Contribution of redox-active properties of compost-derived humic substances in hematite bioreduction
Chao Yang, Lin-Xiao Hou, Bi-Dou Xi, Li-An Hou, Xiao-Song H,*
a State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China
b State Environmental Protection Key Laboratory of Simulation and Control of Groundwater Pollution, Chinese Research Academy of Environmental Sciences,Beijing 100012, China
c School of Environmental Science and Engineering, Tianjin University, Tianjin 300350, China
d College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
e High-Tech Institute of Beijing, Beijing 710025, China
Keywords:Composting Humic substances Reducing capacities Humic acids Hematite bioreduction
ABSTRACT The compost-derived humic substances (HS) can function as electron mediators for promoting hematite bioreduction because of its redox capacity.Humification process can affect redox capacities of compostderived HS by changing its intrinsic structure.However, the redox properties of compost-derived HS linking with hematite bioreduction during composting still remain unclear.Herein, we investigated the redox capacities of compost-derived HS, and assessed the responses of the redox capacities to the hematite bioreduction.The result showed that compost-derived HS (i.e., humic acids (HA) and fulvic acids (FA))were able to accept electrons from Shewanella oneidensis MR-1, and the electron accepting capacity was increased during composting.Furthermore, it could be functioned as electron mediators for promoting the hematite bioreduction, achieving 1.19-2.15 times compared with the control experience.Not only the aromatic structures (quinone) but also the non-quinone structures such as nitrogen- and sulfur-containing functional moieties were served as the redox-active functional groups of compost-derived HS.Our work proved that the aromatic functional groups and the heteroatom structures (especially N) were important to the hematite bioreduction.This study highlights the redox-active properties of compost-derived HS and its impact on the microbial reduction of iron mineral.Redox capacity of compost-derived HS might mitigate the environmental risk of contaminants when the composting production was added into the contaminated soils as low-cost repair materials
The rapid urbanization and population growth accelerate greatly the generation of municipal solid wastes (MSWs) in China[1,2].The transported quantity of MSWs reach 242.1 million tons in 2019, and more than 200 cities are facing the predicament of being surrounded by MSWs [3].MSWs are considered as the misplaced resource, and resource recovery from MSWs is an effective option to overcome the dilemma of the city besieged by waste [4].In China, MSWs are characterized as high water-content,low calorific-value and high organic-composition, which restricted widespread adoption of combustion for obtaining energy recovery[5,6].
Composting is an efficient and eco-friendly method for disposing MSWs [7].It is a biological process in which the organic substances are converted to stable humic substances (HSs) by aerobic thermophilic/mesophilic microorganisms [7,8].Compost can not only obtain resource recovery from MSWs but reduce carbon emission [9].Compost is a stable and organic-rich product, which can be applied to soil.It not only enriches the soil nutrients for the plant growth (e.g., nitrogen, phosphorus, and potassium), but also improves microbial species and accelerates the nutrient mobilization [8,10].Furthermore, compost product is regarded as lowcost materials to remediate contaminated soils.For example, the compost-derived HS can interact with soluble/insoluble contaminant (e.g., Zn(II), U(VI), Cr(VI) and polycyclic aromatic hydrocarbon), resulting in contaminant-complexes with low mobility [11-14].
Presently, numerous studies have been focused on the redox capacity of compost-derived HS because of its great potential in contaminated soil treatment [15,16].Natural HS can be used as the electron shuttle, which enhances the electron transfer from microbial respiration to the terminal electron acceptor (e.g., Feoxide) based on its redox capacities [17,18].For instance, soilderived HS can serve as the additional electron acceptor for anaerobic microbial respiration, which controls the amount of CH4formation [18].HS promotes Fe(II) generation from iron-bearing mineral in the process of microorganism reduction, and then affects anthropogenic contaminant in natural environments, particularly in Fe-rich soils and sediments [17,19,20].Under suboxic/anoxic environments, HS accepts electrons directly from microorganisms(e.g., iron-reducing bacteria, sulfate-reducing bacteria and fermenting bacteria) [19,21-23].Reduced HS further donates electrons to poorly-soluble Fe(III) (oxyhydr)oxides (e.g., hematite, ferrihydrite)[18,23].The microbial reduction of HS and subsequent chemical reduction of poorly soluble iron(III) minerals can contribute to iron redox processes when Fe(III) minerals are present in anoxic natural environments [23].Previous studies show that quinone moieties are the main functional components with mediating electron transport between bacteria and Fe(III) [24,25].Composting is an artificially enhanced organic matter humification process [7].Comparing with natural HS, the structure, precursor transformation and formation environment of compost-derived HS are different [7,16].The humification and aromatization of the compost-derived HS is lower than that of natural HS [7,20,22].Previous reports have indicated that the aromatic humification and aromatization is the crucial index of redox-active capacity for HS [15,16].Thus, the redox capacity of compost-derived HS may be different from that of natural HS.However, to date, the effect of the redox capacity of compost-derived HS remains unclear in microbial dissimilatory iron mineral reduction.
