Roles of Adinandra nitida (Theaceae) and camellianin A in HCl/ethanol-induced acute gastric ulcer in mice
Erdong Yuan, Yingyi Lian, Qiuhua Li, Zhaoxiang Lai, Lingli Sun, Xingfei Lai,Ruohong Chen, Shuai Wen, Junquan Zhu, Wenji Zhang,*, Shili Sun,*
a School of Food Science and Engineering, South China University of Technology, Guangzhou 510641, China
b Tea Research Institute, Guangdong Academy of Agricultural Sciences/Guangdong Key Laboratory of Tea Resources Innovation & Utilization, Guangzhou 510640, China
c Guangdong Society of Plant Protection, Guangdong 510640, China
Keywords:
Adinandra nitida (Theaceae)
Camellianin A
Gastric ulcer
Oxidative stress
Anti-inflammation
A B S T R A C T
Gastric ulcer is a global health concern nowadays. Adinandra nitida, known as Shibi tea, is a flavonoidrich plant found in South China. A. nitida possesses many healthy properties, such as antioxidation and reducing blood pressure. However, its effects on gastric ulcer have not been investigated. This study aimed to investigate the effects of the Shibi tea water extract (STE) and its main flavonoid camellianin A (CA)in hydrochloric acid (HCl) and ethanol (EtOH)-induced acute gastric ulcer in mice. Administration of CA and STE for continuous two days after stimulation by HCl/EtOH significantly attenuated the deterioration of gastric mucosal damage by lowering the gross gastric mucosal index, histopathological injury index,the oxidative stress, the expression of the inflammatory cytokines TNF-α and IL-6, and the expression of inflammatory mediators iNOS and COX-2. Western blotting and immunohistochemistry analysis showed that CA and STE regulated the inflammatory signaling pathway protein levels of IκB-α and NF-κB. Taken together, our study verified that CA and STE have antioxidative and anti-inflammatory effects in gastric ulcer mice. We propose that A. nitida should be developed as natural functional food for acute gastric ulcer patients base on the gastroprotective effects of STE and its mainflavonoid CA.
1. Introduction
Gastric ulcer disease is a common gastrointestinal tract disease provoked by multifactorial factors, includingHelicobacter pyloriinfection, use of non-steroidal anti-inflammatory drugs (NSAIDs),excessive alcohol consumption, smoking, or stress [1-4]. Epigastric pain and dyspeptic symptoms of gastric ulcer affects the quality of life and work productivity of up to 20% of the world population [5,6]. For the treatment of gastric ulcer, there are a variety of drugs with different modes of action, such as aluminum or magnesium hydroxide and calcium carbonate (antacids), cimetidine and ranitidine (H2-receptor antagonists), parecoxib and celecoxib (cyclooxygenase-2 inhibitors) [7].Nevertheless, these remedies are often accompanied with side effects, such as diarrhea, constipation, fatigue, drowsiness, headache,and muscle aches, and can even result in acute liver injury [8-10].Using natural functional ingredients to help healing gastric ulcer is an important alternative way for gastric ulcer patients.
It’s documented thatAdinandra nitida(Theaceae), also named Shibi or Shiya tea, an originally wild plant mainly distributed in south China, has anti-inflammatory and analgesic effects. The leaves ofA. nitidahave been consumed as a healthy tea due to its palatable flavor and healthy functions, as it is an antioxidant [11,12], it has anti-adipogenic activity [13], and it can lower blood pressure [14], as reported by recent research. It should be noted that the most characteristic feature ofA. nitidaleaves is its rich content offlavonoids, with camellianin A (CA), a flavone glycoside with apigenin as its mother nucleus, as the main one accounting up to 25%−35% [12,15]. We previously established that flavonoids play vital roles in the treatment and prevention of peptic ulcer [5].However, the advantages of Shibi tea and CA in gastric ulcer have not been studied. This study firstly establishes Shibi tea and CA as novel functional beverage and bioactive ingredient for gastric ulcer inin vivostudies.
Gastric ulcers are caused by an imbalance between gastric mucosal damage and the immune system. Excessive reactive oxygen species(ROS) which directly cause oxidative damage on cellular components and tissue is the inducement of gastric mucosal damage [16].Myeloperoxidase enzyme (MPO) activity and lipid peroxidation(LPO) are also well-known indicators of oxidative stress[17,18].Reducing oxidative stress and immune pro-inflammatory cytokines defending are usually suggested to be effective ways to treat a gastric ulcer[19]. Oxidative stress and mucosal inflammation are the main factors associated with the pathogenesis of the hydrochloric acid(HCl) and Ethanol (EtOH)-induced gastric ulcer model [20,21],a commonly usedin vivomodel that induces gastric mucosal erosion, bleeding, perforation, and other damage [22,23]. Driven by oxidative stress, the nuclear factor κB (NF-κB) pathway is regulated to amplify the inflammation responses by increasing the release of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and the expression of the downstream inflammation mediators cyclooxygenase-2 (COX-2)and inducible nitric oxide synthase (iNOS) [5]. In this study, we successfully established the HCl/EtOH-induced gastric mouse model and explored the antioxidative and anti-inflammatory effects of Shibi tea water extract (STE) and its mainflavonoid CA in repairing acute gastric ulcer. Consequently, we propose that Shibi tea beverage might serve as functional food with gastroprotective effects and establish CA as a novel natural gastroprotective component that plays a significant role in acute gastric ulcer modifications.
