Immunoregulatory polysaccharides from Apocynum venetum L.flowers stimulate phagocytosis and cytokine expression via activating the NF-κB/MAPK signaling pathways in RAW264.7 cells
Honglin Wang, Changyang M Dongxiao Sun-Waterhouse,Jinmei Wang*, Geoffrey Ivan Neil Waterhouse*, Wenyi Kangd,*
a National R&D Center for Edible Fungus Processing Technology, Henan University, Kaifeng 475004, China
b Functional Food Engineering Technology Research Center, Kaifeng 475004, China
c School of Chemical Sciences, University of Auckland, Auckland 1142, New Zealand
d Joint International Research Laboratory of Food & Medicine Resource Function, Kaifeng 475004, China
Keywords:
Apocynum venetum L. flowers
Immunomodulatory polysaccharide
RAW264.7 cells
NF-κB signaling pathway
MAPK signaling pathway
A B S T R A C T
Two immunomodulatory polysaccharides (Vp2a-II and Vp3) were isolated and identified from Apocynum venetum L. flowers, and their innate immune-stimulating functions and working mechanisms were evaluated in RAW264.7 cells. Both the level of released nitric oxide (NO) and expression of inducible nitric oxide synthase (iNOS) mRNA were significantly enhanced in the RAW264.7 macrophages cells treated by Vp2a-II and Vp3. Vp2a-II (100–800 µg/mL) and Vp3 (400 µg/mL) could significantly increase the phagocytic activity of RAW264.7 cells and the secretion and mRNA expression of TNF-α and IL-6 in a concentrationdependent manner through affecting mitogen-activated protein kinase (MAPK) activity and nuclear factor κB(NF-κB) nuclear translocation. Vp2a-II might activate the MAPK signaling pathways and induce the nuclear translocation of NF-κB p65, whilst Vp3 likely activated the NF-κB and MAPK signaling pathways without influencing the p38 MAPK route.
1 Introduction
The immune system in human is a well-known defense against foreign antigens and external stimulations including virus, and its dysfunction causes autoimmune and inflammatory diseases [1,2].Two types of immune responses, innate immunity and adaptive immunity, monitor the whole body’s immune defenses in human,with the innate system allowing rapid immune responses. These processes for immune regulation involve immune organs such as spleen and thymus, as well as immune cells such as macrophage,natural killer cells and splenocytes. Macrophages are located throughout the body tissues [3]. Macrophages, in collaborations with other types of immune elements (like the neutrophils), exert important regulatory functions in innate and acquired immunity through surveillance, chemotaxis, phagocytosis, secretion of pro-/antiinflammatory cytokines, and inflammatory mediators (such as nitric oxide (NO), tumor necrosis factor α (TNF-α), interleukin (IL) 6 and IL-1β, and subsequent activation of fellow immune cells [4,5]. When macrophage RAW264.7 cells are stimulated, the mitogen-activated protein kinase (MAPK) or nuclear factor κB (NF-κB) signaling pathways in cells were found to be activated through the receptors on the cell membrane [6,7].
Polysaccharides are biomacromolecules that participate in a wide range of physiological functions including immunoregulatory and anti-inflammatory activities, and can be isolated from plants,animals and microorganisms [8-10]. In particular, a number of plant polysaccharides were found to have immune-regulating,anti-inflammatory, antioxidative, antibacterial, antithrombotic,hypoglycemic, hypolipidemic and anti-fatigue functions [11-16]. Plant polysaccharides may support the immune system at multiple levels and by various mechanisms including their capabilities to stimulate immune cells (including macrophages) and enhance their functions(including phagocytic activity) and activate/promote the secretion of cytokines [17,18]. Accordingly, there has been ongoing interest in screening immunomodulatory polysaccharides from edible plants,and growing efforts are being made towards this aspect because of the increasing emergence of viral infections/diseases.
Apocynum venetumL., a wild subshrub of Apocynaceae distributed widely in temperate regions across the continents of Asia,Europe and North America, has demonstrated potent antioxidant,cardiotonic, hepatoprotective, lipid-lowering, anti-anxiety/depression activities [19-25].A. venetumleaves were reported, in the first volume ofChinese Pharmacopoeia(2015 edition), capable of calming the liver and nerves, clearing away heat/fire and boosting diuresis, and their use at a daily dosage (50 mg/person/day) for more than three years has been proven safe with no severe side effects [26,27].A. venetumflowers have significant amounts of antioxidants like flavonoids as well as cardiac glycosides and organic acids [27,28].To fill the knowledge gap in the bioactivities of the polysaccharides fromA. venetumflowers, research has recently been conducted in our laboratory to establish scalable procedures for preparing bioactive polysaccharides, and two polysaccharides, Vp2a-II (with →6)-β-DGlcp-(1→6)-α-D-Galp-(1→ structure) and Vp3 (withα-D-GlcpA-(3→α-D-GalpA structure), were found to exhibit an anticoagulant activity [29]. In this study, the two polysaccharides were subjected to examinations on their immunomodulatory potential in RAW264.7 cells (as the immunological model) with lipopolysaccharide(LPS, 1 μg/mL) as the positive control.