This study was to investigate systematically the construction and redox capacity of compost-deriver HS, and assessed the responses of redox capacity in microbial dissimilatory iron mineral reduction.To test this, the construction of compost-derived HS was analyzed by UV-vis spectra,1H NMR spectra, elemental analysis,and the reversed phase high performance liquid chromatography(RP-HPLC).The details of the analytical methods were supplied in Texts S1-S5 (Supporting information).The specific aims were (i) to determine the redox capacity of compost-derived HS (i.e., humic acids (HA) and fulvic acids (FA)) and (ii) to identify the influence of the redox capacities for hematite bioreduction.Redox capacities of the compost-derived HS were able to mitigate the environmental risk of the contaminants associated with composting production applied in the contaminated soils.
Compost-derived HS (including HA and FA), which were extracted at different composting stages, was reduced by shewanella.oneidensis MR-1 under anaerobic condition, and then reacted with ferric citrate.The HS act as redox carrier by accepting electrons from microbial respiration under anoxic condition.Thus, the microbial reduction method of assessing the redox capacities of compost-derived HS was necessary despite its redox capacities had reported by the mediated electrochemical reduction and oxidation methods [26].Native reducing capacity (NRC), electron accepting capacity (EAC) and microbial reducing capacity (MRC) were used to assess the redox capacities of compost-derived HS (detailed description in Text S2 (Supporting information), supporting information).As shown in Fig.1a, NRC of the HA was increased firstly, then decreased, and reached the maximum in 28-day composting (1.01 mmol/(g C)).Interestingly, NRC of the FA decreased from 0-day (1.99 mmol/(g C)) to 28-day (1.35 mmol/(g C)), and slightly increased to 1.44 mmol/(g C) on 51-day.The EAC of the HA and FA all increased to the maximum on 28-day (2.31 and 1.01 mmol/(g C)), and then slightly decreased on 51-day (Fig.1b).The change of NRC of HA was negative correlation with that of FA during composting (P <0.05), but the change of EAC of HA was significant agreed with that of FA during composting (P <0.01)(Fig.S1 in Supporting information).These results reflected the distributions of the electron-donating groups and electron-accepting groups in inherent structure of the compost-derive HSs.Meanwhile, in order to reveal differences in compost-derived and natural HS among the redox capacity, NRC and EAC of natural HS (including HA and FA) were shown in Figs.S2 and S3 (Supporting information).The results indicated that the redox capacities (NRC and EAC) of compost-derived HS were similar to that of natural HS.Thus, the above result verified that humification process can promote the formation of redox-active functional groups (such as amidogen, phenolic hydroxyl group, quinone-like moieties), resulting in excellent redox capacity of compost-derived HS [15].
The MRC of the compost-derived HS, which derived from the different compost stages, was also evaluated (Fig.1c).Our result showed that MRC of the HA and FA decreased firstly and then increased, and it reached the minimum in the 28-day composting (3.32 mmol/(g C) and 2.36 mmol/(g C)) (Fig.1c).The range of the HA were remarkably higher than that of FA, indicating composting could affect the redox-active capacities of the HS, especially HA.In addition, the NRC of FA (average value was 1.58 mmol/(g C)) was significantly higher than NRC of HA (average value was 0.76 mmol/(g C)), but the EAC of FA (average value was 0.76 mmol/(g C)) was significantly lower than the EAC of HA (average value was 1.58 mmol/(g C)) (Fig.1d), indicating the electronaccepting capacity of HA was higher than that of FA, which were attributed to the difference of the redox-active functional groups in inherent structure of the HA and the FA.