2. Materials and methods
2.1 Chemicals and reagents
Catechin (C, HPLC ≥ 98%) (Cat# B21722), epicatechin (EC,HPLC ≥ 98%) (Cat# B20102), catechin gallate (CG, HPLC ≥ 98%)(Cat# B20350), epicatechin gallate (ECG, HPLC ≥ 98%) (Cat#B20103), epigallocatechin gallate (EGCG, HPLC ≥ 98%) (Cat#B20106), gallocatechin (GC, HPLC ≥ 98%) (Cat# B20849),gallocatechin gallate (GCG, HPLC ≥ 98%) (Cat# B20850),epigallocatechin (EGC, HPLC ≥ 98%) (Cat# B20105), gallic acid(GA, HPLC ≥ 98%) (Cat# B20851) were procured from Yuanye biotechnology, Shanghai, China. Caffeine (CAFF, HPLC ≥ 99.8%)(Lot:1552009) was purchased from TM standard, Jiangsu, China. CA(HPLC ≥ 98%) and camellianin B (CB, HPLC ≥ 98%) were obtained from our previous study [15].
2.2 Preparation of STE
Shibi tea (leaves ofA. nitida) was provided by Pinshang 1962 Tea Industry Co., Ltd. in Yingde, and Taihongyuan Agriculture Co.,Ltd. in Xinyi, Maoming, Guangdong, China. The powdered leaves of Shibi tea were extracted three times for 30 min using boiling distilled water (tea-water = 1:20,m/V). These extracts were combined and centrifuged, concentrated at 60 °C, and lyophilized.
2.3 Component analysis of STE
High-performance liquid chromatography (HPLC) analysis was performed to analyze the constituents of STE. The STE sample and standards, including C, EC, CG, ECG, EGCG, GC, GCG, EGC, GA,CAFF, CA, and CB, were dissolved in 70% methanol and filtered through a 0.45 μmol/L nylon membrane (Millipore). 10 μL of each sample was injected by the instrument of Agilent Technologies Ltd.with a C18column (Agilent, 5 μm, 250 × 4.6 mm), with a flow rate of 1.0 mL/min, while the column temperature was 35 °C and the detection wavelength was 280 nm. The gradient elution started with 93.5% solvent C (0.05% phosphate acidV/V) and 6.5% solvent D(acetonitrile), and changed to 84% solvent C and 16% solvent D within 10 min, then to 80% solvent C and 20% solvent D within 9 min, to 93.5% solvent C and 6.5% solvent D within 1 min, and maintained for another 5 min.
2.4 Generation of HCl/EtOH-induced gastric ulcer animals
7-week-old male Institute of Cancer Research (ICR) mice were purchased from Beijing HFK Bioscience Co., Ltd. and handled under laboratory animals care and use guidelines. Mice were housed in standardized conditions: (23 ± 2) °C temperature, (55 ± 5)% relative humidity, and a 12 h light/dark cycle, withad libitumaccess to food and water throughout the experiment. The animal experiment protocols were approved by the Animal Care and Welfare Committee of the Tea Research Institute, Guangdong Academy of Agricultural Sciences.
After 1 week of acclimatization, 49 mice were randomly divided into 7 groups (n= 7 per group) as follows: (1) normal group; (2)model group; (3) positive group (100 mg/kg bw of cimetidine); (4)L-CA group (30 mg/kg bw of CA); (5) H-CA group (100 mg/kg bw of CA); (6) L-STE group (200 mg/kg bw of STE) (7) H-STE group(700 mg/kg bw of STE). Cimetidine (Hengjian Pharmaceutical Co.,Ltd., Guangdong, China), CA, and STE were dissolved in 0.5% Sodium carboxymethyl cellulose (CMCNa). The acute gastric injury model was induced by intragastric administration of 0.4 mol/L HCl/60% EtOH on the first day [24]. For the next 2 days, vehicle (0.5% CMCNa), 100 mg/kg of cimetidine, 30 mg/kg or 100 mg/kg CA, and 200 mg/kg or 700 mg/kg STE were orally administrated once daily,respectively. The normal group mice were given equivalent amounts of the vehicle at the same time. Mice were sacrificed one hour after the final administration, and stomach samples were collected for experimental analysis.
2.5 Determination of gross gastric mucosal index
The stomachs of the mice were excised and opened along the greater curvature. After a gentle wash with cold saline, the stomachs were spread on a dish and the area of gastric damage was captured using a digital camera. The digital photos were analyzed to calculate the gastric ulcer index using a standard as described [20,25], no lesion (score = 0), tiny spotted erosion(score = 1), lesion < 1 mm (score = 2), 1 mm < lesion <2 mm (score = 3), 3 mm < lesion < 4 mm (score = 4), or lesion >4 mm (score = 5). The sum of the total scores was divided by the number of animals to obtain the mean ulcer index for each group. The digital photos of gastric injury were quantified using Image J software(NIH, Bethesda, MD).
2.6 Histological examination
Half of each stomach was fixed in 10% neutral formalin,dehydrated in graded concentrations of alcohol, and embedded in paraffin wax. The tissues were sectioned into 4 μm slides, then were deparaffinized, rehydrated, and stained with hematoxylin and eosin (H&E) using standard protocols (Cat# CO105S, Beyotime Biotechnology, Shanghai, China). Histological slides of gastric lesions were observed using an Olympus BX-53 microscope(Olympus, Tokyo, Japan). The following scoring system was used to calculate the injury of each sample: epithelial cell loss (score 0−3),mucosal exfoliation and edema (score 0−4), hemorrhage (score 0−4),and inflammatory cell infiltration (score 0−3) [20]. The sum of the total scores was divided by the number of animals to obtain the mean ulcer index for each group.