2 Materials and methods
2.1 Chemicals
Dulbecco’s Modified Eagle’s Medium (DMEM) and neutral red were purchased from Solarbio (Beijing, China). Fetal bovine serum(FBS) was purchased from Gibco (Grand Island, NY, USA). Nitric oxide kit was obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). TNF-α and IL-6 enzyme-linked immunosorbent assay (ELISA) kits were obtained from Beijing 4A Biotech Co, Ltd. (Beijing, China). Primer inducible nitric oxide synthase (iNOS), TNF-α and IL-6 were purchased from Thermo Fisher scientific (Shanghai, China). PrimeScriptTMRT reagent kit with gDNA Eraser kit and TB GreenTMExTaqTMII (Tli RNadeH Plus) as well as Bulk RT kit were purchased from TaKaRa (Dalian,China). Antibody NF-κB p65, phospho-NF-κB p65 (p-p65), p38 MAPK, p-p38 MAPK, stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK), p-SAPK/JNK, p44/p22 MAPK and p-p44/p22 MAPK were purchased from Cell Signaling (Beverly,MA, USA). NF-κB inhibitor (PDTC), extracellular regulated protein kinases (ERK) inhibitor (PD98059), JNK inhibitor (SP600125), p38 inhibitor (SB203580) and LPS were purchased from Sigma-Aldrich(St. Louis, MO, USA).
2.2 Preparation of Vp2a-II and Vp3
Vp2a-II and Vp3 were isolated and purified by the National R&D Center for Edible Fungus Processing Technology, Henan University according to our published method [30]. Brie fly, the driedA. venetumflowers were extracted three times with distilled water at 90 °C, and the obtained extracts were combined and precipitated with 70% aqueous ethanol. The crude polysaccharides were subjected to protein removal by the Sevag method and purification by a DEAE-52 cellulose column and Sephadex G-100 column chromatography to yield two purified polysaccharides, Vp2a-II (average molecular weight: 6.738 × 103g/mol) and Vp3 (average molecular weight:8.564 × 103g/mol). The obtained Vp2a-II and Vp3 did not contain any endotoxin based on the assay results by ToxinSensorTMChromogenic LAL Endotoxin Assay Kit.
2.3 Cell culture and treatment
The murine macrophage cell line RAW264.7 was obtained from Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences, China). The cells were cultured in DMEM medium supplemented with 10% FBS, penicillin (100 units/mL) and streptomycin (100 mg/mL), and incubated in a 5% CO2humidified atmosphere at 37 °C. The complete medium and LPS (1 μg/mL) were used as a normal and positive control, respectively.
2.4 Cell viability assay
The MTT method was used to evaluate the effects of the polysaccharides on the RAW264.7 cells’ viabilities. Briefly, an aliquot (100 μL) of cells suspensions (density: 1 × 104cells/well)was added into the wells of 96-well plates. The cells were cultured at 37 °C and in 5% CO2for 24 h. The supernatant was discarded and the cells were treated with 100 μL of the polysaccharide samples at different concentrations. After a 24-h incubation, an aliquot (10 μL)of MTT solution (1 mg/mL) was added to each well and the resulting mixture was subjected to further incubation for 4 h. Then, the medium was discarded and an aliquot (100 μL) of dimethyl sulfoxide(DMSO) was added to allow standing for 10 min. The absorbance was measured at 490 nm by a spectrophotometer (Thermo Fisher Scientific, catalog number: 1510, USA ).
2.5 Phagocytic activity
The phagocytic activity of RAW264.7 cells was assessed by the neutral red phagocytosis assay [11]. Brie fly, after cells were cultured with Vp2a-II, Vp3 or LPS (1 μg/mL) for 24 h, an aliquot (100 μL) of neutral red solutions (0.075%,V/V) was added into each well for an 1-h incubation. The supernatant was removed and the cells were washed with PBS twice. Then, cell lysis solution (containing 1% acetic acid and anhydrous alcohol at a ratio of 1:1 (V/V), 100 μL) was added to each well, and the plates were incubated at room temperature for 4 h.The absorbance was measured at 540 nm.
2.6 Measurements of NO, TNF-α and IL-6 levels
RAW264.7 cells were cultured with Vp2a-II, Vp3 or LPS(1 μg/mL) at 37 °C for 24 h. The supernatants were collected and the concentrations of the NO and pro-inflammatory cytokines released from the cells were determined by the ELISA kits, according to the manufacturer’s instructions.
2.7 Quantitative real-time polymerase chain reaction (qPCR)
To evaluate the iNOS and pro-inflammatory cytokines mRNA expression levels, total RNA from the RAW264.7 cells media was isolated by the Trizol Reagent and reversed to cDNA using the Prime ScriptTMRT Reagent Kit with the gDNA Eraser. The levels of genes were quantified by qPCR using TB GreenTMExTaqTMII (Tli RNadeH Plus), Bulk kit (all sequences are listed in Table 1). The amplification was carried out using the Thermo Fisher 7500 real-time PCR system.All the samples were assessed in triplicate, and the obtained data were calculated using the 2–ΔΔCtmethod and expressed as the relative abundance ratio of the target genes to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
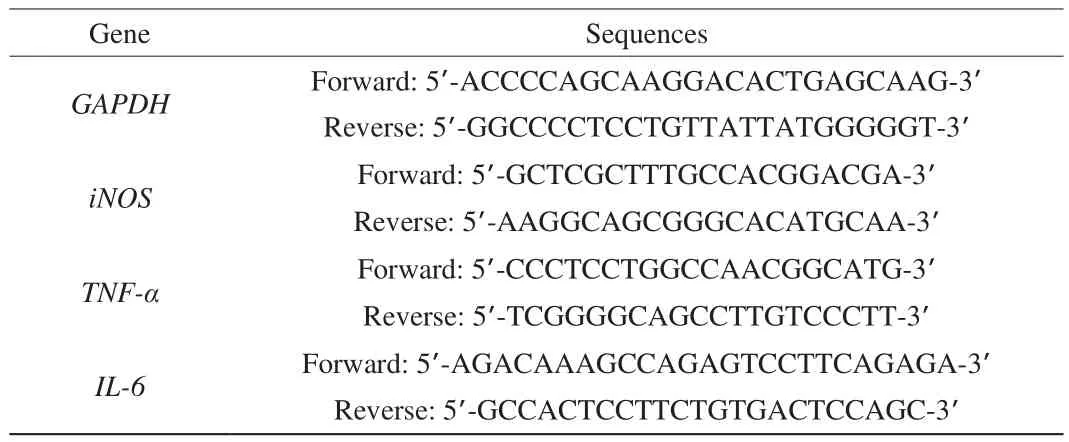
Table 1Primers for real-time RT-PCR.