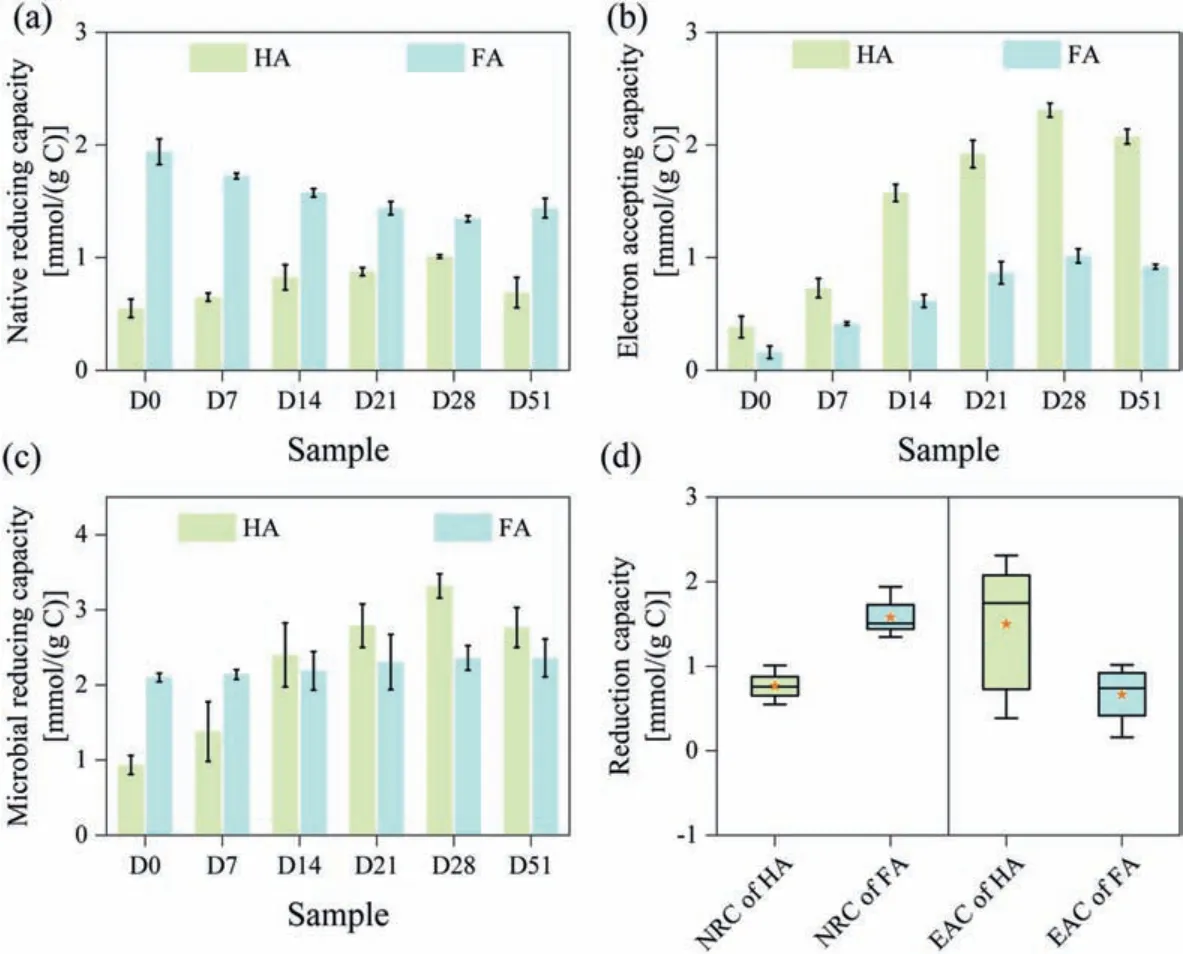
Fig.1.Native reducing capacity (a), electron accepting capacity (b) and microbial reducing capacity (c) of the compost-derived HA and FA and (d) their distribution of the native reducing capacity (NRC) and electron accepting capacity (EAC). ★Represents the average value.
