DSS group as positive control, DSS + low dose group, DSS + medium dose group and DSS + high dose group (the GPP concentrations were the same as described above), and also with 12 animals in each group. GPP of different concentrations was given by oral administration to animals at a fixed time from day 1 to day 30 (control groups with water),separately.
In the DSS-induced mice model parts, animals started to receive DSS from day 23 by drinking 5% (m/V) DSS (relative molecular weight 36 000–50 000; MP Bi medicals) or regular water in control group for 7 days to induce acute colitis. All mice were carefully monitored daily for signs of disease, including weight loss, stool consistency and rectal bleeding. On day 31, mice were humanely sacrificed and colon and feces were harvested. Fresh faces samples of each group of different time point were collected and stored at −80 °C immediately, the samples were subsequently sent to analyze intestinal flora (Novogene, China).
2.3 16S rRNA analysis
The total genome DNA of feces samples was extracted and 16S rRNA of 16S V4 regions were amplified used specific primer 16S V4: 515F – 806R with the barcode. PCR reactions were set up with 15 μL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs); 2 µmol/L of forward and reverse primers, and about 10 ng of template DNA. Thermal cycling consisted of initial denaturation at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 30 s. Finally 72 °C for 5 min. After amplification generation, PCR products were quantitated by 2% agarose gel electrophoresis. The sequencing libraries were generated using TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, USA) following manufacturer’s recommendations and index codes. The library quality was then assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. Finally, the library was sequenced on an Illumina NovaSeq platform and 250 bp paired-end reads were generated. Data were split and assembled by FLASH (V1.2.7) [28].Data filtration was performed with QIIME (V1.9.1) [29]. We used UCHIME algorithm [30]to detect and remove chimera sequences to obtain the effective tags, and adopted Uparse software [31]to perform sequence analysis. Sequences with more than 97% similarity were regarded as the same OTUs. The taxonomic information of OTUs was then annotated with Silva Database [32]based on Mothur algorithm. The phylogenetic relationship of different OTUs was conducted by MUSCLE software (V3.8.31) [33].OTUs abundance information were normalized based on the least sequences sample as a standard of sequence number. Alpha diversity, beta diversity and further analysis were all performed basing on this normalized abundance data.
2.4 Measurement of immune organ indexes
On day 31, mice were weighed and sacrificed. The spleen and thymus were immediately removed and weighed. The spleen or thymus index was calculated according to the following formula:
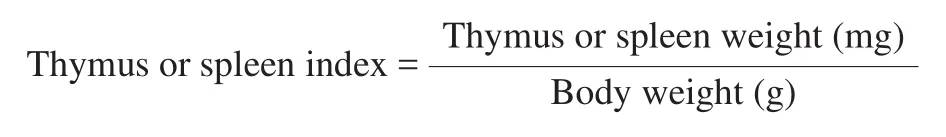
2.5 Macrophage phagocytosis assay
Chicken blood was placed in a conical flask with glass beads,shaken fully in one direction, washed for 3 times with normal saline,centrifuged (2 000 r/min, 10 min) and prepared into chicken red blood cell suspension of 2% (V/V) with normal saline. Each mouse was intraperitoneally injected with 1% chicken red blood cell suspension.After 30 min, the mice were sacrificed by cervical dislocation. Inject 2 mL normal saline intraperitoneally, and gently rub the abdomen of the mice for 20 seconds. Put the mouse into enamel box with wet sand cloth and transfer it to 37 °C incubation box for 30 min. Rinse with normal saline to remove uncoated cells. Dry and fix the cells with 1 : 1 acetone methanol solution, and dye with 4% (V/V) Giemsaphosphoric acid buffer for 3 min, then rinse with distilled water and dry them, and were observed and counted under the microscope.
2.6 The determination of NK cell activity
The splenic cell suspension (effector cells) was prepared at a final concentration of 5 × 106cells/mL with complete culture medium of RPML1640. For NK cell activity detection, both 100 μL target cells(YAC-1 cells) and 100 μL effector cells (with a ratio of 50 : 1) were seeded to U-shaped 96-well culture plate. The cells were cultured at cell incubator of 37 °C with 5% CO2for 4 h. The plate was then centrifuged at 1 500 r/min for 5 min, and 100 μL supernatant was pipetted from each well to a new 96-well culture plate and added with LDH matrix solution. After reaction for 5 min, 30 μL of 1 mol/L HCl was added to each well, and the optical density value at 490 nm were measured.