2.8 Western blot analysis
The preparation of total protein and nuclear protein was conducted through extracting from the RAW264.7 cells pretreated with Vp2a-II,and Vp3 or LPS, the method published previously [11,30]. The bicinchoninic acid (BCA) kits were used to evaluate the protein concentration. Equal amounts of proteins were separated by 8% SDS-PAGE and then transferred to the polyvinylidene di fluoride (PVDF)membranes. After the incubation with the blocking buffer (containing 5% skim milk) at room temperature for 2 h, the membranes were incubated with the phosphorylated or non-phosphorylated antibodies(NF-κB p65, ERK1/2, SAPK/JNK and p38) overnight at 4 °C. After three washes with the Tris-Buffered Saline-Tween 20 (TBST),the membranes were incubated with the horseradish peroxidase(HRP)-conjugated secondary antibody at 4 °C for 4 h. The incubated membranes were washed again with the TBST, and the protein strips were visualized using the enhanced chemiluminescence(ECL) reagents.
2.9 Statistical analysis
All experiments were repeated three times and the results were expressed as mean ± standard deviation (SD). All statistical tests were carried out using the computer program SPSS 19.0 and the statistical significance was calculated by the one-way variance analysis(ANOVA) (statistical significance was set atP< 0.05).
3. Results
3.1 Effects of Vp2a-II and Vp3 on RAW264.7 cells viability
Compared with the contal group, Vp2a-II had no cytotoxicity on RAW264.7 cells at a concentration up to 800 μg/mL, and promoted cell proliferation in a wide concentration (P< 0.01) (Fig. 1A).Accordingly, the concentration range of 100–800 μg/mL was chosen to carry out the subsequent experiments. Vp3 at a concentration in the range of 800–1 600 μg/mL exhibited a cytotoxic effect on the RAW264.7 cells (P< 0.001). Therefore, the concentration range of 50–400 μg/mL was chosen as the most suitable dose for Vp3 (Fig. 1B).
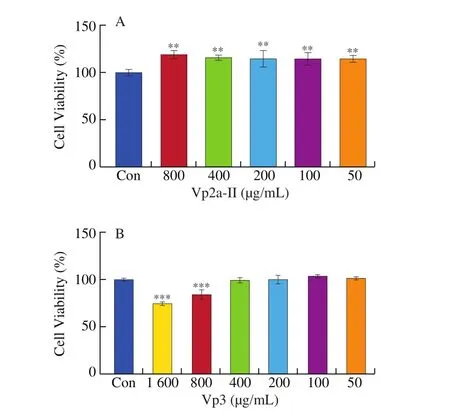
Fig. 1 Effects of Vp2a-II (A) and Vp3 (B) on the viability of RAW264.7cells. Data are presented as “mean ± SD”, n = 3. Compared with the controlgroup, *** indicates a significant difference at P < 0.001; ** indicates asignificant difference at P < 0.01; * indicates a significant difference at P < 0.05.
3.2 Vp2a-II and Vp3 enhanced the phagocytic activity of RAW264.7 cells
In this study, Vp2a-II significantly (P< 0.05) enhanced the pinocytic activity of RAW264.7 cells, compared with the normal control (Fig. 2A). The phagocytosis index of Vp2a-II reached its highest values at concentration of 200 μg/mL. When the concentration up to 800 μg/mL, the phagocytosis index showed a slight decline, but the value was still higher than that of the blank control. However,Vp3 enhanced the phagocytic activity of macrophages only at the high concentration (400 μg/mL) (Fig. 2B). The phagocytosis index of Vp2a-II was higher than that of Vp3 at the concentration of we studied.
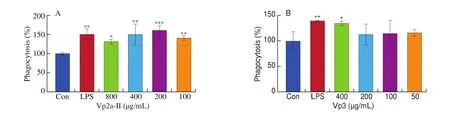
Fig. 2 Effects of Vp2a-II and Vp3 on the phagocytic activity of RAW264.7 cells. Data are presented as “mean ± SD”, n = 3. Compared with the control,*** indicates a significant difference at P < 0.001; ** indicates a significant difference at P < 0.01; * indicates a significant difference at P < 0.05.
3.3 Effects of Vp2a-II and Vp3 on NO production and iNOS expression
In this study, the level of NO released from RAW264.7 cells increased significantly (P< 0.05) and in a dose-dependent manner under the stimulation of Vp2a-II and Vp3, compared with the normal control (Figs. 3A and 3B). Vp2a-II (400 μg/mL) and Vp3(200 μg/mL) could increase the expression ofiNOSmRNA, and the regulatory ability of Vp3 was found stronger than that of Vp2a-II(Fig. 3C). Therefore, Vp2a-II and Vp3 could promote the secretion of NO from RAW264.7 cells by activating the expression of iNOS.
Fig. 3 Effects of Vp2a-II and Vp3 on NO production (A and B) and iNOS mRNA expression (C). Data are presented as “mean ± SD”, n = 3. Compared with the control, *** indicates a significant difference at P < 0.001, ** indicates a significant difference at P < 0.01, * indicates a significant difference at P < 0.05.