Under 15-day anoxic environments, HS accepted electrons directly from microorganisms (MR-1), and then donated electrons to hematite (Fig.2a).Previous studies had been shown that some reduced HS functional groups such as aromatic ketones cannot be oxidized by O2, or some redox-active functional groups may be shielded because the availability of the functional groups is likely to change as a result of molecule aggregation [27,28].Compared to the initial reduction capacity of the HS-free experience, the compost-derived HA and FA all promoted the hematite bioreduction except for day 0 (Figs.2d and e).The ineffectiveness of initial compost-derived HS for hematite bioreduction might be resulted in its complete decomposition by MR-1 under 15-day anaerobic culture.Composting process could change the distribution of the redox-active functional groups in the compost-derived HS [7],which influenced observably the Fe(II) production in the process of the HS-mediating hematite bioreduction.Although an observed decline occurred in the 51-day compost-derived FA, the total HCl-Fe(II) were increased generally with composting time in the process of the HS-mediating hematite bioreduction.Meanwhile, the total HCl-Fe(II) of FA (average value was 0.279 mmol/L) in hematite reduction was higher than that of HA (average value was 0.243 mmol/L) (Figs.2b and c), which was different from distribution of MRC.Previous studies show that HS sorption on iron mineral surfaces will suppress the iron mineral reduction [29,30].The HA and FA, which are isolated based on hydrophobicity, were also regarded as the multi-component mixture of aromatic and aliphatic hydrocarbon structures with polar functional groups (such as carboxyl,hydroxyl, quinone) [31].Thus, the result indicated that the intrinsic physiochemical properties of compost-derived organic matters might influence hematite bioreduction.To investigate this, we tested the physiochemical properties of HA and FA during composting.