2.7 Tlymphocyte proliferation assay
The spleen cell suspension was prepared by filtering with 200-mesh sieve and washed with Hanks solution, and adjusted to a final concentration of 3 × 106cells/ml. For the lymphocyte proliferation reaction, each splenic cell suspension was divided into two wells, one well was added with 50 μL ConA solution (equivalent to 5.0 g/mL)and the other well was used as control. The cells were cultured at cell incubator of 37 °C with 5% CO2for 72 h. Before the end of culture, 0.7 mL of supernatant was gently pipetted from each well and 0.7 mL of RPMI1640 without calf serum was added for 4 h. 50 μL MTT(5 mg/mL) was subsequently added to each well for further culture for 4 h. Discard the supernatant, add 1 mL acidic isopropanol into each well, blow and mix well to completely dissolve the purple crystal.The samples were pipetted into 96-well culture plates with 3 parallel wells. The OD value was determined by a microplate reader at a wave length of 570 nm.
2.8 Other immune function test
Other immune function test like the determination of the hemolytic value (HC50), detection of antibody generating cells, induction of DTH in mice by Sheep Red Blood cell (SRBC), carbon clearance test were also conducted. All the experiments were operated according to the criteria for the enhancement of immune function in the“Technical Specification for Inspection and Evaluation of Health Food”(2003 edition) issued by the Ministry of Public Health of China.
2.9 PAS staining
Paraffin slides were washed by pure xylene, pure ethanol and 75% ethanol, and were kept in tap water. For PAS staining, sections were stained with PAS dye solution B for 10–15 min, PAS A for 25–30 min in the dark, and then were stained with PAS C for 30 s(Wuhan Servicebio technology Co., Ltd, China). Finally, the slides were dehydrated by pure ethanol and pure xylene, and covered with neutral resin. All slices were captured by automatic slides scanner machine (Panoramic SCAN, 3DHISTECH, Hungary).
2.10 Histological analysis
The histological score of each mouse was calculated according to epithelium damage and cell inflltration [34]. The slices stained with PAS and Alcian Blue were analyzed and the scores were con firmed as follows: 0, no significant change; 1, low level of inflammation with scattered inflammatory cells; 2, moderate inflammation with multiple foci; 3, high level of inflammation with marked wall thickening;4, maximal severity of inflammation with transmural leukocyte inflltration and loss of goblet cells [35]. The neutrophil inflltration in colon was also analyzed according to the characteristic of neutrophil,and the number of neutrophil aggregation was also recorded [35].
2.11 Goblet cells quantification assay
The quantification of goblet cells was based on Alcian Blue staining slice scan image. Following the method described by Montrose et al. [36], Fiji/ImageJ (NIH) software was used to perform color deconvolution and Gaussian blur. After color deconvolution,the total mucosal area was selected by Gaussian blur in sigma 20 and threshold auto-selection method “Huang”. The selected area was masked, measured and labeled as “mucosal area”. Similar to the selection of total mucosal area, total goblet cells area was selected by Gaussian blur in sigma 2 and threshold auto-selection method“Default”. Then the selected area was masked, measured and labeled as “goblet cell area”. The ratio of goblet cell area and total mucosal area was regarded as the proportion of mucosal surface occupied by goblet cell as an index to quantify goblet cells.
2.12 Statistical analysis
Results were expressed as mean ± SEM. Comparison of more than two groups was made with one-way analysis of variance (ANOVA).P< 0.05 was considered as statistically significant differences. The histogram was made by GraphPad Prism 6.0 software.
3. Results
3.1 Effect of GPP on intestinal bacteria community in normal mice
In order to investigate whether GPP had an effect on intestinal bacteria community, Chao1 index of intestinal flora in mice was calculated to reflect the abundance and diversity of microbial community among different samples. The GPP was administrated to normal mice for 30 days with different doses, during the GPP treatment, mice feces of different time point were collected for analysis. On day 31, the animals were sacrificed for subsequent experiment (Fig. 1A). As shown in Fig. 1B, with the increase of GPP dose, the species richness of intestinal flora in mice gradually increased, indicating that GPP increased the diversity of intestinal microorganisms in mice. In Fig. 1C, the species richness of intestinal flora in mice increased with the extension of GPP administration time, the treatment of GPP for 15 days significantly enhanced the intestinal flora species diversity. Next, unweighted principal components analysis (PCA) dimensionality reduction analysis was performed for each sample according to GPP doses. PCA with a twodimensional principal component cumulative variance of 29.28% showed that GPP doses had less influence on the species distribution of intestinal flora in mice (Fig. 1D). However, the unweighted PCA on different time showed that GPP administration time can affect the species distribution of intestinal flora in mice. The 15-days group and 30-days group consisted of two apart sections and were significantly distinguished from each other (Fig. 1E). The results suggested that the GPP improved the richness of intestinal microbiota and that GPP doses had less influence on the species distribution compared with the administration time.