3.4 Effects of Vp2a-II and Vp3 on the expression of cytokines and mRNA in RAW264.7 cells.
In present work, the release and corresponding mRNA expression of pro-inflammatory cytokines from the RAW264.7 cells treated with different concentrations of Vp2a-II and Vp3 or LPS (1 μg/mL)for 24 h were evaluated by ELISA and qPCR. As expected, Vp2a-II(400 or 800 μg/mL) and Vp3 (50 μg/mL) significantly promoted the production of TNF-α and IL-6, compared with control (P< 0.05)(Fig. 4A, B and Fig. 4D, E). Besides, the other concentrations of Vp2a-II and Vp3 slight improved the production of TNF-α and IL-6 than the control. Based on the results of various immune indexes,the intermediate concentration of Vp2a-II and Vp3 were selected for qPCR verification. The results showed that Vp2a-II (400 μg/mL) and Vp3 (200 μg/mL) could promote the mRNA expression ofTNF-α,IL-6(P< 0.05) (Fig. 4C, F). According to the above results, Vp2a-II and Vp3 play an immunomodulatory role and can increase the levels of the released pro-inflammatory cytokines through upregulating the levels of their corresponding mRNA expression.
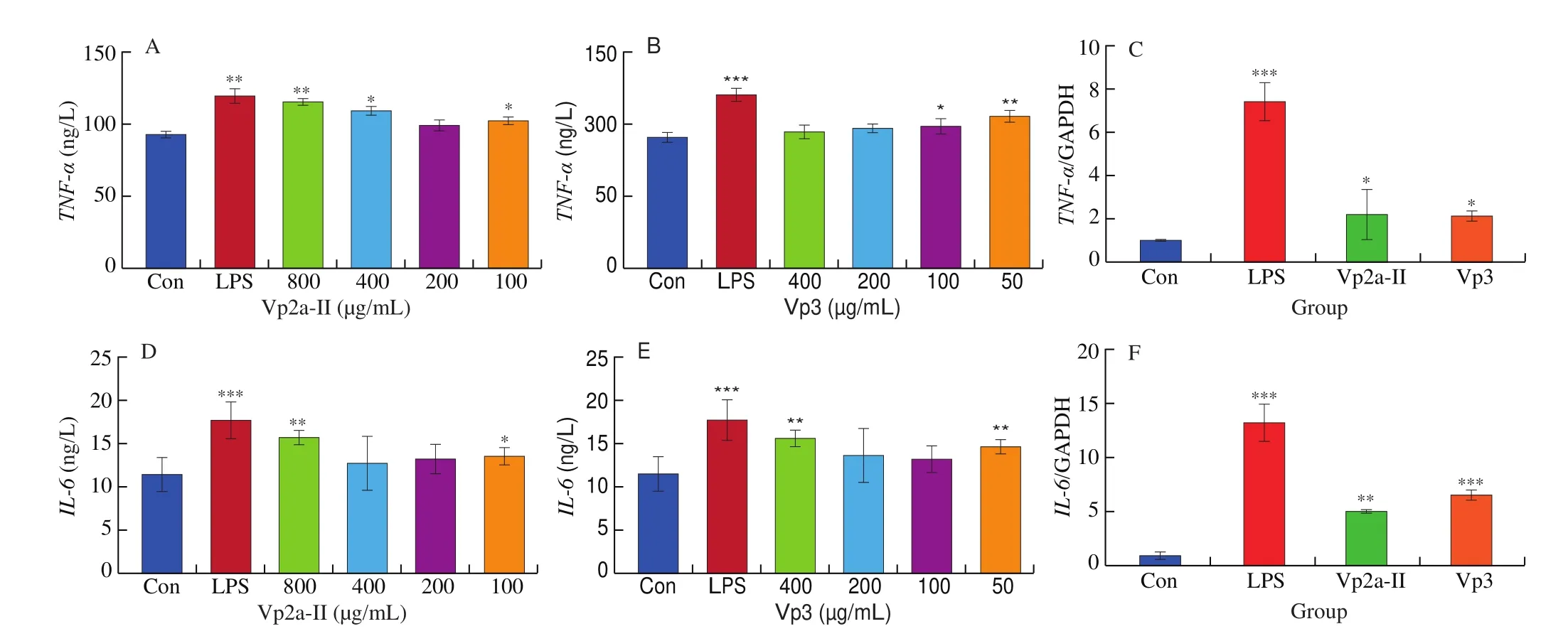
Fig. 4 Effects of Vp2a-II and Vp3 on the expression of TNF-α (A,B), IL-6 (D,E) and TNF-α, IL-6 mRNA (C,F) in RAW264.7 cells. Data are presented as“mean ± SD”, n = 3. Compared with the control, *** indicates a significant difference at P < 0.001, ** indicates a significant difference at P < 0.01, * indicates a significant difference at P < 0.05.
3.5 Vp2a-II and Vp3 induced the activation of MAPKs and NF-κB pathways in RAW264.7 cells
The level of NF-κB p65 protein phosphorylation in RAW264.7 cells significantly increased after LPS stimulation. Both Vp2a-II(100–800 μg/mL) and Vp3 (50–400 μg/mL) promoted the transfer of NF-κB p65 from the cytoplasm to the nucleus in a dose-dependent manner (Fig. 5A, B). In order to verify further the role of NF-κB signaling pathway in the immunomodulatory effects ofA. venetumpolysaccharides, the RAW264.7 cells were also pretreated with NF-κB p65 specific inhibitor PDTC in a separate set of experiments.PDTC (10 μmol/L ) was added into Vp2a-II (400 μg/mL) and Vp3(200 μg/mL), respectively. The obtained results (Fig. 5C) revealed that such a pretreatment with inhibitors reduced the level of protein phosphorylation of NF-κB p65 in both the Vp2a-II-treated group and Vp3-treated group. Taken together, the NF-κB signaling pathway plays an important role in the immunomodulation of the macrophages exerted by Vp2a-II and Vp3.