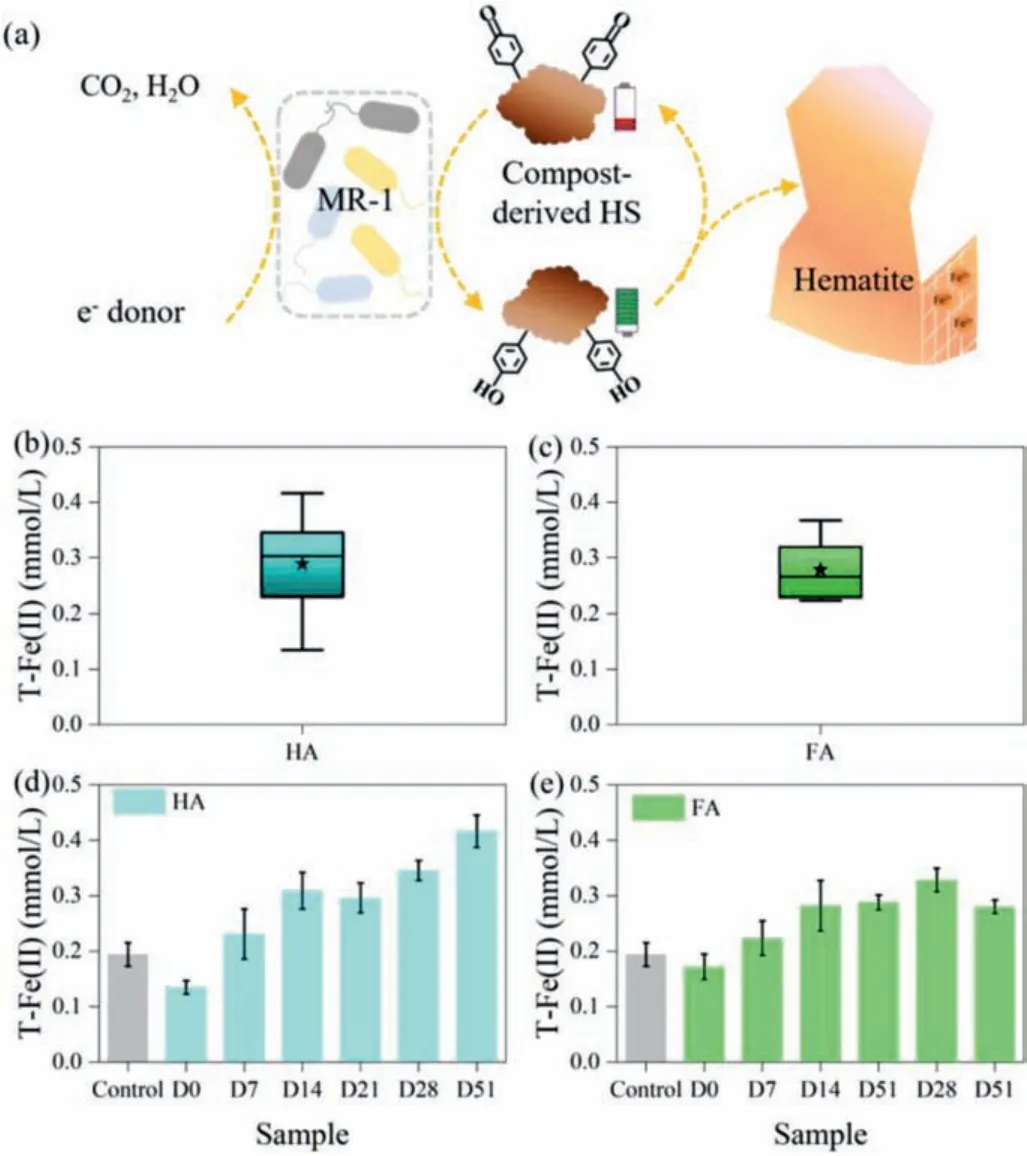
Fig.2.(a) The schematic illustration of hematite bioreduction by compost-derived HS.The distribution of total HCl-Fe(II) of compost-derived HA (b) and FA (c) in hematite reduction.The total amount of Fe(II) in compost-derived HA (d) and FA(e) mediated hematite bioreduction. ★Represents the average value.
The UV spectra,1H NMR spectra and fluorescence spectra were used to analyze chemical structures of the compost-derived HS,and the result showed in Table 1 and Fig.S4 (Supporting information).SUVA254and HIX indicated generally the degree of aromatic degree of HS [19,28].The result showed that the aromatic component of HA and FA increased firstly and then declined during composting.Meanwhile, the SUVA254and HIX of HA were lower than that of FA, which confirmed that the aromatic degree of HA was lower than that of FA (Fig.S4).The phenomenon was also confirmed by the relative amount of aromatic H of HA and FA (the aromatic H of HA and FA were 1.67%-7.66% and 7.36%-8.75%, respectively) (Table 1).The characteristic area of 0-3.0 ppm was relative to the terminal methyl groups of aliphatic structure and protons of aliphatic carbon, and the characteristic areas of 3.0–4.25 ppm was relative to protons attaching to O or N heteroatoms [32,33].The result indicated that the aliphatic structure of HA was decomposed during composting, whereas the O or N heteroatoms and aromatic structure were increased firstly and then decomposed (Table S1 in Supporting informationm).Meanwhile, the aliphatic structure of FA was increased firstly and then decomposed, whereas the O or N heteroatoms were declined during composting.Remarkably, the aliphatic structure was the main constituent in the compost-derived HA and FA during composting (more than 60%).
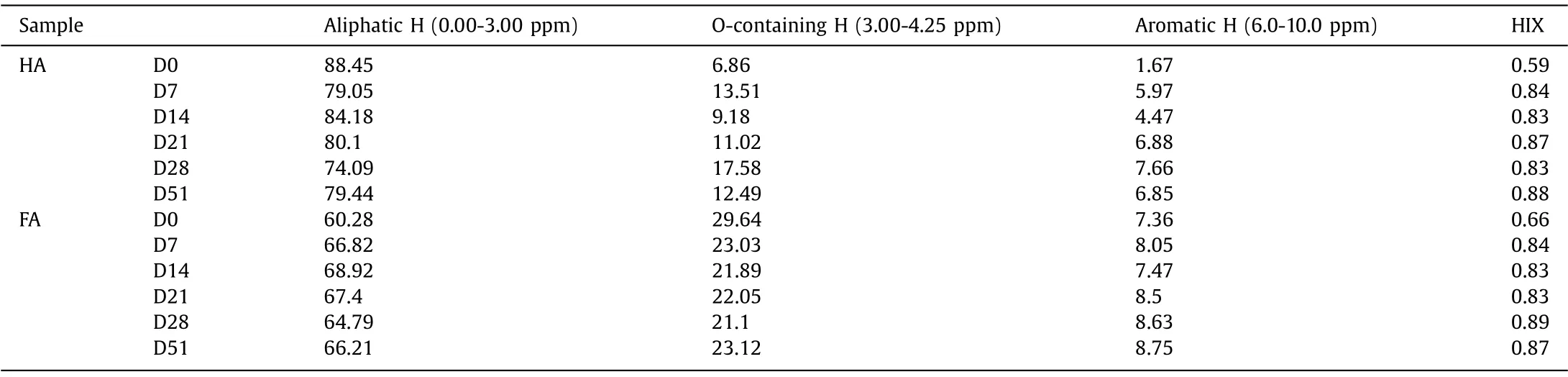
Table 1 Chemical structures of compost-derived HA and FA during composting.