2.7 Determination of ROS, LPO, MPO, GSH, SOD, CAT and NO levels
The stomach tissues were homogenized in phosphate-buffered saline (pH 7.4) at a ratio of 1:9 (m/V) and centrifuged at 2 500 r/min for 20 min at 4 °C. The ROS and NO levels in the supernatants were quantified using commercial ELISA kits (Cat# MM-43700M1; Cat#MM-0658M1, MEIMIAN, China) according to the manufacturer’s instructions. The GSH, SOD, CAT and LPO in the supernatants were quantified using commercial kits (Cat# A006-2-1; Cat# A001-3;Cat# A007-1-1; Cat# A106-1-2, Nanjingjiancheng, China) according to the manufacturer’s instructions. The protein concentration of the supernatants was measured by the bicinchoninic acid (BCA)protein assay kit (Lot: UE284356, Thermo, USA) using bovine serum albumin (BSA) as a standard. The relative quantification of absorbance against protein concentration was used to compare the difference in ROS, LPO, MPO, GSH, SOD, CAT and NO levels in different groups. The MPO levels of the stomach tissues were quantified using commercial kit (Cat# A044-1-1, Nanjingjiancheng,China) according to the manufacturer’s instructions.
2.8 Immunohistochemistry analysis
Immunohistochemical analysis was performed using the SABCHRP/DAB kit (Lot No: P0203, Beyotime, Shanghai, China) according to the manufacturer’s recommendations. Briefly, the sectioned 4 μm slides were deparaffinized, rehydrated, and quenched with 3% hydrogen peroxide for 15 min. After antigen retrieval with Ethylene Diamine Tetra Acetic Acid (EDTA) antigen retrieval solution,the sections were blocked with 5% normal goat serum (Lot No:11K11B09, Boster, Wuhan, China) at room temperature for 40 min.Then the primary antibodies (IL-6: Cat# bs0379, Bioss Antibodies,Beijing, China; TNF-α: Cat# ab6671, Abcam, Cambridge, MA,USA; IκB-α:Cat# 9242, Cell Signaling Technology, Danvers, MA,USA) were incubated overnight at 4 °C, after which a biotinylated secondary antibody (Cat# KPL 074-1506, Seracare Life Sciences Inc,Milford, Germany) was incubated for 1 h at room temperature. Next,the SABC-HRP reagent (30 min) and diaminobenzidine (DAB) were used for color development. Hematoxylin was used as a counterstain for another 2 min. Images of immunostained stomach tissue were taken with an Olympus BX-53 microscope (Olympus, Tokyo, Japan).
2.9 Western blotting analysis
The stomach tissues were homogenized and lysed in a RIPA lysis buffer (Cat# P0013B, Beyotime, Shanghai, China). The tissue lysates were centrifuged at 13 200 r/min at 4 °C for 20 min, and the supernatants were collected for western blotting analysis. The protein concentration was determined using the BCA protein assay kit (Lot: UE284356, Thermo, USA). Equal protein amounts of each sample were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were then blocked with 5% skimmed milk for 2 h at room temperature and incubated overnight at 4 °C with the primary antibodies: COX-2 (Cat# 12282S),IκB-α (Cat# 9242), NF-κB p65 (Cat# 8242S), phospho-NF-κB p65 (Ser536) (Cat# 13346S) (Cell Signaling Technology, Danvers,MA, USA), IL-6, (Cat# SC-1265, Santa Cruz Biotechnology,USA), TNF-α (Cat# ab6671), iNOS (Cat# ab15323), (Abcam,Cambridge, MA, USA), andβ-actin (Cat# A1978, Sigma-Aldrich,St. Louis, MO, USA). After washing three times with TBST, the membranes were incubated with the secondary antibodies for 50 min at room temperature. Positive signals were visualized using a chemiluminescence (ECL) analysis kit (Cat# 170-5061, Bio-Rad,USA), recorded with the Chemi Doc system (Bio-Rad, USA), and quantified using NIH ImageJ software (Bethesda, MD, USA).
2.10 Statistical analysis
All data are presented as the mean ± SD of at least three independent experiments. The means were compared by one-way ANOVA and Tukey’s test using the GraphPad Prism 7.0 software for Windows(GraphPad Software Inc., San Diego, CA, USA).P-values < 0.05 (*/#)and < 0.01 (**/##) were considered statistically significant.
3. Results
3.1 The catechins and camellianin content of STE
HPLC analysis was conducted to investigate the ingredients of STE. As shown in Table 1, we found 23.66% of CA in STE,which was the most abundant flavonoid. A small amount of CB((0.80 ± 0.01)%) and CAFF ((0.31 ± 0.01)%) was detected in STE. Additionally, EC ((18.97 ± 0.33)%) was the main catechin in STE, followed by (1.56 ± 0.03)% of EGC, (0.79 ± 0.09)% of C,(0.46 ± 0.01)% of GCG, (0.40 ± 0.00)% of ECG, and (0.35 ± 0.02)% of EGCG. However, CG, GC, and GA were not detected in STE.

Table 1The catechins and camellianin contents of STE.