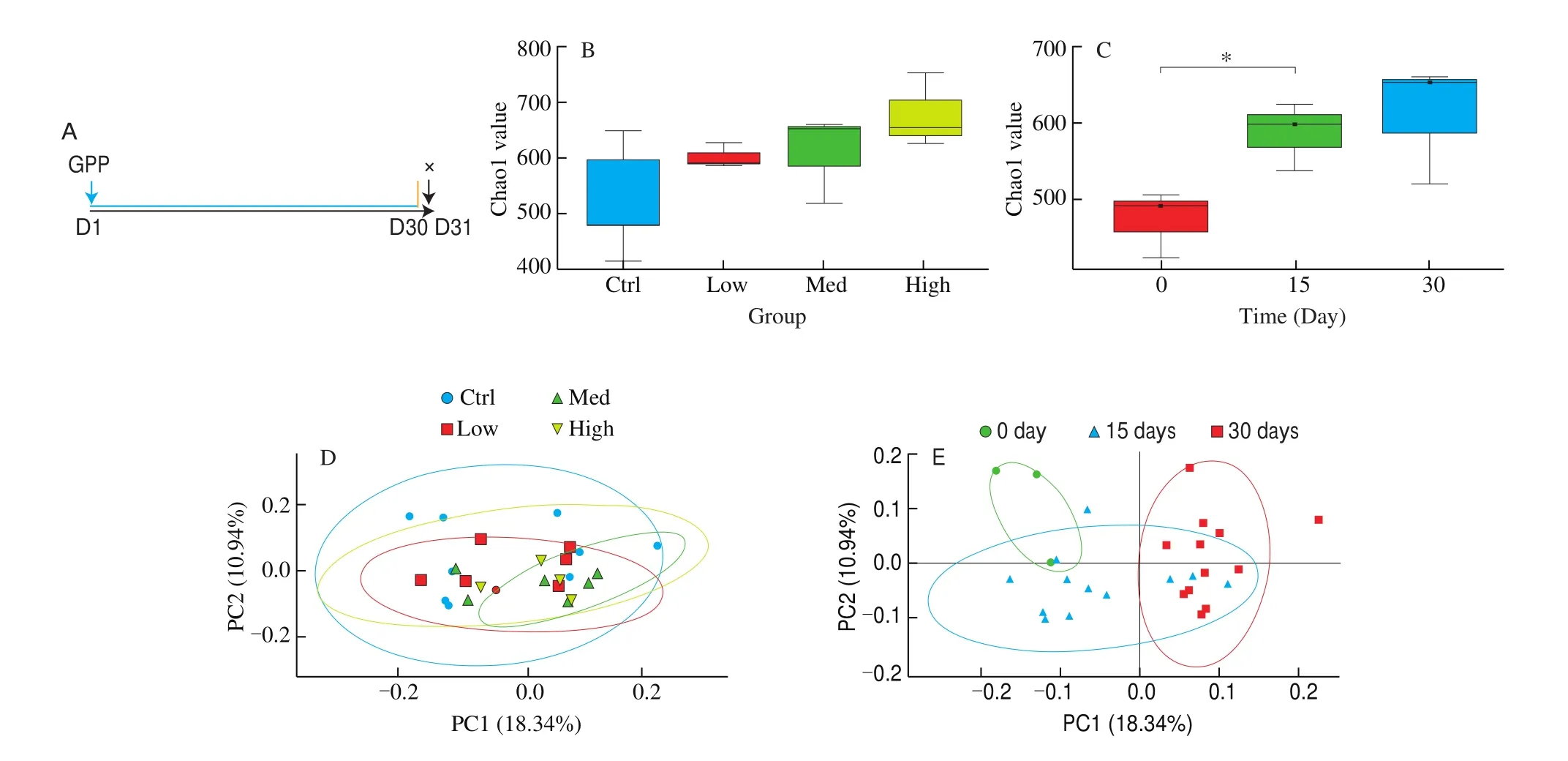
Fig. 1 The alpha and beta diversity index of intestinal flora in mice. (A) Protocol for GPP treatment in normal mice. (B) With the increase of GPP dose, the species richness of intestinal flora in mice gradually increased. (C) The microbial diversity in the intestinal tract of mice increased with the extension of GPP administration time. (D) Unweighted PCA of samples collected with different doses of GPP. (E) Unweighted PCA of samples collected with different time point of GPP treatment (n = 4).