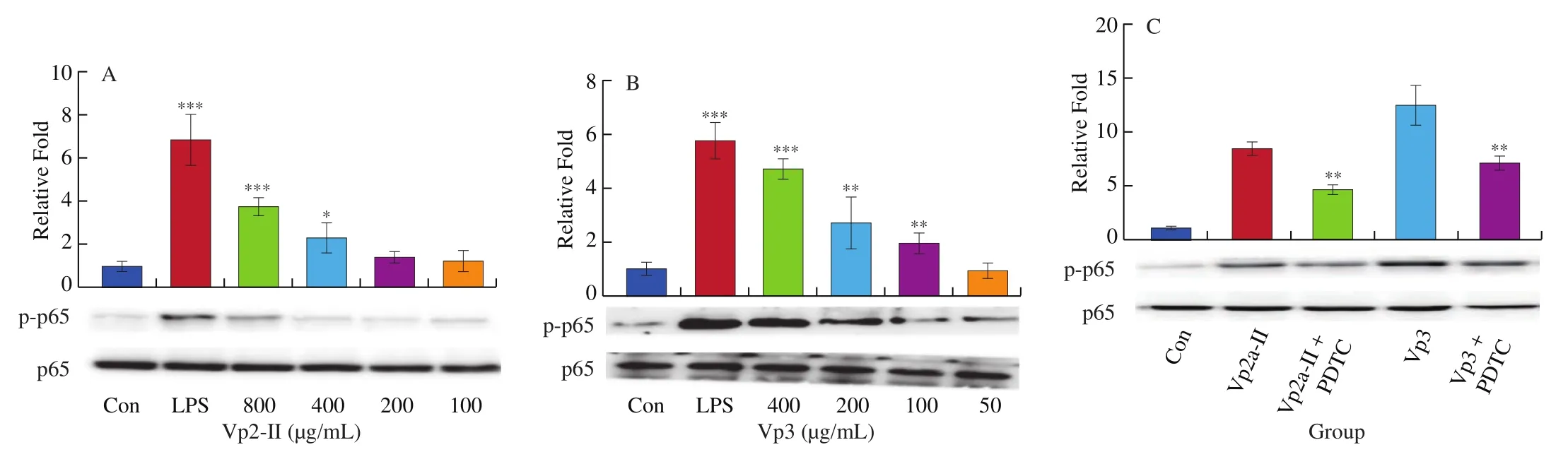
Fig. 5 Effects of Vp2a-II (A) and Vp3 (B) on the expression of phosphorylation NF-κB p65 protein in RAW264.7 cells and NF-κB inhibitors, PDTC (10 μmol/L) (C),on protein expression after activation of Vp2a-II (400 μg/mL) and Vp3 (200 μg/mL). Data are presented as “mean ± SD”, n = 3. Compared with the control, ***indicates a significant difference at P < 0.001, ** indicates a significant difference at P < 0.01, * indicates a significant difference at P < 0.05.
The expression of p-p38, p-ERK and p-JNK protein in the LPS-stimulated group and theA. venetumpolysaccharidetreated groups was significantly higher than that of the normal control group (Fig. 6). When the concentration of Vp2a-II was 100–800 μg/mL, the expression of the phosphorylated proteins associated with the MAPK pathway was significantly enhanced (Fig. 6A).When the concentration of Vp3 was 50–400 μg/mL, the degree of phosphorylation of ERK1/2 and JNK increased significantly whilst the effect of the phosphorylation of p38 MAPK protein was not obvious(Fig. 6B). To further verify the activation exerted by Vp2a-II and Vp3, MAPK inhibitors, including PD98059 (ERK inhibitor,30 μmol/L), SP600125 (SAPK/JNK inhibitor, 3 μmol/L) and SB203580 (p38 inhibitor, 10 μmol/L), were applied to Vp2a-II(400 μg/mL) and Vp3 (200 μg/mL) (Fig. 6C–E). As expected,the application of MAPKs inhibitors significantly reduced the phosphorylation of ERK1/2, JNK and p38 induced by Vp2a-II in RAW264.7 cells, in contrast with the outcome arising from the stimulation by Vp2a-II alone. The application of MAPKs inhibitors significantly decreased the phosphorylation of ERK1/2 and JNK induced by Vp3 in RAW264.7 cells, but did not reduce the phosphorylation of p38 MAPK protein. The data in Figs. 6C-E verified the results in Fig. 6B. Taken together, Vp2a-II and Vp3 could activate the MAPKs signaling pathway in RAW264.7 cells.