3.2 CA and STE alleviate HCl/EtOH-induced gastric mucosal damage
Oral administration of 0.4 mol/L HCl and 60% EtOH caused severe visible hemorrhagic necrosis and burning damage with increased ulcer index (12.14 ± 3.39) compared to the normal group without macroscopic lesions (Fig. 1). In addition, the gastric tissues were getting thin and loose in the model group. However, 2-day treatment of cimetidine, CA (30 and 100 mg/kg) or STE (200 and 700 mg/kg) significantly (P< 0.01) ameliorated the mucosal damage caused by HCl/EtOH and reduced the ulcer index scores to 8.67 ± 1.53, 3.86 ± 0.89, 1.50 ± 1.00, 5.17 ± 1.33 and 2.67 ± 1.16,respectively. Notably, compared with the positive cimetidine group, all CA and STE treatment groups showed dose-dependent therapeutic effects with lower ulcer index scores and fewer injuries in the stomach, with the best results in the group receiving the higher dose of CA (100 mg/kg) (Fig. 1b). These results suggest that CA and STE treatments alleviate the gastric mucosal damage induced by HCl/EtOH in mice.

Fig. 1 Macroscopic appearance of gastric mucosa in mice. (a) Gastric mucosal of different treatment groups: Normal (water + 0.5% CMCNa), Model (0.4 mol/L HCl/60% EtOH + 0.5% CMCNa), Positive (0.4 mol/L HCl/60% EtOH + 100 mg/kg bw cimetidine), L-CA (0.4 mol/L HCl/60% EtOH +30 mg/kg bw of CA), H-CA (0.4 mol/L HCl/60% EtOH + 100 mg/kg bw of CA), L-STE (0.4 mol/L HCl/60% EtOH + 200 mg/kg bw of STE) and H-STE(0.4 mol/L HCl/60% EtOH + 700 mg/kg bw of STE), same as follow. (b) Changes in the mean gross gastric mucosal damage index. The data is expressed as the mean ± SD (n ≥ 5). **P < 0.01 compared with the model group.
3.3 CA and STE mitigate HCl/EtOH-induced gastric histopathological alterations
Histological examination showed that normal group mice had normal mucosal architecture with an intact epithelial surface,submucosa, and muscularis layers (Fig. 2a). Unlike the normal control, the model group exhibited severe gastric damage with a high hemorrhagic injury score re flecting hemorrhagic necrosis, submucosal edema, epithelial cell loss, and inflammatory cell inflltration (Fig. 2).Compared to the model group, treatment with CA (30 and 100 mg/kg)and STE (200 and 700 mg/kg) caused a dose-dependent reduction in these damages and lowered the histological index, which was analogous to the cimetidine group (P< 0.01).

Fig. 2 Histopathological damage of gastric mucosa in mice. (a) Original magnification (100 ×) of gastric mucosal of different treatment groups: Normal group mice showed intact mucosa (mu), submucosa (subm), and muscularis (ml) layers; model group mice showed hemorrhagic necrosis, submucosal edema (ede),epithelial cell loss, and inflammatory cell inflltration (arrows). (b) The gastric mucosal histological index in different groups. The data is expressed as the mean ± SD. **P < 0.01 compared with the model group.
3.4 CA and STE decrease HCl/EtOH-induced oxidative stress
Since gastric damage is typically accompanied with oxidative stress in gastric tissues, we investigated the ROS, MPO and LPO levels of different group mice. As shown in Fig. 3, administration of HCl and EtOH significantly provoked oxidative stress with increases in ROS level in the model group (P< 0.01). Treatment with CA (30 and 100 mg/kg) and STE (200 and 700 mg/kg) reduced the gastric ROS level in a dose-dependent manner. But there was no significant difference of MPO and LPO levels among all groups. In order to explore the effects of antioxidant defenses on the ulceration process, the antioxidant levels (GSH, SOD and CAT) were evaluated.Administration of HCl and EtOH significantly decreased the GSH and SOD levels of the model group (P< 0.01). Treatment with cimetidine(100 mg/kg) and STE (700 mg/kg) significantly (P< 0.05 andP< 0.01,respectively) increased the GSH level, and CA (30 mg/kg) and STE(700 mg/kg) significantly (P< 0.05) increased the SOD activity.However, there were no significant difference of CAT level among different groups.