3.2 Effect of GPP on the composition of the intestinal micro flora in normal mice
To investigate whether the dominant microflora and their abundance changes with the different doses of GPP treatment, the top 20 microflora at phylum level and top 10 microflora at genera level were plotted (Figs. 2A, 2B), even though the proportion of each intestinal microflora has changed after GPP treatment of different doses, the dominant flora were consistent on phylum level.In the phylum level, the the dominant flora were Bacteroidetes and Firmicutes (Fig. 2A). In the genera level, the dominant flora wereLactobacillus,Clostridium, Lachnospiraceae, etc.The results showed that GPP did not affect the whole intestinal microorganism abundance and diversity, indicating that GPP may have influence on the balance of intestinal flora.
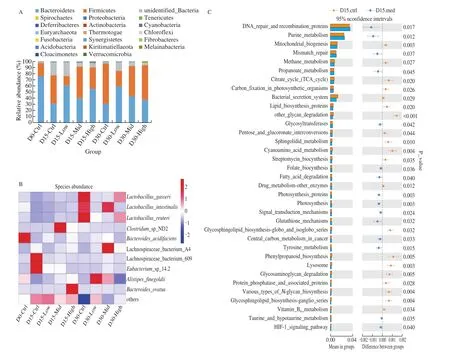
Fig. 2 (A) The effect of GPP on bacterial phylum alternation during the 15-days and 30-days treatment. (B) Effect of GPP on bacterial genera alternation during the 15-days and 30-days treatment. (C) Comparisons of the predominant gene pathways of the bacterial microbiota in different groups. Orange stands for control group and blue stands for GPP medium treatment group (n = 4).
16S rRNA marker gene sequences were used to predict the functional profiling of microbial communities. After treated by medium concentration of GPP for 15 days, DNA repair and recombination related proteins and base mismatch repair pathways were up-regulated, which also indicate the protect role of GPP on maintaining the homeostasis of intestinal microenvironment (Fig. 2C).
3.3 Daily GPP intake attenuated DSS-induced colon injury
Since GPP can both improve immune function and maintain the homeostasis of intestinal flora, we hope to explore whether GPP has identical biological effect on disease model. A classical colitismouse-model induced by DSS was established to investigate the effect of GPP on immune function and the composition of intestinal flora (Fig. 3A). The DAI score of each group were calculated and the scores in DSS-induced groups significantly increased compared with control group, and the treatment of GPP significantly decreased the score of DAI (Fig. 3B), indicating that GPP protected the colon from the damage of DSS. In the control group, goblet cells were evenly distributed, the structure of mucous layer was integrated,and there were no obvious inflammatory cells (Fig. 3C). In the DSS treated group, severe hyperplasia of goblet cells was observed,along with increased secretion of mucin, which eventually formed the vacuolated structure (Fig. 3D). The submucosa became swelled and widened, and some part of which showed necrosis and exfoliation (Fig. 3D). The number of monocytes in the columnar epithelium was increased, and the inflammatory response was obviously observed (Fig. 3E). After DSS induction, the colon structure of the GPP treatment group was more integrated than that of the DSS treated group, no necrosis or exfoliation was observed in submucosa. Goblet cells formed vacuole structure can only be found in low dose of GPP administration group, but the volume of vacuole was significantly smaller than that of DSS positive control group(Fig. 3F-3H). The degree of mononuclear cell infiltration is also lowered in GPP administration group compared with DSS group.These results demonstrated that GPP intake can attenuate colon injury induced by DSS.
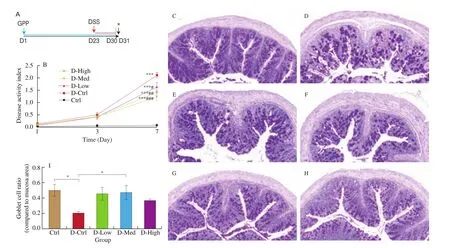
Fig. 3 Pathological changes in colitis mouse model induced by DSS. (A) Protocol for GPP treatment. (B) Change of DAI score in each group. (C–H) Colon tissues from mice were stained with PAS for histological analysis. (C) Control; (D–E) DSS (D-Ctrl); (F) DSS + 36 mg/kg BM of GPP (D-Low); (G) DSS + 120 mg/kg BM of GPP (D-Med); (H) DSS + 360 mg/kg BM of GPP (D-High). (I) The quantification of goblet cells. Data were expressed as mean ± SEM (n = 12).*P < 0.05,**P < 0.01, ***P < 0.001 compared with control group. #P < 0.05, ##P < 0.01, ###P < 0.001 compared with D-Ctrl group.
3.4 GPP treatment in DSS-induced mice affected the spleen index and protected the splenic lymphocyte proliferation ability
Spleen and thymus are both important immune organs, the organ index of which is often used as a preliminary indicator to evaluate the immunopharmacology of drugs [37]. As a result, both the spleen index and thymus index of mice were detected after being treated with different doses of GPP for 30 days, which is also the last day of DSS treatment.