HS was consisted mainly of protein-like component with Em<380 nm and humic-like components with Em>380 nm based on fluorescence spectra.Recent studies suggested that the redoxactive functional groups (e.g., quinone), which were used as electron shuttle for microbially mediated Fe(III) reduction, mainly existed in humic-like components [5,24,34].Therefore, the RP-HPLC detectors with measuring wavelength at 280 nm were used to analyze the molecular weight distribution of the aromatic substances(Figs.S5 and S6 in Supporting information).After peak fitting, the areas and retention times of different molecular weight peaks were shown in Table S2 (Supporting information).Fig.S7 (Supporting information) revealed the change in percentage of high molecular weight (HMW) (retention time<2 min) and low molecular weight(LMW) (retention time>2 min) during composting.The percentage of HMW of the HA and FA increased in the initial process of the 28-day compost, whereas it showed a slight decrease in the 51-day compost (Fig.S7).Besides, the HMW’s percentage of the HA was higher than that of FA (Fig.S7).
In order to determine the relationship between the reducing capacities (NRC and EAC) and intrinsic physiochemical properties, we employed a wide set of parameters to reflect the chemical structure.SUVA254, HIX and aromatic H could be used to predict the aromatic structure [23,28,35].NRC could reflect the capacity of donating electron.The NRC of HA was positive related to the aromatic construction (Fig.3), and it negatively related to aliphatic HA.However, the NRC of FA was negatively related to the aromatic construction, but it significantly positive to O-containing functionalities and S/C (Fig.3).Meanwhile, the NRC of HA and FA was all significantly positive to S and S/C.N and N/C influenced distinctly NRC (Fig.3).The result indicated that the aromatic structure of the compost-derived HA and FA were not mainly functional groups.The heteroatom structures (e.g., N or S) were also used as electron donator for transferring electron to Fe(III).
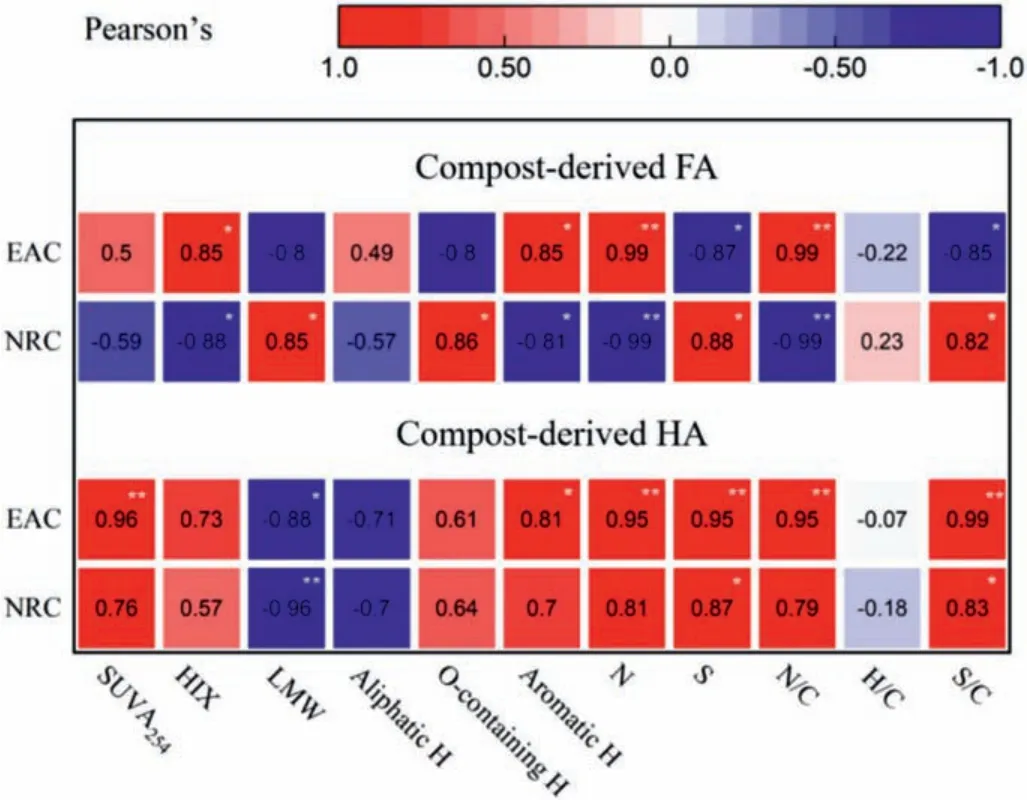
Fig.3.Correlation between redox capacity of HS and its inherent structures.The correlation coefficient (R2) between redox capacity and inherent structures was indicated by color and numbers.Significance (P) of the correlations (*) and (**) were indicated at the level of 0.05 and 0.01, respectively.