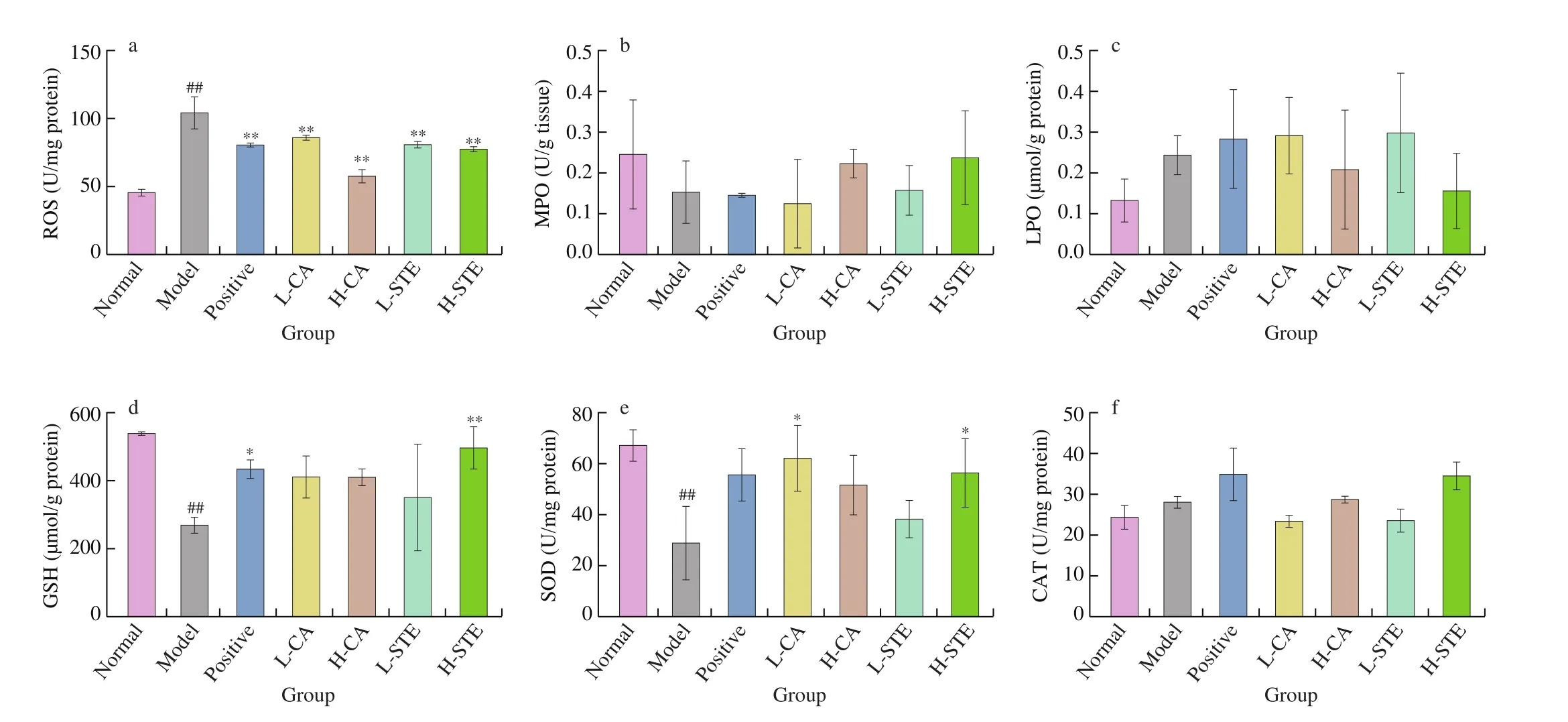
Fig. 3 Effects of CA and STE on the gastric ROS (a), MPO (b), LPO (c), GSH (d) levels and SOD (e) and CAT (f) activities. The data is expressed as the mean ± SD. ##P < 0.01 compared with the normal group, and **P < 0.01, *P < 0.05 compared with the model group.
During gastric ulceration, NO and the protein expression of inducible nitric oxide synthase (iNOS) can be upregulated in macrophages and neutrophils, promoting oxidative damage [26-28].As shown in Fig. 4, the gastric NO level of model group was significantly (P< 0.01) increased, and both western blotting and immunohistochemistry results showed that the gastric iNOS expression levels significantly (P< 0.01) increased after oral administration of 0.4 mol/L HCl and 60% EtOH. After treatment with CA (30 and 100 mg/kg) and STE (200 and 700 mg/kg) for two days,the gastric NO and the iNOS protein levels significantly decreased(P< 0.01), an effect that was slightly stronger than that seen in the positive cimetidine (100 mg/kg) group. These results suggest that both CA and STE decreased HCl/EtOH-induced oxidative stress in mice with gastric ulcers.
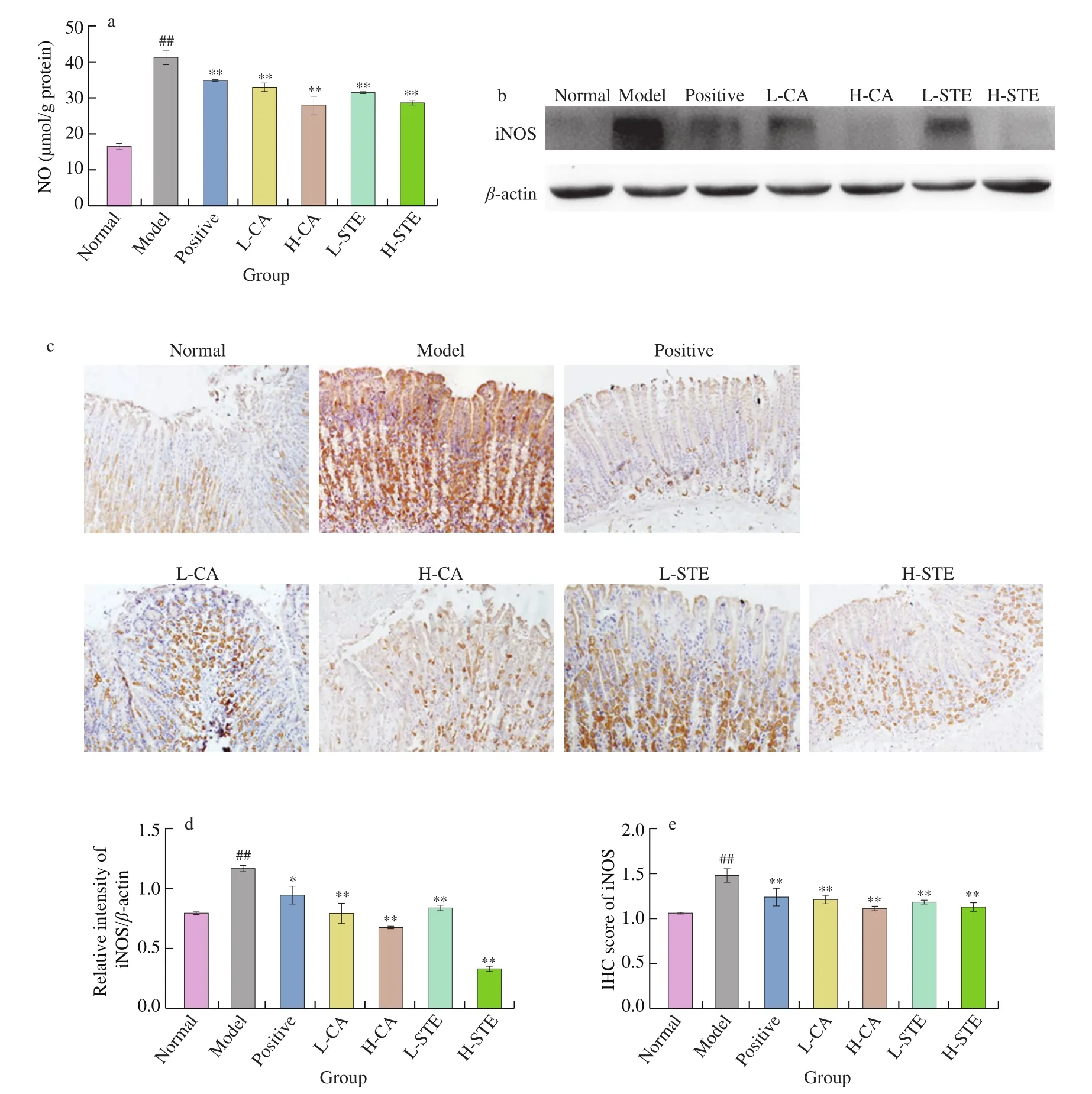
Fig. 4 Effects of CA and STE on gastric NO level and iNOS expression. (a) Gastric NO level, (b) Western blotting analysis of iNOS expression in gastric tissues and (d) densitometric quantification (n = 3 independent experiments). (c) Immunohistochemical staining of gastric iNOS and (e) densitometric quantification(n = 3 independent experiments). The data is expressed as the mean ± SD. ##P < 0.01 compared with the normal group, and **P < 0.01, *P < 0.05 compared with the model group.
3.5 CA and STE inhibit the induction of gastric COX-2 expression
COX-2 is a major inflammatory mediator that is only rapidly induced in inflammatory status and regulates the inflammatory response in the development of gastric ulcers [23,29]. It increases leukocyte adhesion and neutrophil activation, which aggravates gastric ulceration [30]. Fig. 5 shows that after administration of HCl and EtOH, the gastric COX-2 expression was significantly upregulated (P< 