The results showed that the spleen index is significantly increased compared with control group, indicating an increased inflammatory response after DSS induction. However, the treatment of 120 mg/kg BW GPP reversed the upregulation, suggesting that GPP may have protection function for the inflammatory response induced by colitis(Fig. 4A). At the same time, the thymus index in each dose group did not changed compared with the control group, indicating that GPP does not affect the size of thymus (Fig. 4B).
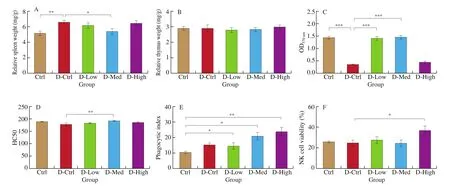
Fig. 4 The effects of GPP on immune functions of DSS-induced model mice. The effect of GPP on spleen index (A) and thymus index (B) after treatment with 36, 120, 360 mg/g BW. GPP for 30 days in the 5 groups (n = 12). (C) The effect of GPP on lymphocyte proliferation and (D) hemolytic value. (E) GPP increased peripheral phagocytes activity in mice. The uptaking capacity of peripheral phagocytes was detected by mouse peritoneal macrophage phagocyte chicken erythrocytes test, and the phagocytic index represented the phagocytosis of macrophages. (F) LDH releasing assay was used to detect the NK cell activity of the spleen. Data were expressed as mean ± SEM (n = 12). * P < 0.05, ** P < 0.01, *** P < 0.001.
Lymphocyte are active immune cells, the proliferation and differentiation of lymphocytes are important phases during the immune response. As a result, the detection of lymphocytes proliferation can be used to evaluate the lymphocyte function in cellular immunity. As shown in Fig. 1C, DSS treatment significantly reduced the lymphocyte proliferation ability, but low-dose and medium-dose of GPP treatment group significantly increased the lymphocyte proliferation ability compared with DSS group(Fig. 4C), illustrating a protective effect of GPP on splenic lymphocytes. However, high-dose of GPP treatment does not enhance the lymphocyte proliferation in both normal and DSS treatment group(Fig. 4C, Fig. S1B), indicating that GPP can enhance the lymphocyte proliferation only within a certain concentration range.
3.5 GPP enhanced the serum hemolysin synthesis
Normal cells can produce hemolysin (anti-erythrocyte antibody)after being immunized with sheep erythrocyte, which can dissolve red blood cells (RBCs) and release hemoglobin when incubatedin vitrowith sheep blood cell complement. The RBC hemolysis is related to the amount of hemolysin, which can be regarded as an indicator to evaluate the humoral immune function. In normal mice treatment groups, the HC50 level does not change with the administration of GPP (Fig. S1C). However, in DSS-induced groups, medium-dose of GPP treatment significantly enhanced the HC50 level compared with DSS-treatment group (Fig. 4D), indicating that GPP may enhance humoral immune function in colitis model mice.
3.6 GPP increased peripheral phagocytes and NK cell activity
In order to investigate the role of GPP on the innate immune system of DSS-treated mice, the phagocytosis of peritoneal macrophages and the NK cell activity of spleen were detected after the 30-day GPP treatment.
Both the phagocytic rate and phagocytic index significantly enhanced in low, medium and high dose groups compared with the control group (Fig. 4E), which was the same as in normal mice GPP treatment groups (Fig. S1A). The results suggested that DSS treatment have less effect on the phagocytic rate and phagocytic index, while GPP can enhance the phagocytosis of peritoneal macrophages in both normal and DSS-treated mice (Fig. 4E, Fig. S1A). Moreover,the cytotoxic activity of NK cells against YAC-1 tumor cells was significantly increased in the high dose GPP group compared with the DSS-control group (Fig. 4F), indicating that GPP protected the NK cell activity in DSS-treatment group. The above experimental results suggested that GPP was involved in the regulation of innate immunity in mice.
3.7 Daily GPP intake changed the composition of the intestinal micro flora in colitis model mice.
To investigate whether GPP had an effect on intestinal bacteria community in colitis model mice, Chao1 index and Shannon value of intestinal flora in mice was calculated. As shown in Figs. 5A and 5B, with the increase of GPP doses, the species richness of intestinal flora does not show significant change. However, the Chao1 value of high dose group and the Shannon value of medium and high dose group were similar as control group, indicating that GPP dose might affect the diversity of intestinal microorganisms in model mice by certain concentrations. Next, unweighted PCA dimensionality reduction analysis was also performed for each sample according to GPP doses. The result showed that the intestinal microflora significantly changed under DSS treatment (Fig. 5C).GPP treatment of medium dose were consisted of different sections and were significantly distinguished from DSS-control group, which was consistent with Shannon result. The results showed that GPP treatment had changed the species distribution of intestinal flora caused by DSS.