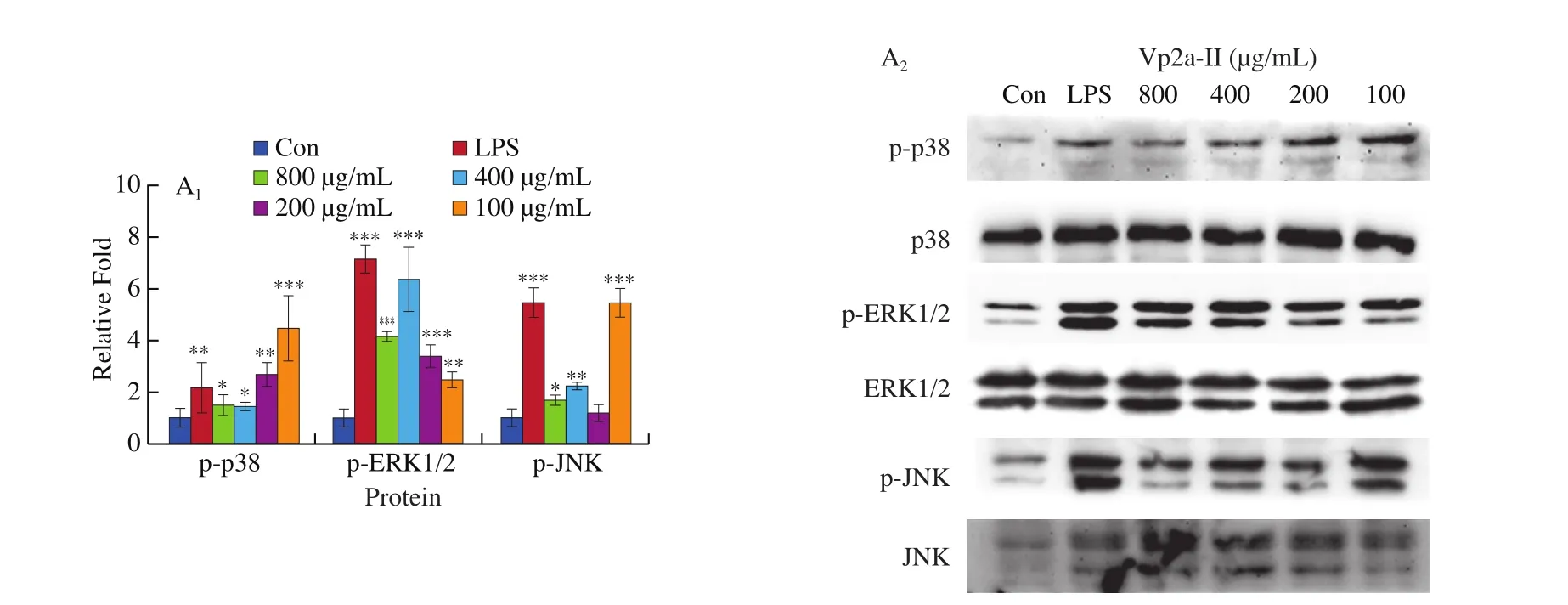
Fig. 6 Effects of Vp2a-II (A) and Vp3 (B) on the expression of phosphorylation MAPK proteins, including p-p38, p-ERK, and p-JNK, in RAW264.7 cells.Effects of specific inhibitors, including SB203580 (p38 inhibitor), PD98059 (ERK inhibitor), and SP600125 (JNK inhibitor) on Vp2a-II (400 μg/mL) and Vp3(200 μg/mL) inhibit p-p38(C), p-ERK (D) and p-JNK(E) expression in RAW 264.7 cells. Data are presented as “mean ± SD”, n = 3. Compared with the control,*** indicates a significant difference at P < 0.001, ** indicates a significant difference at P < 0.01, * indicates a significant difference at P < 0.05
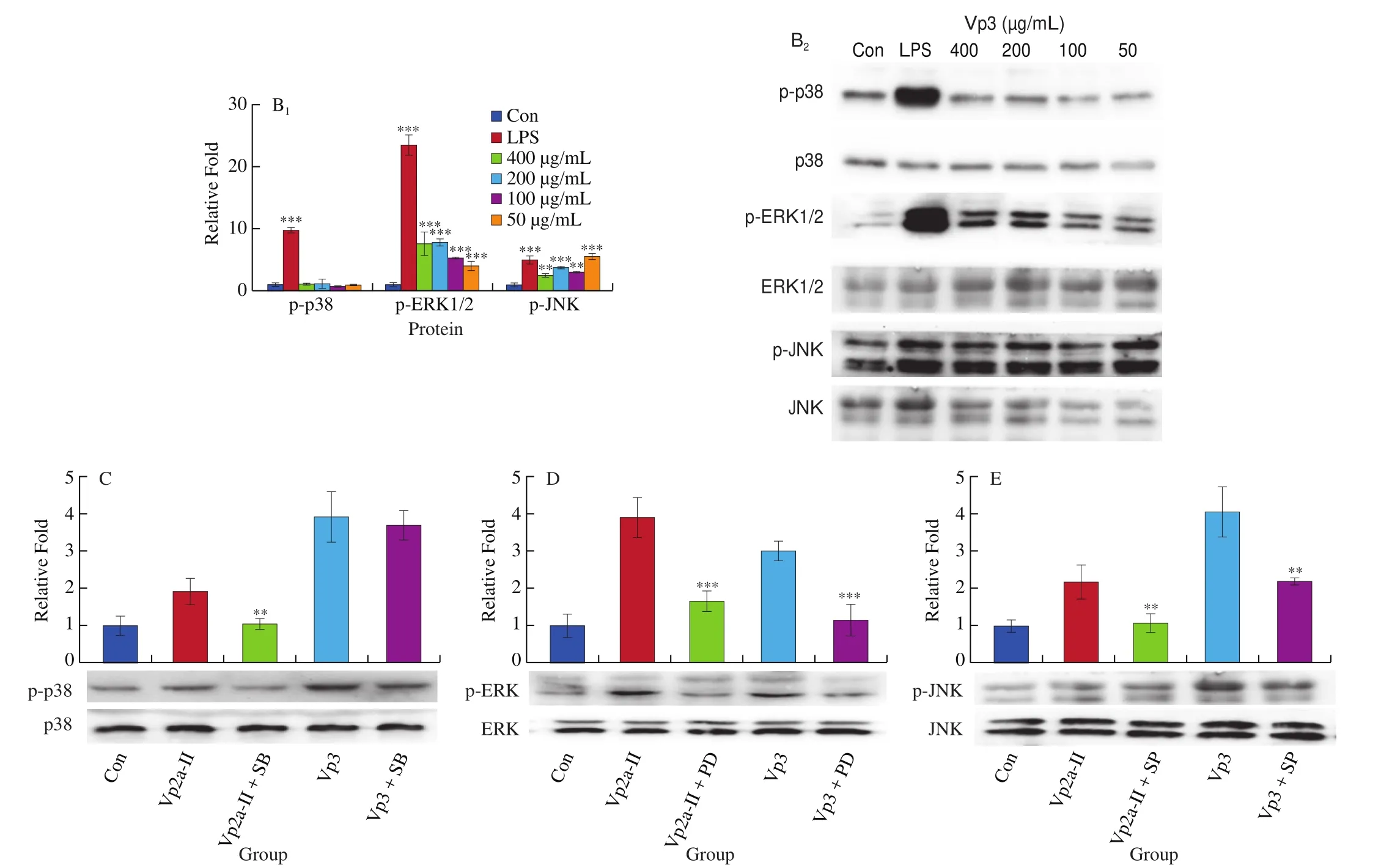
Fig. 6 (Continued)
4. Discussion
Previous studies have demonstrated that certain polysaccharides from different plants could enhance the function of macrophages [31].The research on the mechanisms underlying the immunoregulatory function of polysaccharides has reached a point where events at the molecular level and even the receptor level. Plant polysaccharides were found capable of activating macrophages by binding to specific receptors on the surfaces of cells, including Toll-like receptor 4(TLR4), CD14, complement receptor 3 (CR3), scavenger receptor(SR), mannose receptor (MR), and Dectin-1 [17]. The activation of macrophage receptors can initiate a series of intracellular signaling cascades, including the MAPK and NF-κB pathways, which lead to the transcriptional activation and production of inflammationrelated cytokines such as IL-6 and TNF-α, and ultimately an inflammatory response [32]. The MAPK and NF-κB pathways are common inflammatory signaling pathways and can regulate the production of molecules such as NO, TNF-α and IL-6 engaging in immune response and inflammation, and MAPKs that are activated by inflammatory stimuli contribute directly to the activation of NF-κB [33,34]. The mechanism of immunomodulatory pathway was showed in the Fig. 7.
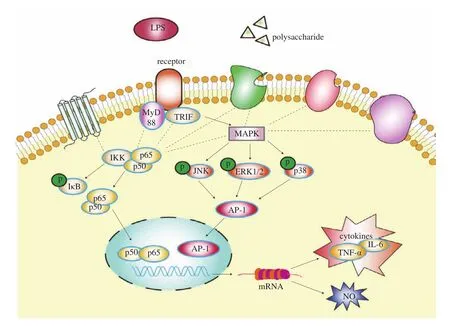
Fig. 7 Schematic diagram of the immune regulatory mechanism of polysaccharides.