0.01). Oral administration of CA(30 and 100 mg/kg) and STE (200 and 700 mg/kg) for two days significantly reduced (P< 0.01) COX-2 expression in a dosedependent manner. Likewise, the positive drug cimetidine (100 mg/kg)also significantly suppressed COX-2 expression. These results indicate that CA and STE can inhibit the induction of COX-2 expression in gastric ulcers.

Fig. 5 Effects of CA and STE on gastric COX-2 expression. (a) Western blotting analysis of COX-2 expression in gastric tissues and (c) densitometric quantification (n = 3 independent experiments); (b) immunohistochemical staining of gastric COX-2 and (d) densitometric quantification (n = 3 independent experiments). The data is expressed as the mean ± SD. ##P < 0.01 compared to the normal group, and **P < 0.01, *P < 0.05 compared to the model group.
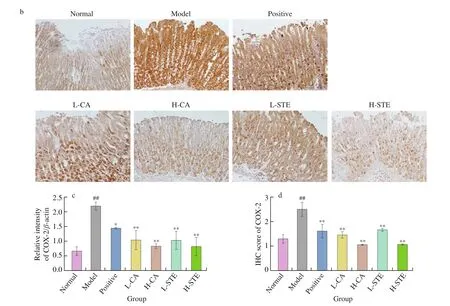
Fig. 5 (Continued)
3.6 CA and STE suppress the expression of pro-inflammatory cytokines
Neutrophil inflltration leads to the production of pro-inflammatory cytokines such as TNF-α and IL-6 that play vital roles in the acute phase inflammation [31]. In the model group, oral administration of HCl and EtOH caused a significant increase in the expression levels of TNF-α and IL-6 when compared with those in the normal group(P< 0.01) (Fig. 6). The model mice treated with CA (30 and 100 mg/kg)and STE (200 and 700 mg/kg) had a significant decrease in TNF-α and IL-6 expression in a dose-dependent manner, a similar effect as found for the positive control cimetidine (P< 0.01 andP<0.01,respectively). These data indicate that CA and STE suppress pro-inflammatory cytokines expression to inhibit inflammation.

Fig. 6 Effects of CA and STE on the gastric TNF-α and IL-6 expression. (a) Western blotting analysis of TNF-α and IL-6 expression in gastric tissues and (b,c)densitometric quantification (n = 3 independent experiments); (d) immunohistochemical staining of gastric TNF-α and IL-6 and (e, f) densitometric quantification(n = 3 independent experiments). The data is expressed as the mean ± SD. ##P < 0.01 compared to the normal group, and **P < 0.01 compared to the model group.