Fig. 5 (A-B) The α-diversity index and the Shannon value of intestinal flora in mice. (C) Unweighted PCA of sample collected with DSS and different dose of GPP. (D) Effect of GPP on bacterial phylum alternation during the 7-days DSS treatment. (E) Effect of GPP on bacterial genera alternation during the 7-days DSS treatment (n = 3).
The composition of the intestinal micro flora also had undergone changes. In the phylum level, Campilobacterota was decreased after DSS stimulation, and the level of Bacteroidota undergone slightly increase but decreased after medium dose of GPP treatment(Fig. 5D). In the genera level, the content ofLactobacillusin the intestinal tract was significantly reduced compared with control group after DSS stimulation, while the content ofLactobacillusin the medium dose group was significantly increased after the administration of GPP (Fig. 5E).Clinical reports have shown that the increase ofLactobacilluscontent can enhance the immune ability and resist intestinal disorders [38].In addition, the content ofDesulfovibrioandAlistipes_ finegoldiialso increased after DSS stimulation but were significantly decreased after GPP treatment (Fig. 5E). Previous studies have shown thatDesulfovibriois closely related with ulcerative enteritis [39], andAlistipes_finegoldiiis related with inflammation and cancer [40].These results indicates the protective role of GPP on intestinal microorganism.
4. Discussion
Gut microbiota is a complicated ecological environment that has numerous essential roles in human physiology and immune system,and is related to many chronic diseases [16,41]. Previous study of acute oral toxicity test demonstrated that GPP belonged to the non-toxic grade [14]. In our research, orally intake GPP of different doses for 30 days does not change the dominant micro flora in normal mice, the top micro flora at phylum level and top micro flora at genera level did not show much difference compared with control group (Fig. 2), this relative stable status indicated that GPP does not have deleterious effect on micro flora, which was consistent with the oral toxicity test result [14]. Moreover, after treated by medium concentration of GPP for 15 days, the comparisons of the predominant gene pathways of the bacterial microbiota showed that DNA repair and recombination related proteins and base mismatch repair pathways were up-regulated in GPP treatment group (Fig. 2C), indicating the protective role of GPP on maintaining the homeostasis of intestinal microenvironment in normal mice.
By the metabolism of gut microbes, polysaccharide went through degradation into simple sugars and short chain fatty acids (SCFAs),which improved the efficiency of energy utilization. SCFAs are the energy source material of the epithelial cells of colon and small intestine, which are able to maintain intestinal morphology and function. Previous studies have highlighted the importance of SCFAs such as acetate, propionate and butyrate in amelioration of chronic inflammatory diseases and promotion of colonocyte health [19,42]. For example, SCFAs were reported to ameliorate colitis by suppressing the production of pro-inflammatory cytokines,enhancing interleukin-10 (IL-10) expression and activating Treg cells [19,43]. In our research, the comparisons of the predominant gene pathways of the bacterial microbiota showed that the purine and propionic acid metabolic pathways were up-regulated in GPP treatment group in normal mice, indicating an upregulated metabolism of SCFAs (Fig. 2C). These results indicated that GPP might maintain the homeostasis of intestinal microenvironment through a SCFAs dependent way.
It has been reported widely that decreased richness or diversity of bacterial species were found both in fecal samples of human patients with ulcerative colitis and Crohn’s disease, and in fecal samples of rats with dextran sulfate sodium DSS-induced colitis [21,44,45].In our research, both Chao1 and unweighted PCA showed that the microbial diversity in the intestinal tract of mice increased with GPP doses and the extension of GPP administration time (Fig. 1). The improved richness of intestinal microbiota is consistent with the enhanced immune function in GPP treatment groups [14].
The mononuclear macrophage system is an important part of innate immunity, which is composed of mononuclear cells,macrophages and their precursor cells in bone marrow [46]. The NK cell is an important innate immune cell, which has a strong cytolytic function to tumor cells, virus infected cells and other physiological stress cells [47]. In our last research works, GPP increased bothin vitroandin vivophagocytosis, and enhanced the activity of NK cells,indicating a specific influence of GPP on innate immune cells [14].