The phagocytic activity is one of the most important parameters for assessing immunotoxicity and changes in the function of macrophages, because it represents the first and imperative defense function of macrophages in immune response [35]. Foreign matters and pathogens are broken down at low pH values, and ingested and degraded by macrophages. Once the pathogen-derived products are presented to T cells and/or B cells, the acquired immunity system is activated [36]. Recently, it has been shown that macrophagemediated therapies could treat many diseasesviaphagocytosis.Macrophages facilitate the uptake, degradation and clearance of the invading nanoparticles from the circulatory system [37,38]. Bioactive polysaccharides were found capable of binding to pattern recognition receptors (like TLRs) on the surface of macrophages and then initiating relevant signaling pathways to further activate macrophages [39]. In this study, compared with the normal control group, Vp2a-II at the examined dose range significantly enhanced the pinocytic activity of RAW264.7 cells while Vp3 enhanced the phagocytic activity only at a high concentration (like 400 μg/mL). The reason for phagocytosis index of Vp2a-II was higher than that of Vp3 at the concentration may be that Vp2a-II could ificantly promote the cell proliferation, while Vp3 could not. The cell proliferation rate had certain influence on the determination of phagocytic activity of macrophages.
NO is a highly reactive free radical produced from the conversion ofL-arginine by three isoforms of nitric oxide synthase (NOS): eNOS,iNOS, and nNOS [40]. iNOS is the major isoform of NO existing in macrophages but generally not expressed under normal physiological conditions. The iNOS can be induced to express by endotoxin or some cytokines, such as TNF-α, IL-1, IFN-γ. The released NO can kill virally infected cells, tumor cells, invading microorganisms and parasites, thereby protect the body against external adverse reactions and invading events [41]. In this study, Vp2a-II (100–800 μg/mL) and Vp3 (50–400 μg/mL) exhibited direct promotion on the secretion of NO in a dose-dependent manner. Besides, Vp2a-II (400 μg/mL) and Vp3 (200 μg/mL) could increase the expression ofiNOSmRNA. The above results indicated that Vp2a-II and Vp3 could play an immun modulative role by regulating the release of NO.