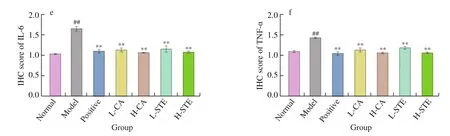
Fig. 6 (Continued)
3.7 CA and STE suppress IκB-α degradation and NF-κB activation
The data mentioned above con firmed the antioxidative properties and anti-inflammatory activity of CA and STE in HCl/EtOH-induced gastric ulcer. Next, we studied the regulation of CA and STE on the NF-κB signaling pathway, which is vital in inflammatory responses(Fig. 7) [20,21,32]. Using western blotting and immunohistochemistry,we found that gastric IκB-α in the model group was significantly(P< 0.01) depressed after administration of HCl and EtOH when compared with the normal group. Treatment with CA (30 and 100 mg/kg) and STE (200 and 700 mg/kg) significantly suppressed IκB-α degradation, like the effects of cimetidine (100 mg/kg).Additionally, NF-κB phosphorylation was significantly activated by HCl and EtOH, while the positive drugs cimetidine, CA, and STE inhibited the phosphorylation of NF-κB, suggesting that CA and STE ameliorate gastric ulcers by regulating the IκB-α/NF-κB inflammatory pathways to block the promotion of inflammation.

Fig. 7 Effects of CA and STE on gastric IκB-α and NF-κB expression. (a) western blotting analysis of IκB-α and NF-κB expression in gastric tissues and(c, d) densitometric quantification (n = 3 independent experiments); (d) immunohistochemical staining of gastric IκB-α and (e) densitometric quantification(n = 3 independent experiments). The data is expressed as the mean ± SD. ##P < 0.01 compared to the normal group, and **P < 0.01 compared to the model group.
4. Discussion
Gastric ulcers affect millions of people and are considered as a common global health concern nowadays. However, treatment of gastric ulcers remains challenging due to the incidence of relapses and side effects of the available drugs. Shibi tea is a plant rich in CA in China, which has antioxidative, anti-proliferative, and antiadipogenic effects [13]. In this present study, we evaluated the gastroprotective effects of Shibi tea and CA in HCl/EtOH-induced acute gastric ulcers in mice and, for the first time, evaluated its mechanism of action. Our results indicate that STE and CA can significantly reduce the HCl/EtOH-induced gastric oxidative stress that suppressed the NF-κB pathway which affects the expression of the downstream pro-inflammatory cytokines TNF-α and IL-6 and inflammatory mediators iNOS and COX-2, therefore, helps ameliorating gastric ulcer damage (Fig. 8).
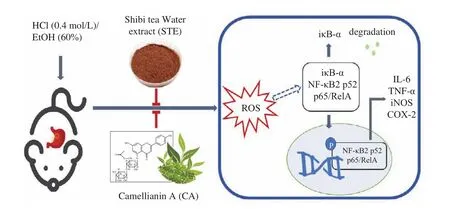
Fig. 8 Diagram depicting the ameliorative actions of STE and CA in HCl/EtOH-induced acute gastric ulcer in mice.
Shibi tea and CA could be safe and healthy choices for HCl/EtOH-induced acute gastric ulcer. Firstly, Shibi tea and CA effectively help ulcers healing. As shown in Fig. 2, mice that treated with CA and STE had less gastric mucosal damage, while the model group mice still showed severe gastric mucosal damage with gastric erosions, mucosal necrosis, and loss of epithelial cells after 2 days of HCl and ethanol consumption, regarded as the leading cause of gastric ulcer in humans [33]. These data show that STE and CA help deal with stomach ulcers caused by an acrimony excitant diet.Secondly, CA and STE are safe for the individuals. Shibi tea has been consumed as a health tea for hundreds of years in South China [12].The concentration of 30 and 100 mg/kg CA and 200 and 700 mg/kg STE, far lower than the concentration offlavonoid monomers causing toxicity or side effects (> 500 mg/kg) [5], efficiently promoted the recovery of gastric mucosal injury caused by HCl and EtOH (Fig. 1).Furthermore, orally administrated dosages of 200 and 700 mg/kg STE are in accordance with the dosage taken by drinking tea (small and normal intake). The CA doses (30 and 100 mg/kg) generally correspond to the CA content in STE (200 and 700 mg/kg). More importantly, during the experimental period, the mice treated with CA and STE showed no irregular behavioral symptoms. These data provide a theoretical basis for the effects of daily Shibi tea drinking on stomach protection and confirm Shibi tea as natural functional food for patients with acute gastric ulcers. Thirdly, CA was found to exert gastroprotective effects in gastric ulcers for the first time and showed better efficacy than Shibi tea, indicating that CA is bioactive ingredient with gastroprotective effect.
Flavonoids are well-known antioxidants, which can act as ROS scavengers [21,34]. Scavenging of ROS has been regarded as an important mechanism in the healing of gastric ulcers [35]. Therefore,we investigated the oxidative stress status of gastric tissues. In this study, CA and STE reduced the gastric ROS levels in a dosedependent manner (Fig. 3a). However, there was no significant difference of LPO and MPO levels among all groups (Fig. 3). Liu et al. [14]found that the ethanol extract ofA. nitidaleaves and CA showed dose-dependent 1,1-diphenyl-2-picrylhydrazyl free radical(DPPH) scavenging activities, and the IC50 of CA was 14.74 μg/mL.Cellular-based antioxidant capacity assays also showed thatA. nitidaleaves had strong cellular antioxidant activity, especially after stirfrying the leaves [11]. In this study, STE (700 mg/kg) significantly(P< 0.05 andP< 0.01) increased GSH level, and CA (30 mg/kg) and STE (700 mg/kg) significantly (P< 0.05) increased the SOD activity(Fig. 3). In addition, we detected the content of NO, another marker of oxidative stress. During inflammation, iNOS are upregulated in macrophages and neutrophils to increase the NO levels, causing cytotoxic effects and gastric oxidative damage [4,36-38]. We found that the damage-induced NO levels were significantly reduced by CA and STE (Fig. 3). These results indicate that CA and STE might exert gastroprotective effects via the oxidative stress balancing mechanism,which might through direct free radical scanning action or by protecting and activating antioxidant enzymes or by synergism effect.