In DSS-induced mice model, daily GPP intake attenuated DSS-induced colon injury, including the reduction of DAI score and histological injury (Fig. 3). DSS induction did not change the phagocytic index or NK cell viability compared with control group.GPP treatment of different doses significantly increased peripheral phagocytes activity in DSS-induced mice (Fig. 4E), which was consistent with what has been observed in normal mice (Fig. S1A).The NK cell viability was also increased in high-dose of GPP treatment in DSS-induced mice (Fig. 4F), which was also consistent with what has been observed in normal mice [13]. These results demonstrated that GPP can enhance innate immune function of both normal and colitis mice, and the function of GPP was not affected by DSS. The mechanism how GPP influence the innate immune still needs further investigation.
GPP treatment in DSS-induced mice also protected the splenic lymphocyte proliferation ability (Fig. 4C) and enhanced the serum hemolysin synthesis (Fig. 4D), which was different from what we have observed in normal mice GPP treatment groups [14], indicating a more significant immune protective effect of GPP in DSS-induced model mice. After DSS-induction, the spleen index has increased (Fig. 4A),and the splenic lymphocyte proliferation ability has decreased significantly (Fig. 4C), which may be due to the inflammatory reaction in spleen after DSS inducement. GPP-treatment has reversed the phenomenon (Fig. 4C), indicating that GPP have protective function for the spleen and somehow reduce the inflammatory accumulation in spleen. The HC50 level is commonly regarded as an index for humoral immunity, different from what we have observed in normal mice(Fig. S1C), medium-dose of GPP treatment significantly enhanced the HC50 level compared with DSS-treatment group (Fig. 4D),indicating that GPP may also enhance humoral immune function.
SCFAs, as mentioned above, were reported to ameliorate colitis by suppressing the production of pro-inflammatory cytokines. They are produced mainly from fermentation of polysaccharides by gut bacteria, such asBacteroides[19], which is a dominated intestinal microbiota in both normal and GPP treatment mice. Previous studies also showed that polysaccharides derived fromG. lucidumincreased the production of TNF-α, IL-2 and interferon-γ (IFN-γ) in primary cultures of human peripheral blood mononuclear cells [48,49]and improved the levels of serum IL-2, IL-4 in rats [21,50], which are key cytokines against pathogen infections in innate and adaptive immunity.G. lucidumis the main component of GPP, comparisons of the predominant gene pathways of the bacterial microbiota in different groups illustrated that DNA repair and recombination, base mismatch repair pathways is stronger, and the purine and propionic acid metabolic pathways were up-regulated in GPP treatment group,representing a higher DNA repair ability and upregulated metabolism of SCFAs in GPP treatment group. However, further exploration is needed to figure out how the GPP derived SCFAs regulate splenic lymphocyte proliferation ability and the synthesis of serum hemolysin.
In our research work, GPP also changed the intestinal bacteria community in colitis model mice. According to the PCA dimensionality reduction analysis, the species distribution of intestinal flora significantly changed under DSS treatment (Fig. 5C). GPP treatment groups, especially the medium dose of GPP treatment group, are consisted of different sections and were distinguished from DSS-control group, indicating a significant change in the gut microbiota of GPP treatment group.
The increase ofLactobacilluscontent can enhance the immune ability, prevent the production of inflammatory cytokines and resist intestinal disorders [38,51]. In the genus level, the content ofLactobacillusin the intestinal tract was significantly reduced compared with control group after DSS stimulation, while the content ofLactobacillusin the medium dose group was significantly increased after the administration of GPP (Fig. 5E).Desulfovibriois predominant member of sulphate-reducing bacteria inhuman gut microbiota [52], it is closely related with the pathopoiesis of ulcerative enteritis [39], patients withDesulfovibrioinfections are usually elderly men with abdominal disorders, especially hepatobiliary diseases [53].Alistipes_finegoldiiis regarded as a novel microbial driver of colitis and tumorigenesis, which is related with inflammation and cancer [40,54]. In our research, the content ofDesulfovibrioandAlistipes_ finegoldiihas increased after DSS stimulation but was significantly decreased after GPP treatment (Fig. 5E), indicating the protective role of GPP on intestinal microorganism.
Taken together, the results indicated the protective role of GPP in regulating the homeostasis of intestinal flora in DSS-induced colitis mice model. These findings suggested that GPP regulated immune function in both health and colitis model and has a positive effect on maintaining intestinal flora homeostasis. A schematic diagram was made to represent the effect of GPP on the immune function and intestinal flora of colitis model mice (Fig. 6).