Cytokines are small signaling proteins secreted by the cells associated with the innate and adaptive immune systems, and act as the intercellular messengers to integrate immune-related cells to construct a coherent response and regulate immunity, inflammation,viral pathogenesis, tumorigenesis and hematopoiesis [42]. RAW264.7 cells regulate the innate and adaptive immunity systems by releasing cytokines such as IL-1β, IL-6, and TNF-α and by improving cell crosstalk, cell growth, and cell differentiation [43]. IL-6 as both a proinflammatory cytokine and an anti-inflammatory myokine plays an important role in promoting the differentiation of T cells and B cells [44].TNF-α as a multifunctional pro-inflammatory cytokine and a major orchestrator of inflammation plays important roles in apoptosis,inflammation and immunity, thereby inducing tumor cell death [45].The ELISA results showed Vp2a-II (400 or 800 μg/mL) and Vp3(50 μg/mL) significantly promoted the production of TNF-α and IL-6. There were many reasons for the difference in ELISA results,such as kits, human operation, sample treatment, etc. qPCR was adopted to further detect the mRNA expression levels of related cytokines. The qPCR results showed that Vp2a-II (400 μg/mL) and Vp3 (200 μg/mL) could promote the mRNA expression ofTNF-αandIL-6. The above results indicated that the addition of Vp2a-II and Vp3 could influence the release of TNF-α and IL-6 in RAW264.7.
Phytogenic polysaccharides were found capable of activating the macrophages and then the downstream pathways, including the MAPK and NF-κB signaling pathways [46]. MAPK and NF-κB regulate a variety of cellular processes, including oxidative stress, immune response, cell proliferation and apoptosis, as well as the transcription and expression of a variety of pro-inflammatory genes [47]. MAPKs are a family of serine threonine kinases and can transduce signals from a diverse array of extracellular stimuli including oxidative stress and cytotoxic factors and contribute to the activation of transcription factors such as NF-κB [48]. NF-κB is a group of pleiotropic transcription factors consisting of five members (p50, p52, p65(RelA), RelB, c-Rel). These NF-κB members function as switchlike agents at the single cell level in B and T cells as well as nonimmune cells, and can be activated by more than 400 stimuli [40,49].The NF-κB members usually exist in a quiescent state in the cytoplasm through combining with the NF-κB inhibitor (IκB). Once macrophages are activated, different upstream pathways converge on the high molecular weight IκB kinase (IKK) complex. The complex is critical for IκB phosphorylation and signal transduction to NF-κB,which can lead to the release of NF-κB from the cytoplasm and translocation into the nucleus to activate specific target genes [50,51].The MAPK family mainly includes ERK (p42/p44), p38, and JNK (p46/p54) [52]. The MAPK pathways were mainly activated by the phosphorylated key proteins to regulate immune reaction.Polysaccharides from RadixAstragaliwere found to enhance immune functions of RAW264.7 cells through activating the MAPK pathway and inducing nuclear translocation of NF-κB p65 [53].
In this study, Vp2a-II promoted the transfer of NF-κB p65 from the cytoplasm to the nucleus and the phosphorylation of MAPK pathway-related proteins. Vp3 induced NF-κB and MAPK signaling pathways without influencing the p38 MAPK pathway-related protein.To further explore the mechanisms underlying the function of Vp2a-II and Vp3 to activate macrophages, the downstream signaling pathways were blocked using corresponding protein inhibitors in this study.Protein inhibitors only inhibit the expression level of specific pathway proteins and have a little effect to other signaling pathway proteins.Compared with the polysaccharide group, if the addition of protein inhibitor decreasing the corresponding protein phosphorylation expression level, it indicates that the polysaccharide plays an immunomodulatory role through this signaling pathway. The obtained results from protein inhibitors studies further support our conclusion.The average molecular weights of Vp2a-II and Vp3 were 7 and 9 kDa,respectively. The backbone of Vp2a-II was composed of →6)-β-DGlcp-(1 → 6)-α-D-Galp-(1→ residues, while Vp3 was composed ofα-D-GlcpA-(3 →α-D-GalpA residues. Their backbone are formed by glucose and galactose, but the connection of monosaccharides is different. The branched chain of Vp2a-II was composed of arabinose,while Vp3 mainly were composed of arabinose and mannose.Vp2a-II and Vp3 have different molecular weight, monosaccharide composition, conformation, and branching degree, which all may cause differences in their immune activity.
5. Conclusions
This study demonstrated that Vp2a-II and Vp3 obtained from the flowers ofA. venetumcould activate RAW264.7 cells by promoting cell viability phagocytosis, and enhancing the NO secretion and mRNA expression ofiNOS,IL-6andTNF-α. Moreover, Vp2a-II and Vp3 could trigger the MAPK signaling pathway and then induce the nuclear translocation of NF-κB p65. The findings obtained in this study provide a better understanding of the molecular mechanism underlying the functions of immunoregulatory agents. Collectively,bioactive polysaccharides, Vp2a-II and Vp3, are potential immunostimulants and promising therapeutics for immune disorders and immune-related diseases.
Competing interests
The authors declare that they have no competing interests.
Acknowledgment
This work was supported by Research on Precision Nutrition and Health Food, Department of Science and Technology of Henan Province (CXJD2021006)
- 食品科学与人类健康(英文)的其它文章
- Dietary bioactives and essential oils of lemon and lime fruits
- Green tea, epigallocatechin gallate and the prevention of Alzheimer’s disease: clinical evidence
- Simultaneous quantification of 18 bioactive constituents in Ziziphus jujuba fruits by HPLC coupled with a chemometric method
- A systematic study on mycochemical profiles, antioxidant, and anti-inflammatory activities of 30 varieties of Jew’s ear (Auricularia auricula-judae)
- GPP (composition of Ganoderma lucidum polysaccharides and Polyporus umbellatus polysaccharides) protects against DSS-induced murine colitis by enhancing immune function and regulating intestinal flora
- MLST analysis of genetic diversity of Bacillus coagulans strains to evaluate effects on constipation model