Oxidative stress could mediate the stimulation of IκB that induces proteasomal breakdown of IκB-α and activates NF-κB [39].NF-κB then translocates into the nucleus and triggers the transcriptional activation of pro-inflammatory cytokines, such as TNF-α and IL-6, and enzymes like iNOS and COX-2, which induce inflammation [21,39]. Our data showed that administration of CA and STE significantly increased the IκB-α level and suppressed NF-κB activation. Furthermore, the NF-κB downstream factors,including pro-inflammatory cytokines levels of TNF-α and IL-6,iNOS and COX-2 levels, were significantly suppressed by STE and CA. TNF-α plays a role in the activation and recruitment of immune cells, and the generation of other pro-inflammatory cytokines, while IL-6 stimulates neutrophils, macrophages, and lymphocytes at the inflammatory site to produce various harmful substances [40].Except for these two critical inflammatory cytokines, CA and STE also regulate iNOS and COX-2. During inflammation circumstance,iNOS is upregulated in macrophages and neutrophils to increase the NO level and break the NO balance, causing further damages[36-38], and COX-2 increases leukocyte adhesion and neutrophil activation, which aggravates peptic ulceration [30]. COX-2 is also an essential enzyme in the biosynthesis of prostaglandins (PGs), which are the primary arachidonic acid metabolites that regulate gastric acid, mucus, and bicarbonate production, improve mucosal blood flow, and promote mucosal healing [5,41,42]. However, normal gastric mucosa does not express COX-2, while expression of COX-2 is rapidly induced and regulates the inflammatory response in gastric ulcer development [23,29]. Our results showed that iNOS and COX-2 were significantly induced by HCl and ethanol, and treatment with CA and STE attenuated the increased expression. These mechanisms indicate that CA and STE can regulate ROS-stimulated NF-κB and its downstream factors to relieve inflammation in the gastric area (Fig. 8). The oxidative stress/NF-κB pathway is also found to play crucial roles in other inflammatory diseases such as hepatitis [43,44]and enteritis [45,46], indicating that Shibi tea and CA might also serve as functional food or bioactive ingredients against these inflammatory diseases.
5. Conclusions
In summary, our study demonstrates that CA and STE can ameliorate HCl/EtOH-induced gastric ulcers in mice. The gastroprotective effects of CA and STE are primarily attributed to the reduction of oxidative stress and anti-inflammatory properties.By reducing ROS levels, CA and STE inhibit the activation of the NF-κB pathway and subsequently suppress the levels of iNOS, COX-2,and the pro-inflammatory cytokines TNF-α and IL-6, to reduce inflammation. Also, CA and STE might exert gastroprotective effects by synergistic action and other mechanism could be involved. More importantly, the current study pinpoints the functional benefits ofA. nitidaand CA as bioactive ingredient for the management of gastric ulcers. Additionally, the confirmed anti-inflammatory activity ofA. nitidaand CA suggests these could also serve as healthy choices for other inflammatory diseases, like hepatitis and enteritis.
Conflicts of interests
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgement
This study was funded by the “14thFive-Year Plan” team-building projects of Guangdong Academy Agricultural Sciences (202126TD),National Natural Science Foundation of China (81903319,81803236, 31800295), Guangdong Science and Technology program(2018KJYZ002), Guangdong Basic and Applied Basic Research Foundation (2020A1515011266), Qingyuan Science and Technology Program (DZXQY021, 181022114566189, 181022114566189,2019A039, 2020KJJH042), Shaoguan Science and Technology Program (2018CS11902, 2018sn081), Guangzhou Science and Technology Program (202002030202), Zhaoqing Science and Technology Program (2019N001, 2019N013), Maoming Science and Technology Program (mmkj2020045), Zhanjiang Science and Technology Program (2020A03014), Yingde Science and Technology Board (JHXM2018029), Innovation Fund projects of Guangdong Academy of Agricultural Sciences (202115), Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (R2019PY-JX004, R2018YJ-YB3002, R2016YJYB3003, R2018PY-QF005, R2018QD-101).
- 食品科学与人类健康(英文)的其它文章
- Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats
- Sucrose-free hawthorn leathers formulated with fructooligosaccharides and xylooligosaccharides ameliorate high-fat diet induced inflammation,glucose and lipid metabolism in liver of mice
- Polygonatum sibiricum polysaccharides protect against obesity and non-alcoholic fatty liver disease in rats fed a high-fat diet
- Trehalose ameliorates autophagy dysregulation in aged cortex and acts as an exercise mimetic to delay brain aging in elderly mice
- Deep eutectic solvents and alkaline extraction of protein from seabuckthorn seed meal: a comparison study
- Isolation of a novel characterized Issatchenkia terricola from red raspberry fruits on the degradation of citric acid and enrichment offlavonoid and volatile profiles in fermented red raspberry juice