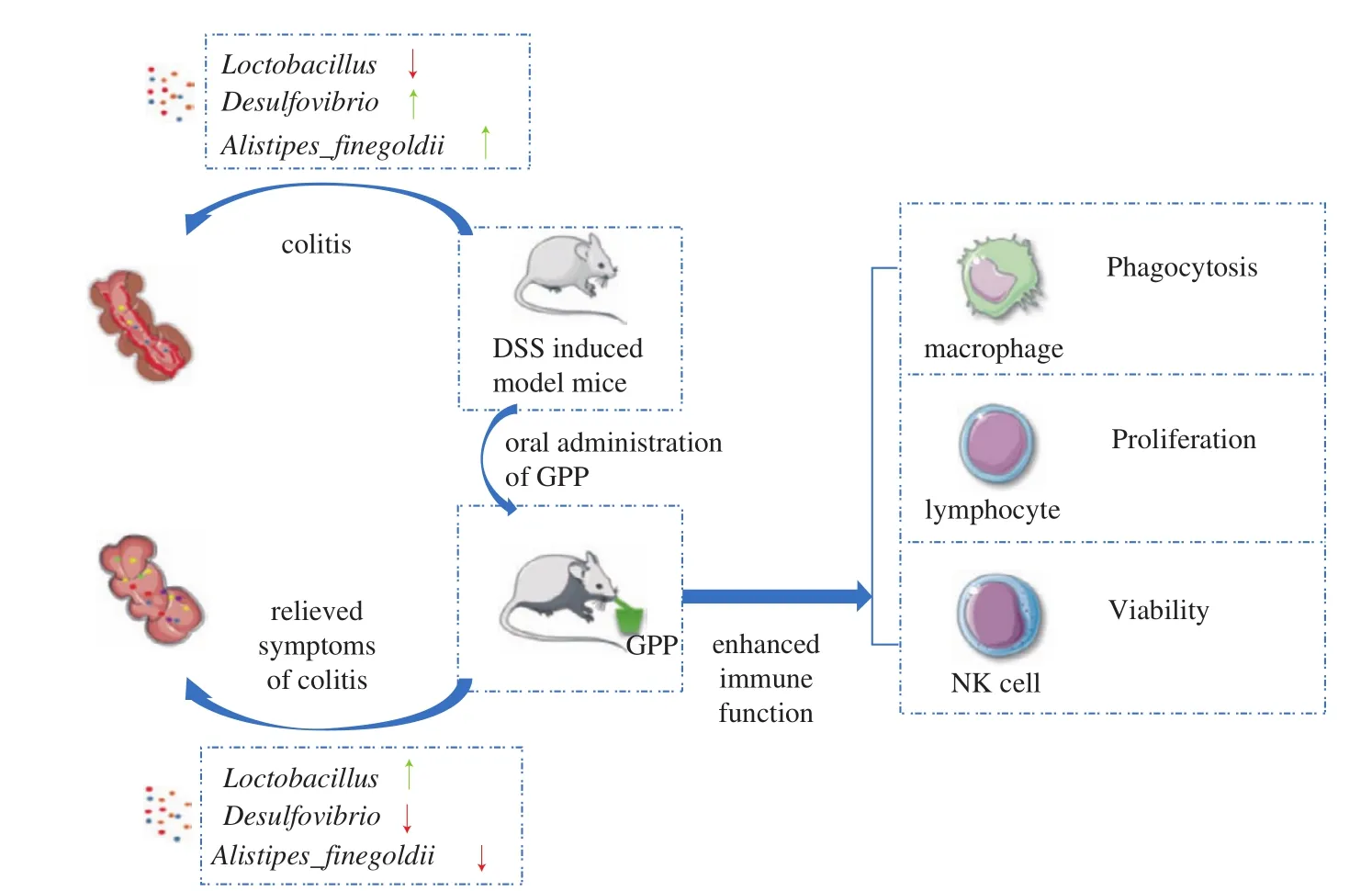
Fig. 6 The schematic diagram represents the effect of GPP on the immune function and intestinal flora of colitis model mice.
5. Conclusions
In summary, our research showed that GPP increased the diversity of intestinal microorganisms without influencing the dominant intestinal microbiota constitution in normal mice, and attenuated DSS-induced colon injury, protected the splenic lymphocyte proliferation ability, enhanced the serum hemolysin synthesis, and increased peripheral phagocytes and NK cell activity in DSS-induced model mice. Our findings revealed that GPP regulated immune function in both normal and colitis model, and had a positive effect on maintaining intestinal flora homeostasis. Our research will provide theoretical basis for the development of immune-enhancing healthy food and contribute to the further utilization of GLP and PUP in enhancing immune function.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This study was financially supported by grants from the National Key R&D Program of China (2018YFD0400204), the National Natural Science Foundation of China (81974503, 81871095),and the Key International S&T Cooperation Program of China(2016YFE113700).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.03.010.
- 食品科学与人类健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study