Correlation analyses revealed that the EAC of the HA and FA were associated significantly with SUVA254, HIX and aromatic H.Meanwhile, the EAC of the HA and FA was also positive correlation with the N, S, N/C and S/C (Fig.3).These results indicated that the redox-active functional groups were not only dependent on aromatic construction, but also may be attributed to a variety of functional groups, such as heteroatom structures (e.g., N or S) with multiple oxidation state and inner-sphere metal-organic charge transfer complexes [27,36,37].Meanwhile, the acceptingelectron groups of the HA and FA might distribute the HMW of the aromatic substances (Fig.3).The electron-donating groups of FA existed in the LMW of the protein-like substances, whereas that of HA existed in the HMW of humic-like substances.Overall, chemical structures mightn’t have linear relationship with the MRC of the compost-derived HA and FA, which possibly due to the diversity redox-active functional groups.
Dissimilatory iron mineral reducing processes were linked to the cycling of elements (such as carbon, nitrogen), and it reduced environmental risks of pollutants in the natural environment [17,19].Thus, we concluded that some redox-active groups in the compost-derived HS could be accepted from shewanella.oneidensis MR-1, but it could not be used as the electron shuttle to transfer electrons which generated by oxidation of sodium lactate and transferred eventually to hematite [38,39].This observation could be supported by previous studies [19].
Correlation analyses were used to investigate the influence of physicochemical property on the hematite reduction.HIX, SUVA254and aromatic H could predict the degree of condensation in the aromatic rings, which were used to indicate quinonoid structures[23,27].As shown in Fig.4, HIX, SUVA254, aromatic H of HA and FA were all significantly associated with the T-ETC.The heteroatom structures (e.g., N or S) of HA and FA also influenced its T-ETC.N and N/C of HA and FA were significantly associated with the TETC.S and S/C were negative relationship with T-ETC of FA, but it was positive with T-ETC of HA.Thus, our results could indicate that the aromatic systems, such as quinone, functioned as the major electron-transfer moieties in the process of hematite bioreduction.The heteroatom structures (especially N) were also function as electron mediators for hematite bioreduction.
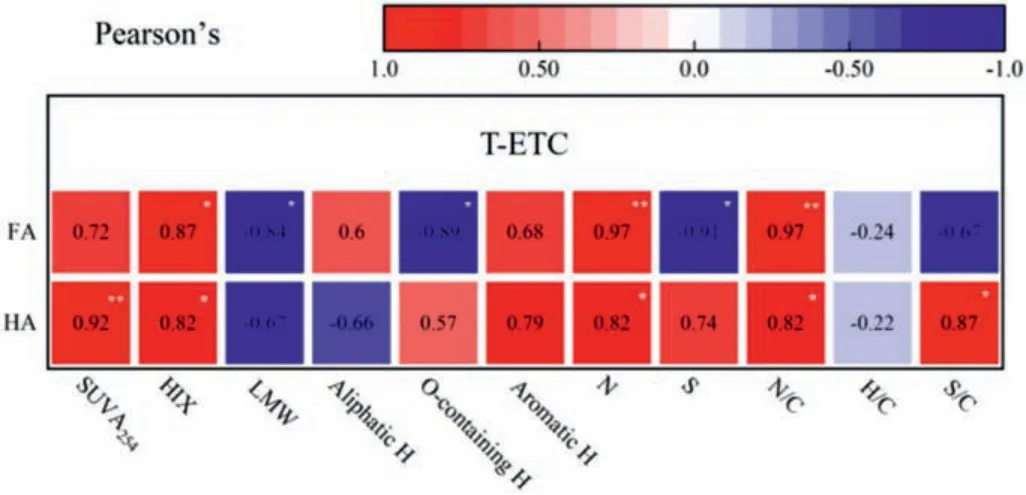
Fig.4.Correlation between Chemical structures of HS and the total-Fe(II) generating the compost derived HA and FA mediating hematite bioreduction (T-ETC).The correlation coefficient (R2) between redox capacity and inherent structures was indicated by color and numbers.Significance (P) of the correlations (*) and (**) were indicated at the level of 0.05 and 0.01, respectively.
Meanwhile, the T-ETC of HA had a positive correlation with the O-containing functional groups, but T-ETC of FA had a negative relationship with the O-containing functional groups (Fig.4).The O-containing functional groups were likely to arise from CHOH and CH2-OH functional groups, which may indicate the presence of methoxyphenylpropyl repeating units, typical molecules found in lignin and lignin-like matter [40,41].The extent to which lignin-derived phenols of compost-derived HA and FA affected the hematite reduction was not easily discernible and required further investigation.T-ETC of the HA and FA were significantly negatively related to LMW of the aromatic substances (Fig.4), indicating that the redox-capacity functional group could mainly exist in the HMW of the humic-like substances.
Redox cycling of HS is believed to significantly promote bioreduction of various Fe(III) minerals, and then influence the cycling of elements (such as carbon, nitrogen) and the degradation of pollutants in the natural environment.Our study showed that the compost-derived HS could accept electrons from microorganism,and the compost-derived HS was used as electron mediators for promoting the hematite bioreduction.The hematite bioreduction increased 1.19-2.15 times when compost-derived HS was added.The enhancement of hematite bioreduction was mainly attributed to the aromatic structures (such as quninones) and the heteroatom structures (especially N).Compost was the important method to recycle resource from MSWs, and could suppress significantly the emissions of greenhouse gases (CO2and CH4) [1,42].This study allowed us to accept that the compost could be used as the ecofriendly and low-cost product for restoring the contaminated soil.Meanwhile, to obtain excellent remediate efficiency, we suggested that the composting materials should be added a more amount of lignin, considering that it could be converted into the aromatic structures in the process of compost.Overall, these findings can help us to clarify the effect of specific component of compostderived HS on their redox-active property.
The research focused on the redox-active property of compostderived HS during composting, and investigated the impact of the reducing capacities in the dissimilatory hematite reducing processes.Compost-derived HS could accept the electrons from microorganism.The aromatic structures were the contributors to redox-active groups in the compost-derived HS, but the contributions of non-quinone structures, such as nitrogen and sulfur functional moieties, cannot be disregarded.Owing to the reversible reduction-reoxidation, the compost-derived HS can accelerate the bioreduction of hematite, increasing by 1.19-2.15 times compared with the control experiment.The enhancement of hematite bioreduction is mainly attributed to the aromatic structures (such as quninones) and the heteroatom structures (especially N).Our work could help us to better understand the importance of the compostderived HA redox cycling in the dissimilatory hematite reducing processes.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
This work was supported by Central Research Institutes of Basic Research and Public Service Special Operations of Chinese Research Academy of Environmental Sciences (No.2019YSKY-023).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.08.115.
 Chinese Chemical Letters2022年5期
Chinese Chemical Letters2022年5期
- Chinese Chemical Letters的其它文章
- Recent advances in enhancing reactive oxygen species based chemodynamic therapy
- An integrative review on the applications of 3D printing in the field of in vitro diagnostics
- Recent developments of droplets-based microfluidics for bacterial analysis
- Dynamics and biological relevance of epigenetic N6-methyladenine DNA modification in eukaryotic cells
- Recent progress in advanced core-shell metal-based catalysts for electrochemical carbon dioxide reduction
- Recent advances in carbon-based materials for electrochemical CO2 reduction reaction
